Abstract
In this paper, ketoprofen and ketoprofen lysinate were used as model drugs in order to investigate release profiles of poorly soluble and very soluble drug from sodium alginate beads manufactured by prilling. The effect of polymer concentration, viscosity, and drug/polymer ratio on bead micromeritics and drug release rate was studied. Ketoprofen and ketoprofen lysinate loaded alginate beads were obtained in a very narrow dimensional range when the Cross model was used to set prilling operative conditions. Size distribution of alginate beads in the hydrated state was strongly dependent on viscosity of drug/polymer solutions and frequency of the vibration. The release kinetics of the drugs showed that drug release rate was related with alginate concentration and solubility of the drug. Alginate solutions with concentration higher than 0.50% (w/w) were suitable to prepare ketoprofen gastro-resistant formulation, while for ketoprofen lysinate alginate, concentration should be increased to 1.50% (w/w) in order to retain the drug in gastric environment. Differential scanning calorimetry thermograms and Fourier transform infrared analyses of drug-loaded alginate beads indicated complex chemical interactions between carboxyl groups of the drug and polymer matrix in drug-loaded beads that contribute to the differences in release profile between ketoprofen and ketoprofen lysinate. Total release of the drugs in intestinal medium was dependent on the solubility of the drug and was achieved between 4 and 6 h.
Key words: controlled release, encapsulation, ketoprofen, microparticles, prilling
INTRODUCTION
Ketoprofen is widely used for the treatment of inflammation, pain, and rheumatisms. It is part of the group of non-steroidal anti-inflammatory drugs (NSAIDs) with low solubility (95 µg/ml, in distilled water at pH 7) and slow dissolution rate. Ketoprofen, as most NSAIDs, produce adverse effect to the upper GI tract (1–4) that can be reduced by altering the conventional dosage forms into a modified drug delivery system able to enhance drug bioavailability and reduce adverse effect (5–7). In fact, sustained-release as well as delayed-release dosage forms can be designed to reduce NSAIDs side effect and improve the patient compliance (8–10).
Microencapsulation is one of the most effective tools to manufacture particles for controlled drug delivery systems. Among the different physical methods for microencapsulation such as the well-known spray drying, fluid bed coating, extrusion, etc., an innovative technique known as prilling or laminar jet breakup is able to produce microparticles or beads with very narrow dimensional range and high encapsulation efficiency by breaking apart a laminar jet of polymer solution into a row of mono-sized drops by means of a vibrating nozzle device (11, 12). The resultant droplets fall into a polymer gelation solution in which they are solidified as beads.
Various polymers have been used for the development of microparticles for controlled drug delivery. Sodium alginate is a naturally occurring biopolymer used successfully in the food and beverage industry as thickening agent, gelling agent, and a colloidal stabilizer (13, 14). Alginate is finding increasing applications in the manufacturing of delivery systems for drugs, proteins (15, 16), genes, and cell immobilization (17–20) due to the possibility of producing beads in mild operative conditions by ionotropic gelation in the presence of bivalent cations (21) as well as its enteric and stabilization properties (22, 23).
In a previous study, it has been shown that solution properties (viscosity, density, and surface activity) and flow rate were mainly responsible for alginate bead micromeritics when manufactured by prilling. Moreover, it has been demonstrated that the mechanism involved in particle degradation was not pH-dependent, but particle swelling and degradation was dependent on calcium depletion from the bead structure (24).
The aim of this work was to study the release kinetics of both poorly soluble and very soluble drugs from alginate beads manufactured by prilling technology using ketoprofen and ketoprofen lysinate as model drugs. The objectives of the present work were to investigate the effects of relevant process variables such as drug concentration, polymer concentration, and microparticle micromeritics on the drug release profiles.
MATERIALS AND METHODS
Sodium alginate European Pharmacopoeia 4 (MW ≈ 160 kDa; Carlo Erba, I) was used as purchased, without further purification. The viscosity of ketoprofen/alginate suspensions and ketoprofen lysinate/alginate solutions was measured by rotational rheometer (Bohlin Instruments Division, UK)
CaCl2 anhydrous, granular (Merck, Germany) was used as purchased.
Ketoprofen and ketoprofen lysinate were kindly donated by Dompé (Dompé pha.r.ma. s.p.a., I).
Drug-Loaded Bead Preparation
An appropriate amount of sodium alginate was dissolved in distilled water at room temperature under gentle stirring for 18 h in order to obtain 100 ml of polymer solution with concentrations ranging between 0.50% and 2.50% (w/w). Different amounts of ketoprofen or ketoprofen lysinate were either dissolved or suspended into the polymer solution and stirred for 2 h in order to obtain different drug/polymer ratios (between 0.1 and 0.5). Beads were manufactured by a vibrating nozzle device (Nisco Encapsulator Unit, Var D; Nisco Engineering Inc., CH) equipped with a syringe pump (model 200 Series, Kd Scientific Inc. USA), pumping the drug/polymer solution through the nozzle. The experiments were performed at different volumetric flow rate, between 15 and 20 ml/min. The vibration frequency used to break up the liquid jet was set between 250 and 450 Hz, amplitude of vibration 100%, and the nozzle diameter was 400 µm. The distance between the vibrating nozzle and the gelling bath was fixed at 25 cm. A stroboscopic lamp was set at the same amplitude as the frequency in order to visualize the falling droplets. Drug/polymer droplets fell into an aqueous solution 0.3 M CaCl2 or 0.3 M CaCl2 ethanol solution when ketoprofen or ketoprofen lysinate was used, where they were gelified under gentle stirring. The beads were held into the gelling solution for 15 min at 25°C then recovered and rinsed thoroughly with distilled water. Finally, the beads were dried at room temperature by exposure to air (22°C; 67% RH) for several hours (12–18 h) until constant weight was reached.
Bead Size and Morphology
Hydrated and dried bead size distribution was measured by laser light scattering spectroscopy (Coulter LS 13320, Beckman Coulter, Inc., USA) and optical microscopy (Citoval 2, Alessandrini, I).
Pictures of hydrated beads were taken with a video camera (CCD-1, JVC, J) attached to the optical microscope. Scanning electron microscopy was performed on dried beads using a Jeol 6400 microscope (Jeol, J) on a deposition of a 200–400 Å thick carbon layer. Analysis was conducted at 15 KeV, and the magnification was ×100.
Projection diameter was obtained by image analysis (Image J software, Wayne Rasband, National Institute of Health, USA). At least 75 microparticle images were analyzed for each preparation, and the length number mean diameter was calculated. The average of mean diameters and relative standard deviations were calculated for at least three different prilling processes.
The perimeter and projection surface area obtained by image analysis were used to calculate a sphericity coefficient (SC) by the following equation (25):
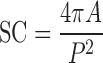 |
where A is the bead surface area and P its perimeter.
Calorimetric Analysis
Thermal characteristics of drug-loaded beads were determined by differential scanning calorimetry (DSC; Mettler Toledo DSC 822e module controlled by Mettler Star E software, USA) and compared with the thermal profiles of both blank beads and drugs as raw material. A scanning rate of 10°C/min was employed between 25°C and 260°C.
Thermogravimetric analysis (TGA) was carried out in order to evaluate water content in the different batches of dried beads (TG50, Mettler Toledo). Analysis was conducted at 10°C/min heating rate between 25°C and 200°C. Both DSC and TGA were performed in nitrogen atmosphere at a flow rate of 100 ml/min.
FTIR Analysis
Fourier transform infrared (FTIR) analysis was performed in a FTIR spectrophotometer (FT-IR Nexus, Thermo-Nicolet, USA) equipped with a mercury–cadmium–telluride detector. The samples, ketoprofen, blank alginate beads, and drug-loaded beads, were combined with small amount of potassium bromide and pressed to 3 tons in a manual press (OMCN s.p.a., I). The thin compacts produced were analyzed using 256 scans with a 1-cm−1 resolution step.
Swelling Kinetics of Dried Beads
Swelling experiments were performed in a USP 27 dissolution apparatus II: paddle, 100 rpm, 37°C (Sotax AT7 Smart–Sotax, CH) using simulated gastric fluid, simulated intestinal fluid (SIF; USP 27). In order to evaluate the swelling profiles, dried beads were placed in a vessel containing 1 l of buffer under stirring, then using an optical microscope equipped with a video camera, the bead volume was measured in swollen state at different times and compared with the volume in dry state. The degree of swelling, or swelling ratio, was calculated as the ratio between the bead volume in swollen and dry states.
Drug Content and Encapsulation Efficiency
Dried bead samples of each manufactured batch (50 mg) were dissolved under vigorous stirring in SIF. Ketoprofen and ketoprofen lysinate drug content were obtained by analyzing the solution spectrophotometrically at λ 254 nm and λ 259 nm, respectively (Lambda 25 UV/VIS Spectrometer, PerkinElmer, USA). Encapsulation efficiency was calculated as the ratio of actual to theoretical drug content. Each analysis was performed in triplicate, and the results were expressed as mean ± standard deviation. Both drug content and encapsulation efficiency were calculated correcting the weight for the residual water and calcium chloride contained into the beads previously determined by titration.
Kinetics of Drug Release
In vitro dissolution/release tests were conducted in sink conditions on 20 mg of drug using a USP 27 dissolution apparatus II: paddle, 100 rpm, 37°C (Sotax AT7 Smart–Sotax, CH) online with a UV spectrophotometer (Lambda 25 UV/VIS Spectrometer, PerkinElmer). Briefly, dried beads were added to the dissolution medium, 750 ml 0.1 M HCl for 2 h, then 250 ml of 0.20 M Na3PO4 was added and pH adjusted to 6.8 as described in the USP 27/NF monograph “Drug release from delayed-release articles.” Data were analyzed spectrophotometrically at λ 254 nm for ketoprofen and λ 259 nm for ketoprofen lysinate, respectively. Dissolution tests were conducted on six different batches of particles, and mean values were reported.
RESULTS AND DISCUSSION
Manufacturing and Characteristics of Drug-Loaded Beads
Ketoprofen and ketoprofen lysinate loaded beads were manufactured using sodium alginate solutions at different concentration from 0.50% to 2.50% (w/w), nozzle with a diameter of 400 µm, and a variable volumetric flow rate (between 15 and 22 ml/min). In accordance with data obtained in a previous study, different sets of operative conditions as volumetric solution rate and nozzle vibration frequency were selected in order to obtain microparticles in a narrow dimensional range with diameters around 750 µm in the hydrated state. To be more specific, operative conditions were selected in accordance with the viscosity of drug/polymer solutions or drug/polymer suspensions at the nozzle calculated by an equation derived from the Cross model (26):
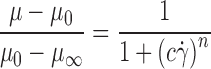 |
where µ is the viscosity at the shear rate applied to the solutions inside the nozzle; μ0 and 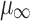 are the viscosity extrapolated at zero and infinite shear rate, respectively; and c and n are rheological parameters. The shear rate at the nozzle,
are the viscosity extrapolated at zero and infinite shear rate, respectively; and c and n are rheological parameters. The shear rate at the nozzle, 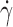 , was dependent on volumetric flow rate and the nozzle radius. Flow rate was modified in accordance with the viscosity extrapolated at zero and infinite shear rate of the solutions in order to maintain viscosity at the nozzle out of the range of turbulence (27). In fact, in that range of viscosity, laminar jet regime was transformed into a vibrated assisted dripping process due to the tendency of the solution to adhere to the nozzle (24). Moreover, because of the pseudoplastic behavior of the drug polymer mixtures, the higher the flow rate, the lower was the viscosity at nozzle.
, was dependent on volumetric flow rate and the nozzle radius. Flow rate was modified in accordance with the viscosity extrapolated at zero and infinite shear rate of the solutions in order to maintain viscosity at the nozzle out of the range of turbulence (27). In fact, in that range of viscosity, laminar jet regime was transformed into a vibrated assisted dripping process due to the tendency of the solution to adhere to the nozzle (24). Moreover, because of the pseudoplastic behavior of the drug polymer mixtures, the higher the flow rate, the lower was the viscosity at nozzle.
In order to obtain microparticles with desired diameter from concentrated polymer solutions, the frequency of vibration applied to the nozzle was increased from 250 to 450 Hz (see Table I). Although higher values of the vibration of the frequency allow the production of beads from very coherent solutions coming out from the nozzle, the frequency was kept as low as possible in order to avoid the formation of satellite droplets leading to beads with different diameter (11). In fact, the higher the frequency used during the prilling process, the higher was the fraction of undesired particles.
Table I.
Operative Conditions, Viscosity at Nozzle, and Mean Diameter of Beads Manufactured by Prilling
| Sodium alginate solution (%, w/w) | Flow rate (ml/min) | Viscosity at nozzle (m Pas) | Frequency of vibration (Hz) | Mean diameter (µm) | |
|---|---|---|---|---|---|
| Ketoprofen/Polymer | |||||
| 1:10 | 0.50 | 15 | 24 ± 0.9 | 250 | 745 ± 19 |
| 1:5 | 15 | 26 ± 1.2 | 250 | 748 ± 21 | |
| 1:3 | 15 | 27 ± 1.1 | 250 | 753 ± 21 | |
| 1:10 | 1.50 | 18 | 73 ± 2.6 | 250 | 742 ± 25 |
| 1:5 | 18 | 75 ± 2.4 | 250 | 749 ± 26 | |
| 1:3 | 18 | 76 ± 2.4 | 250 | 751 ± 19 | |
| 1:10 | 2.50 | 15 | 356 ± 9.5 | 280 | 748 ± 36 |
| 1:5 | 15 | 358 ± 9.9 | 280 | 754 ± 33 | |
| 1:3 | 15 | 362 ± 9.8 | 280 | 751 ± 36 | |
| Ketoprofen lysinate/Polymer | |||||
| 1:10 | 0.5 | 15 | 29 ± 1.3 | 250 | 746 ± 29 |
| 1:5 | 20 | 36 ± 1.8 | 250 | 755 ± 32 | |
| 1:3 | 20 | 49 ± 1.6 | 250 | 752 ± 31 | |
| 1:2 | 20 | 85 ± 2.6 | 250 | 751 ± 28 | |
| 1:1 | 20 | 118 ± 3.4 | 250 | 748 ± 32 | |
| 1:10 | 1.50 | 20 | 74 ± 2.8 | 250 | 752 ± 23 |
| 1:5 | 20 | 126 ± 4.3 | 250 | 754 ± 28 | |
| 1:3 | 18 | 180 ± 4.8 | 250 | 756 ± 25 | |
| 1:2 | 15 | 250 ± 6.6 | 270 | 749 ± 42 | |
| 1:1 | 15 | 395 ± 8.4 | 290 | 754 ± 43 | |
| 1:10 | 2.50 | 15 | 370 ± 7.9 | 280 | 748 ± 41 |
| 1:5 | 15 | 446 ± 9.3 | 300 | 749 ± 46 | |
| 1:3 | 15 | 575 ± 9.9 | 320 | 752 ± 43 | |
| 1:2 | 18 | 768 ± 12.3 | 380 | 755 ± 50 | |
| 1:1 | 15 | 986 ± 15.9 | 450 | 756 ± 52 |
Each point represents the mean ± SD (n = 3)
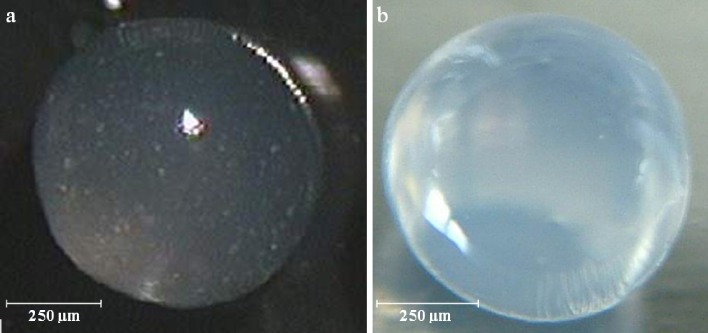
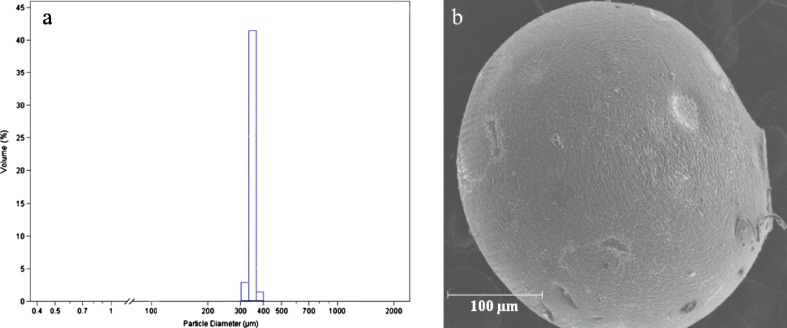
Ketoprofen and ketoprofen lysinate-loaded alginate beads were obtained in a narrow dimensional range using any set of operative conditions obtained by the Cross model. In fact, the diameters of the beads obtained by laser scattering particle size analysis in hydrated state indicated a relative deviation lower than 7% of the mean diameter for each batch. Moreover, variation on mean diameter was directly proportional to the viscosity at nozzle as expected from the model. The surface of the beads was observed to be smooth and regular in all the batches manufactured. Spots of ketoprofen particles were recognized in beads in the hydrated state due to the low solubility of the drug in the aqueous alginate solution. On the contrary, all ketoprofen lysinate beads appeared almost transparent, even the most concentrated in drug as shown in Fig. 1a, b, demonstrating that the solubility of the drug in the polymer matrix and in water was not significantly different. All batches of hydrated beads both loaded with ketoprofen and ketoprofen lysinate demonstrated good sphericity coefficient, 0.98 ± 0.02 (1 corresponds to a sphere), independently of either sodium alginate or drug concentration. Drying process was conducted by exposing the microparticles to air in normal conditions of temperature and humidity. Beads mean diameter was reduced to 350 ± 22 µm because of the shrinking of volume due to the loss of water. In the drying process, the loss of water was homogenous in any part of the beads; in fact, the reduction in diameter did not affect significantly the dimensional distribution (p < 0.005) and shape in dried beads (see Fig. 2). Sphericity coefficient of dried beads was found to be 0.92 ± 0.02.
Fig. 1.
Hydrated sodium alginate beads obtained by 2.50% (w/w) alginate solution loaded with a 8% ketoprofen and b 8% ketoprofen lysinate by optical microscopy analysis
Fig. 2.
a Laser scattering analysis and b SEM microphotograph of dried sodium alginate beads obtained by prilling and dried by exposure to air in standard condition after 18 h
Drug content, encapsulation efficiency, and water content of both ketoprofen and ketoprofen lysinate beads formulated in different operative conditions are reported in Table II. As expected, ketoprofen drug content increased by increasing drug/polymer ratio used to formulate the beads (9). Unfortunately, when drug/polymer ratio reached 1:3, the jet coming out of the nozzle jammed. This phenomenon was due to stacking of bulk ketoprofen particles (mean diameter 96 ± 28 µm) inside the nozzle when higher concentration of the drug was used. In order to avoid the stall into the nozzle, drug–polymer suspensions were prepared using ketoprofen powder with smaller particle size (mean diameter, 45 ± 12 µm). Unfortunately, reduction of drug particle size did not prevent nozzle jamming because of the aggregation of the drug particles during the preparation of the suspensions, as demonstrated by laser scattering analysis.
Table II.
Feed Solution, Drug Content, Encapsulation Efficiency, and Water Residue of Alginate Beads Manufactured by Prilling at Different Operative Conditions
| Loaded alginate beads | Drug/Polymer (feed solution) | Drug content | Encapsulation efficiency | Water residue |
|---|---|---|---|---|
| Ketoprofen | 1:10 | 8% ± 0.3a | 93% ± 1.2a | 15% ± 0.6 |
| 1:5 | 16% ± 0.4a | 94% ± 1.4a | 13% ± 0.7 | |
| 1:3 | 25% ± 0.9a | 94% ± 1.2a | 11% ± 0.6 | |
| 1:2 | Nozzle-jammed | |||
| Ketoprofen lysinate | 1:3 | 1% ± 0.1a | 5% ± 0.1a | 8% ± 0.9 |
| 1:10 | 3% ± 0.2b | 30% ± 0.8b | 11% ± 0.8 | |
| 1:5 | 8% ± 0.3b | 32% ± 0.8b | 8% ± 0.8 | |
| 1:3 | 12% ± 0.3b | 34% ± 0.9b | 7% ± 0.9 | |
| 1:2 | 16% ± 0.4b | 35% ± 0.9b | 7% ± 0.8 | |
| 1:1 | 27% ± 1.0b | 38% ± 0.9b | 7% ± 0.9 | |
Each point represents the mean ± SD (n = 3)
aCaCl2 in water as gelling solution
bCaCl2 in ethanol as gelling solution
Drug content and encapsulation efficiency of ketoprofen lysinate microparticles were low when manufacturing of beads occurred using CaCl2 aqueous solution as gelling agent. In fact, since ketoprofen lysinate was very soluble in water, most of the drug was able to diffuse from beads during the gelling process (28). In order to reduce diffusional transport of the drug into the hardening bath, a new gelling solution was prepared, dissolving CaCl2 in ethanol 96°. The values of drug content and encapsulation efficiency were raised in accordance with the drug/polymer ratio, reaching 27% and 38%, respectively, when a feed solution with drug/polymer ratio 1:1 was used (see Table II). Moreover, when ethanolic gelling solution was used, water content was lower in ketoprofen lysinate-loaded alginate beads than in ketoprofen-loaded alginate microparticles. In fact, the penetration of ethanol inside the aqueous beads during the gelling phase leads to the formation of a hydro-alcoholic solution inside the beads which was easy to eliminate during the drying process.
Thermal and FTIR Analysis
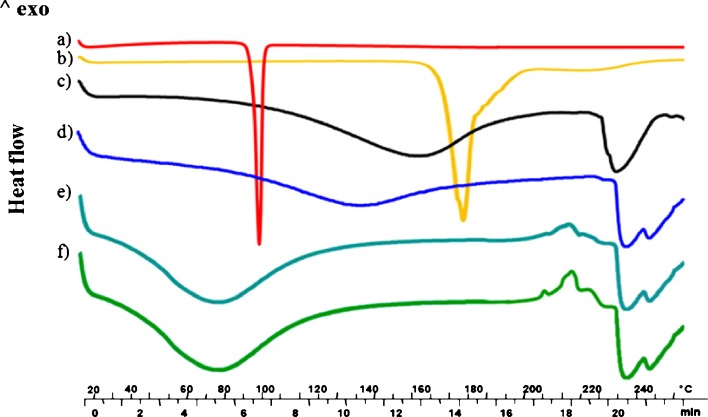
DSC thermograms of blank alginate microparticles, of both ketoprofen and ketoprofen lysinate as raw materials, and of ketoprofen and ketoprofen lysinate-loaded alginate beads are shown in Fig. 3. Ketoprofen and ketoprofen lysinate exhibited endothermic peaks at 97°C and 177°C, respectively, due to the melting of the crystalline state (a and b in Fig. 3). Blank alginate beads thermogram (c in Fig. 3) exhibited a very broad peak between 120°C and 180°C due to the loss of bound water from polymer matrix, as demonstrated by TGA analysis (data not reported) and a peak at 230°C that was related to the endothermic melting process before decomposition of the polymer materials. The disappearance of drug melting peaks, detection of the endothermic melting peak of the polymer material shifted at higher temperature (∼235°C), and the appearance of a shoulder peak at 245°C might be correlated to a drug–polymer interaction in both ketoprofen and ketoprofen lysinate-loaded beads, as shown in d–f in Fig. 3. Furthermore, in the thermograms of ketoprofen lysinate-loaded alginate beads (e and f in Fig. 3), a group of exothermic peaks directly correlated with drug content was detected between 205°C and 220°C.
Fig. 3.
Differential scanning calorimetry thermographs of a ketoprofen; b ketoprofen lysinate; c blank alginate beads; d 6% ketoprofen loaded alginate beads; e 16% loaded ketoprofen lysinate alginate beads, and f 25% loaded ketoprofen lysinate alginate beads
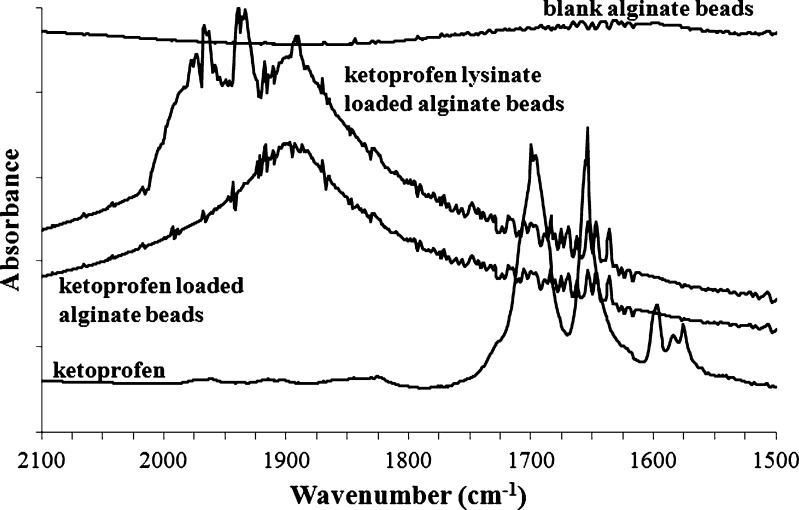
All batches of drug-loaded beads were analyzed by FTIR spectroscopy in order to obtain further information on the solid state of the drug into the formulation. FTIR spectra of the drug-loaded alginate beads were compared with those of the pure ketoprofen, ketoprofen lysinate, and blank alginate beads. In Fig. 4 are reported the spectra in the ν(C=O) stretching region of blank alginate beads, pure ketoprofen-, and ketoprofen-loaded alginate beads. The sharp peaks at 1,697 and at 1,655 cm−1 represent the stretching vibration of the carbonyl group in the dimeric carboxylic acid and the stretching vibration of the carbonyl group in the ketonic group, respectively. In both ketoprofen- and ketoprofen lysinate-loaded alginate beads, these peaks are shifted to higher wavenumbers (1,887 cm−1) due to the breakage of the interaction between the ketoprofen molecules and the formation of hydrogen bond between the alginate carbonyl group and the oxydryl group of the ketoprofen molecule and interactions between ketoprofen hydroxyl group and Ca+2 inside the polymer matrix.
Fig. 4.
FTIR spectra of ketoprofen, blank alginate beads, and drug-loaded alginate beads in the C=O stretching region
Release Studies
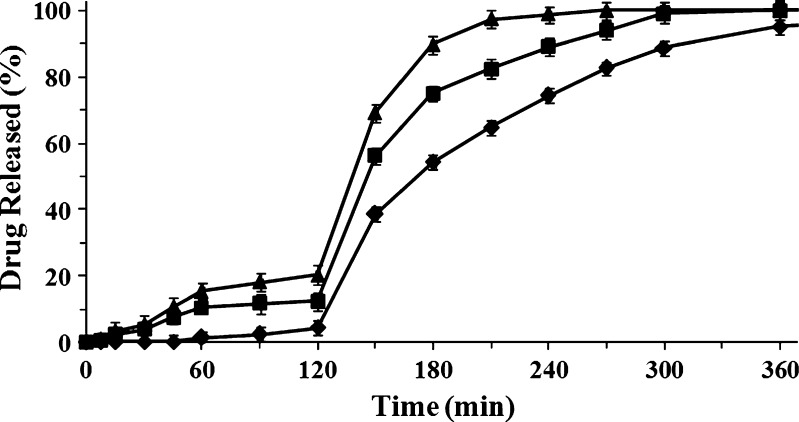
Release profiles of ketoprofen and ketoprofen lysinate from beads manufactured with alginate solution at different concentrations are shown in Figs. 5 and 6. Beads loaded with ketoprofen released low amount of the drug in acidic medium, even those manufactured with lower concentrated alginate solution. Ketoprofen release was kept under 20% in beads produced with 0.50% (w/w) alginate solution, while beads formulated with 2.50% (w/w) alginate solution were able to keep drug release under 5% in the simulated gastric fluid as shown in Fig. 5.
Fig. 5.
Release profiles of dried beads loaded with ketoprofen 16% (w/w), manufactured with solutions at different concentration of sodium alginate 0.5% (w/w, triangle); 1.5% (w/w, square), and 2.50% (w/w, diamond). Mean ± SD; n = 6)
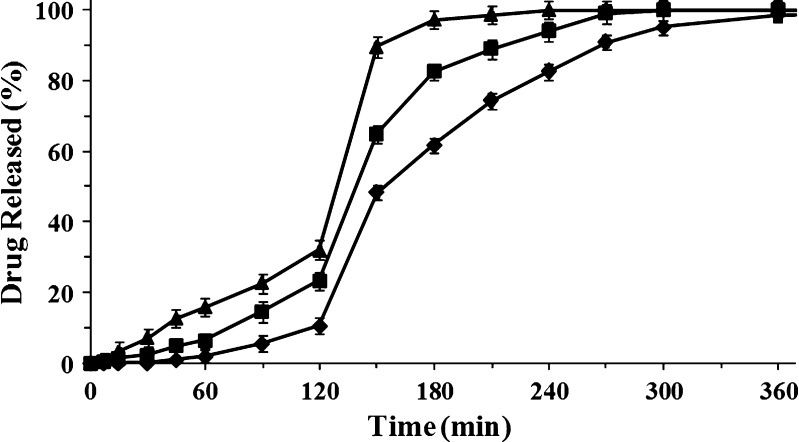
Fig. 6.
Release profiles of dried beads loaded with ketoprofen lysinate 16% (w/w) manufactured with solutions at different concentrations of sodium alginate 0.5% (w/w, triangle), 1.5% (w/w, square), and 2.50% (w/w, diamond). Mean ± SD; n = 6)
Ketoprofen lysinate was released from beads with similar release profiles, but because of its high solubility in aqueous media, release rate of ketoprofen lysinate was higher than ketoprofen and strongly dependent on the concentration of the polymer solution used to manufacture the beads (29). In simulated gastric fluid, the amount of ketoprofen lysinate released from microparticles was around 35% in beads produced with 0.50% (w/w) alginate solution and around 10% for beads manufactured with 2.50% (w/w) polymer solution (see Fig. 6). This effect might be explained by an interaction between the alginate matrix and the drug encapsulated stronger in the case of ketoprofen lysinate than ketoprofen (22, 30). Moreover, a time lag was present in each release profile for both ketoprofen and ketoprofen lysinate. Time lag was longer as polymer concentration increased. This phenomenon can be explained by considering that the number of “egg box” inside the beads is directly correlated with the concentration of the polymeric materials used to produce the beads. In fact, the higher the number of “egg box” in the gelled polymer matrix, the lower is permeability and swelling properties of the material.
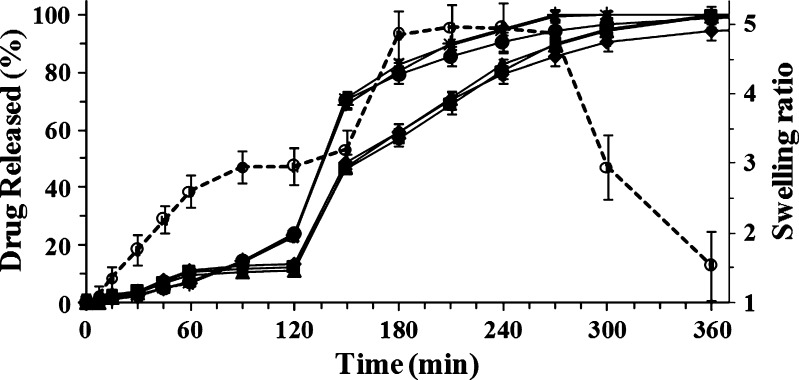
Similar drug release profiles were obtained by dissolution studies of microparticles manufactured with the same polymer solution. Figure 7 show the release profile of both ketoprofen and ketoprofen lysinate from beads manufactured with 1.50% (w/w) polymer solution. Dissolution profiles of beads loaded with different amounts of either ketoprofen or ketoprofen lysinate were almost superimposable. The amount of ketoprofen released from each batch of beads was lower than 15% in simulated gastric fluid. This value was reached in 1 h as burst effect followed by a phase where no release was observed until the change of the medium to simulated intestinal fluid (2 h). On the contrary, beads loaded with ketoprofen lysinate exhibited a slow release behavior linear over time (R2 = 0.985) that did not exceed 23% of the drug content in the same conditions.
Fig. 7.
Swelling ratio (empty circle) and release profiles of dried beads manufactured with the same sodium alginate solution (1.50%, w/w) with different drug content: 8% ketoprofen lysinate (asterisk), 16% ketoprofen lysinate (plus sign), 27% ketoprofen lysinate (filled circle), 8% ketoprofen (triangle), 16% ketoprofen (square), and 25% ketoprofen (diamond), respectively. Mean ± SD; n = 6)
When the pH was changed (pH 6.8), drug release rate increased, leading to a complete liberation of the drug in the next 2 h for ketoprofen lysinate and 4 h for ketoprofen, respectively. Moreover, all the release profiles were consistent with swelling behavior in acidic medium and swelling/disintegration of the beads in the simulated intestinal fluid due to the Ca+2 depletion from polymer matrix in this medium (24). Thus, differences in both drug release profile and release rate were recognizable in simulated gastric fluid (first 2 h), while in simulated gastric fluid, there was no significant variation in the release rate from ketoprofen- and ketoprofen lysinate-loaded beads. Moreover, drug release rate was mainly dependent on the concentration of the polymer solution used to manufacture beads; the higher the concentration of the polymer solution, the lower was the release rate of the drug.
CONCLUSIONS
This study showed that prilling technique could be used as a simple method to prepare microparticles with narrow particle size distribution loaded with ketoprofen and ketoprofen lysinate as model drug for NSAIDs with low solubility and high solubility, respectively. As previously observed for blank beads, the main variable affecting drug-loaded bead micromeritics was confirmed to be the viscosity of the feed solution. Cross model can be used to calculate viscosity at nozzle of the polymer solution and to modify other process variables in order to obtain microparticles loaded with both poorly soluble and very soluble drugs with desired diameters. Drug encapsulation efficiency of manufactured beads was affected only by drug polymer ratio when gelling solution was fixed. Ketoprofen encapsulation efficiency reached high values (about 94%) with CaCl2 aqueous solution as gelling agent, while good encapsulation efficiency was obtained for ketoprofen lysinate-loaded beads (among 30% and 38%) when CaCl2 ethanolic solution was used. The release of both ketoprofen and ketoprofen lysinate from the calcium alginate beads was found to be diffusion-controlled and dependent on swelling/degradation of the beads and correlated with alginate concentration. In fact, the higher the concentration of the solution used to manufacture the beads, the slower was the release rate of the drug. The difference in DSC thermograms and FTIR spectra between drug-loaded alginate beads and pure drugs and blank beads indicated complex chemical drug–polymer interactions between carboxyl groups of the drug and the gelled polymer matrix that might explain the difference in drug release profile between ketoprofen and ketoprofen lysinate in gastric simulated fluid. Thus, only alginate solutions with concentration higher than 0.50% (w/w) were suitable to prepare ketoprofen gastro-resistant formulation, while when ketoprofen lysinate was encapsulated, alginate concentration should reach at last 1.50% (w/w).
References
- 1.de la Lastra CA, Nieto A, Motilva V, Martin MJ, Herrerias JM, Cabre F, Mauleon D. Intestinal toxicity of ketoprofen-trometamol vs its enantiomers in rat. Role of oxidative stress. Inflamm Res. 2000;49(11):627–632. doi: 10.1007/s000110050640. [DOI] [PubMed] [Google Scholar]
- 2.Sommerauer M, Ates M, Guhring H, Brune K, Amann R, Peskar BA. Ketoprofen-induced cyclooxygenase inhibition in renal medulla and platelets of rats treated with caffeine. Pharmacology. 2001;63(4):234–239. doi: 10.1159/000056139. [DOI] [PubMed] [Google Scholar]
- 3.Luna SP, Basilio AC, Steagall PV, Machado LP, Moutinho FQ, Takahira RK, Brandao CV. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res. 2007;68(3):258–264. doi: 10.2460/ajvr.68.3.258. [DOI] [PubMed] [Google Scholar]
- 4.Savage RL, Moller PW, Ballantyne CL, Wells JE. Variation in the risk of peptic ulcer complications with nonsteroidal antiinflammatory drug therapy. Arthritis Rheum. 1993;36(1):84–90. doi: 10.1002/art.1780360114. [DOI] [PubMed] [Google Scholar]
- 5.Giunchedi P, Torre ML, Maggi L, Conti B, Conte U. Cellulose acetate trimellitate ethylcellulose blends for non-steroidal anti-inflammatory drug (NSAID) microspheres. J Microencapsul. 1996;13(1):89–98. doi: 10.3109/02652049609006805. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Onishi H, Machida Y. In vitro and in vivo evaluation of sustained release chitosan-coated ketoprofen microparticles. Yakugaku Zasshi. 2001;121(3):239–245. doi: 10.1248/yakushi.121.239. [DOI] [PubMed] [Google Scholar]
- 7.Roda A, Sabatini L, Mirasoli M, Baraldini M, Roda E. Bioavailability of a new ketoprofen formulation for once-daily oral administration. Int J Pharm. 2002;241(1):165–172. doi: 10.1016/S0378-5173(02)00230-2. [DOI] [PubMed] [Google Scholar]
- 8.Cerdeira AM, Goucha P, Almeida AJ. Hydroxypropyl methylcellulose phtalate beads containing a model non-steroid antiflammatory drug. Int J Pharm. 1998;164:147–154. doi: 10.1016/S0378-5173(97)00432-8. [DOI] [Google Scholar]
- 9.Yamada T, Onishi H, Machida Y. Sustained release ketoprofen microparticles with ethylcellulose and carboxymethylethylcellulose. J Control Release. 2001;75(3):271–282. doi: 10.1016/S0168-3659(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang R, Wang Y, Zheng X, Meng J, Tang X, Zhang X. Preparation and evaluation of ketoprofen hot-melt extruded enteric and sustained-release tablets. Drug Dev Ind Pharm. 2008;34(1):83–89. doi: 10.1080/03639040701580572. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Hoshino N. Production of uniform droplets by longitudinal vibration of audio frequency. J Chem Eng Jpn. 1980;13(4):263–268. doi: 10.1252/jcej.13.263. [DOI] [Google Scholar]
- 12.Sakai T, Sadataka M, Saito M, Matsushita K. Studies of disintregation of liquid column between production of uniform size droplets by vibration method. ICLASS-85. 1985, 7B. p. 2–15.
- 13.Gómez-Díaz D, Navaza JM. Rheology of food stabilizers blends. J Food Eng. 2004;64(2):143–149. doi: 10.1016/j.jfoodeng.2003.09.024. [DOI] [Google Scholar]
- 14.Mancini M, Moresi M, Sappino F. Rheological behaviour of aqueous dispersions of algal sodium alginates. J Food Eng. 1996;28(3–4):283–295. doi: 10.1016/0260-8774(95)00068-2. [DOI] [Google Scholar]
- 15.Singh M, O’Hagan D. The preparation and characterization of polymeric antigen delivery systems for oral administration. Adv Drug Delivery Rev. 1998;34:285–304. doi: 10.1016/S0169-409X(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 16.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. Adv Drug Delivery Rev. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Koch S, Schwinger C, Kressler J, Heinzen C, Rainov NG. Alginate encapsulation of genetically engineered mammalian cells: comparison of production devices, methods and microcapsule characteristics. J Microencapsul. 2003;20(3):303–316. doi: 10.1080/0265204021000058438. [DOI] [PubMed] [Google Scholar]
- 18.Mahler S, Desille M, Fremond B, Chesne C, Guillouzo A, Campion JP, Clement B. Hypothermic storage and cryopreservation of hepatocytes: the protective effect of alginate gel against cell damages. Cell Transplant. 2003;12(6):579–592. doi: 10.3727/000000003108747181. [DOI] [PubMed] [Google Scholar]
- 19.De Ceuninck F, Lesur C, Pastoureau P, Caliez A, Sabatini M. Culture of chondrocytes in alginate beads. Methods Mol Med. 2004;100:15–22. doi: 10.1385/1-59259-810-2:015. [DOI] [PubMed] [Google Scholar]
- 20.Luo D. A new solution for improving gene delivery. Trends Biotechnol. 2004;22(3):101–103. doi: 10.1016/j.tibtech.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Bajpai SK, Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004;59:129–140. doi: 10.1016/j.reactfunctpolym.2004.01.002. [DOI] [Google Scholar]
- 22.Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K, Mishra AK. Alginate microspheres of isoniazid for oral sustained drug delivery. Int J Pharm. 2007;334(1–2):71–77. doi: 10.1016/j.ijpharm.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Simonoska Crcarevska M, Glavas Dodov M, Goracinova K. Chitosan coated Ca-alginate microparticles loaded with budesonide for delivery to the inflamed colonic mucosa. Eur J Pharm Biopharm. 2008;68(3):565–578. doi: 10.1016/j.ejpb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Del Gaudio P, Colombo P, Colombo G, Russo P, Sonvico F. Mechanisms of formation and disintegration of alginate beads obtained by prilling. Int J of Pharm. 2005;302:1–9. doi: 10.1016/j.ijpharm.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Almeida-Prieto S, Blanco-Mendez J, Otero-Espinar FJ. Image analysis of the shape of granulated powder grains. J Pharm Sci. 2004;93(3):621–634. doi: 10.1002/jps.10572. [DOI] [PubMed] [Google Scholar]
- 26.Soong D, Shen M. Kinetic network model for nonlinear viscoeleatic properties of entangled monodisperse polymers. I. Steady state flow. J Rheol. 1981;25:259–273. doi: 10.1122/1.549644. [DOI] [Google Scholar]
- 27.Yildirim ÖE, Basaran OA. Dynamics of formation and dripping of drops of deformation-rate-thinning and -thickening liquids from capillary tubes. J Non-Newtonian Fluid Mech. 2006;136(1):17–37. doi: 10.1016/j.jnnfm.2006.02.009. [DOI] [Google Scholar]
- 28.Holte O, Onsoyen E, Myrvold R, Karlsen J. Sustained release of water-soluble drug from directly compressed alginate tablets. Eur J Pharm Sci. 2003;20(4–5):403–407. doi: 10.1016/j.ejps.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Colombo P, Bettini R, Catellani PL, Santi P, Peppas NA. Drug volume fraction profile in the gel phase and drug release kinetics in hydroxypropylmethyl cellulose matrices containing a soluble drug. Eur J Pharm Sci. 1999;9(1):33–40. doi: 10.1016/S0928-0987(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 30.Mladenovska K, Cruaud O, Richomme P, Belamie E, Raicki RS, Venier-Julienne MC, Popovski E, Benoit JP, Goracinova K. 5-ASA loaded chitosan-Ca-alginate microparticles: preparation and physicochemical characterization. Int J Pharm. 2007;345(1–2):59–69. doi: 10.1016/j.ijpharm.2007.05.059. [DOI] [PubMed] [Google Scholar]