Abstract
The objectives of this study were to determine the concentrations of free benzalkonium chloride (BAC) and apparent partitions coefficients (Km) in micelle solutions and to explore its application in formulation development. Ultrafiltration (UF) was carried out using 10K Nanosep® devices and centrifugation at 5,000 rpm for 5 min. The separation of free BAC from micellar solutions was also conducted using ultracentrifugation (UC) method for the comparison with UF method. Capillary electrophoresis method was used for the identification of micelles. Results showed that a UF method was applicable for quantitatively evaluating BAC–micelle interaction in micellar solutions. Unlike UF, UC could not completely separate free BAC from the micelles. The free BAC concentrations in the micelle solutions decreased with increasing surfactant concentrations. Among polysorbate 80, cremophor EL, and tyloxapol, BAC had the highest Km in polysorbate 80 solutions. The Km was significantly lower in non-buffered aqueous solutions than that in citric buffers. Moreover, increasing surfactant concentrations led to reducing antimicrobial activity. The UF is a rapid and accurate method that minimally alters the micellar equilibrium for the determination of free BAC and Km in micellar solutions. In conclusion, free BAC concentration, which is a function of surfactant type, surfactant concentration, and ion strength of solution, is likely associated with the antimicrobial activity.
Key words: apparent partitions coefficients (Km), benzalkonium chloride (BAC), micelle, ultrafiltration (UF)
INTRODUCTION
Preservatives used in multi-dose ophthalmic formulations play a key role in the prevention of inadvertent bacterial contamination of the formulations at in-use conditions. The requirement for preservative activity is to suppress microbials which may contaminate the product. Despite the known physiological side effects of preservatives in liquid ophthalmic preparations, the use of preservatives is still necessary because of the potential in-use microbial contamination. Benzalkonium chloride (BAC), a mixture of C12–C16 or C12–C18 alkylbenzyldimethylammonium chlorides with a potent biocide character, is the most commonly employed preservative in various dosage forms including aqueous ophthalmic formulations (1). The level of BAC used in pharmaceutical preparations is generally in the range of 0.002–0.02%, but could be up to 0.2% in some cases, depending on various attributes in ophthalmic formulations. BAC is a surface active agent, and it’s likely to interact with other ingredients, especially surfactants. Surfactants are one of the common excipients used in ophthalmic formulations primarily to increase drug solubility. In aqueous solutions, surfactants form aggregates known as micelles once the surfactant concentrations are above the critical micelle concentrations (CMC). When BAC is added in a micellar formulation, it has a tendency to partition into micelles to form mixed aggregates. This decreases the concentration of free BAC in the aqueous phase and hence possibly diminishes its preservative activity. However, the quantitative impact of micelles in the formulations on BAC antimicrobial activities has not been well understood, perhaps due to challenges in separating and quantitating low levels of free BAC in micellar solutions. In order to gain a better understanding, it is critical to develop an assay which could be used to measure free BAC at low concentrations without substantially affecting micelle association/dissociation equilibrium and consequently estimate the partition of BAC in the micelle systems.
During sample preparation to determine free BAC concentrations in micelle systems, the micelle separation process may result in the disruption of micelle association/dissociation equilibrium. This makes it difficult to accurately measure the BAC partitioning constants in the untreated systems. Several size-dependent approaches have been developed for measuring free solutes in micelle solutions, such as gel chromatography, dialysis, ultrafiltration (UF), and polymer partitioning based on the mechanical or physicochemical isolation of a micelle phase from its surrounding aqueous phase. These approaches require the use of membranes, selectively permeable gels, or other materials that may interact with the components in the equilibrium solution so to alter the partition equilibrium (2, 3). Among size-dependent approaches, UF appeared to be the most attractive option for separating solutes from micelle solutions. However, UF was only applied as a micelle-enhanced method to enrich the solutes from aqueous phases in the literature (4). It has not been reported that UF could be used for the determination of solute partition in micellar solutions. As an alternative for the size-dependent approaches, ultracentrifugation (UC) method was reported for the separation of micelles based on the gravity of the components. As one of the advantages, UC method allowed the separation of micelles from the starting solutions without the use of membranes (5, 6). While UC was less likely to alter the micellar equilibrium, its efficiency to separate solutes from micelles has not been verified or compared with other methods. Therefore, it is desirable to investigate and further modify these previously used methods for the determination of free BAC concentrations with or without minimal alteration of drug partitioning equilibrium between micelles and surrounding aqueous phases. The overall objectives of this study were to compare UF with UC method and to further optimize UF method for a rapid and efficient determination of the free BAC concentrations and BAC partition coefficients in micelle systems. In addition, the correlation of free BAC concentrations with the antimicrobial activity of the formulations was also investigated.
MATERIALS AND METHODS
Materials
Benzalkonium chloride (BAC mix, 98.1%) was from Fluka (Ronkonkoma, NY). Benzyldimethylhexadecylammoniumchloride (BAC-C16), benzyldimethyltertradecylammoniumchloride (BAC-C14), and benzyldimethyldodecylammoniumbromide (BAC-C12) were obtained from USP (USP Convention, Inc., Rockville, MD). Cremophor EL was purchased from BASF (Florham Park, NJ), and polysorbate 80 was purchased from Sigma-Aldrich (St. Louis, MO). Tyloxapol was obtained from MP Biomedicals (Solon, OH). All other reagents were purchased from Sigma-Aldrich. Deionized water was obtained from a Reverse Osmosis system (Milli-Q).
Preparation of Micellar Solutions
Polysorbate 80, cremophor EL, and tyloxapol are commonly used surfactants for commercial ophthalmic formulations, and thus, they were selected for the study. The surfactants and other excipients were weighed and added in water or as solutions at room temperature under stirring speed at 50 rpm overnight, and no foaming was observed in the micellar solutions. The glass vials containing the clear solutions were slowly rotated for 24 h before testing. The CMC of surfactants in aqueous solutions is 15, 80, or 81 mg/L for polysorbate 80, cremophor EL, and tyloxapol, respectively. The concentrations of the surfactants used in this study were all above the CMCs.
Ultracentrifugation
Ultracentrifuge tubes (Beckman Ultra-Clear tubes) containing 12 mL of the sample solution (polysorbate 80 solutions containing 200 µg/mL of BAC) were centrifuged at 25°C at 41,000 rpm (2,888 kg) using a BACKMAN-L70 apparatus (SW41T1 rotor). Various centrifugation time points (8 to 72 h) were applied to understand the separation efficiency. Immediately after centrifugation, each of the 2-mL samples was carefully collected from the top to the bottom of the ultracentrifuge tubes using a pipette. The accuracy of each 2-mL sample was checked by weighing. The variation of the withdrawn volume was less than 10%. BAC concentrations in the six fractions collected from each tube were analyzed using a high-performance liquid chromatography (HPLC) method. According to a previous study (5), the BAC/micellar BAC equilibrium was not likely to be changed by ultracentrifugation.
Ultrafiltration
Nanosep® centrifugal filters were obtained from Pall Life Sciences Company (Ann Arbor, MI). The device with appropriate molecular weight cutoff (MWCO) membrane was selected according to the estimated membrane pore size. According to the information from suppliers, the 10K or 30K Nanosep® device are recommended for the separation of 100K molecules (Pall Life Sciences), and the micelle molecule weight of Polysorbate 80 (Tween 80) is estimated as 75K (Sigma). In addition, the diameters of the micelles in the 0.25% polysorbate 80 and 0.02% BAC solutions were 7–10 nm, measured with dynamic light scattering and small-angle X-ray scattering methods. These results are close to the data (10–15 nm) from a reference (7). The micellar size was not noticeably changed before and after ultrafiltration. Although the pore size data of Nanosep® are not available from the supplier, the pore diameter of a 10K device would be approximately 1 nm based on the extrapolation from the Microsep Centrifuge Devices (a 1000K device with a pore size of 100 nm and a 500K device with a pore size of 50–55 nm, Pall Life Science). Therefore, UF was carried out with a 10K MWCO device which filtered the micelles but allowed the free BAC to pass through the filter after centrifugation with a VWR-Calaxy-16 centrifuge instrument. The solution introduced into the filter device served as the donor and that passing through the filter became a filtrate. The BAC concentrations in the initial and remaining donor solutions as well as the filtrates were analyzed using an HPLC method.
Determination of BAC
The BAC detection was modified from a method reported in a previous publication (8). The commercially available BAC was a mixture of alkylbenzyldimethylammonium chloride homologs with n-C12H25, n-C14H29, and n-C16H33 comprising a major portion of the alkyl groups present. Quantitative analysis of the samples were performed using an HPLC apparatus (Waters 2695, USA) equipped with a C18 column (Waters symmetry shield RP-18, 4.6 × 100 mm) at 50°C and UV–Vis detector (Waters model 2487), which could differentiate and quantitatively analyze the homologs C12, C14, and C16 in the BAC mixture. An isocratic mixture (69:31, v/v) of methanol and 0.03 M potassium phosphate monobasic buffer (adjusted to pH 3.0) was used as a mobile phase, and the monitor wavelength was set at 208 nm. The injection volume was 50 µL. The C-12, C-14, and C-16 homolog peaks in the samples were within a linear range to the concentrations. The HPLC results were processed using the Waters Empower 2 chromatography software. The concentrations of BAC-C12, BAC-C14, and BAC-16 were 66.5%, 33.0%, and 0.4%, respectively.
Detection of Polysorbate 80 Micelles by CE
Capillary electrophoresis (CE) has been applied for the detection of the nonionic surfactants in complex systems (9). An Agilent G1600A CE instrument was used for the present study. The electrophoretic results were processed using the Chemstation software. A relatively higher response was obtained by detecting the polysorbate 80 at 208 nm comparing to those at other wavelengths. The capillary was rinsed with carrier electrolyte for 3 min between runs and stored after rinsing with water. Electrokinetic injection of analyte was performed at 15 kV for 5 s, followed by 5 kV, 3 s with a borate buffer (20 mM, pH 9.3).
Determination of Apparent Micelle–Water Partition Coefficient (Km)
In micellar solutions, BAC molecules distribute between aqueous phase and the micellar phase. The apparent micelle–water partition coefficient (Km) was estimated using the free BAC concentration (Cf) and the total BAC concentration (CT) in the equilibrated micellar solutions
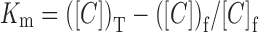 |
where Km is the apparent partition coefficient (unitless), CT is the total BAC concentration in micelle solution (mol/L), and Cf is the free BAC concentration in micelle solution (mol/L).
Antimicrobial Effectiveness Testing
Antimicrobial effectiveness testing (AET) was performed using microorganisms according to EP and USP methods: Candida albicans (ATCC no. 10231), Aspergillus niger (ATCC no. 16404), Escherichia coli (ATCC No. 8739), Pseudomonas aeruginosa (ATCC no. 9027), and Staphylococcus aureus (ATCC no. 6538). AET was tested by a contract lab (Lancaster, PA) and was determined following USP 51 and PharmEur 5.1.3.
The criteria for passing preservative efficacy testing include: (1) after inoculation of the bacteria, it requires not less than 1-log reduction at 7 days, 3-log reduction at 14 days, with no increase from the day 14 counts at 28 days (USP) and not less than 2-log reduction at 6 h, 3-log reduction at 24 h, and no recovery after 28 days (EP-A). (2) After inoculation of the yeast and mold, it requires no increase from the initial at 14 and 28 days (USP) and not less than 2-log reduction at 2 days and no recovery at 28 days (EP-A).
Statistical Analysis
Data were compared with a one-way ANOVA and processed using Origin 6.0 software (Microcal Software, Inc., Northampton, MA, USA).
RESULTS AND DISCUSSION
Measurement of Free BAC Using UC
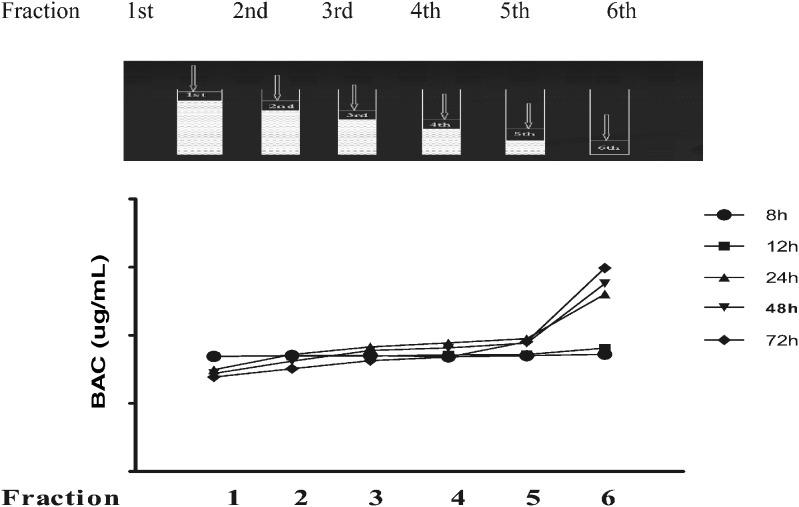
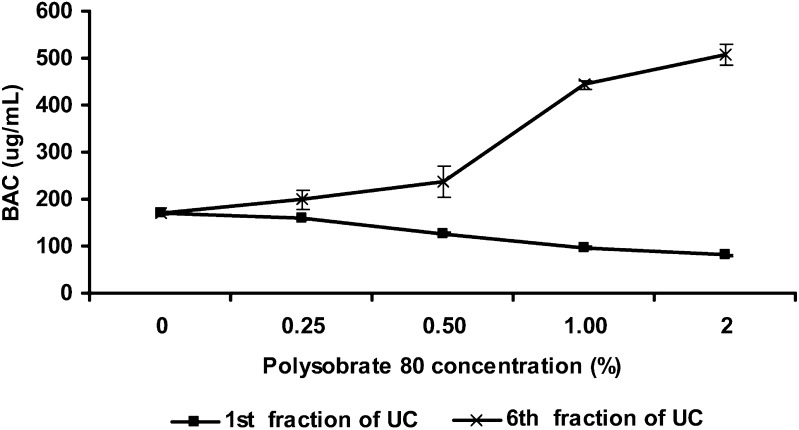
A previous study showed that UC was used to determine the molecular weight, size, molecular interactions of micelles, and partition coefficients of solute in micelle systems without altering the solution equilibrium (5). In the present study, the samples containing 0.02% BAC and 0.25% polysorbate 80 in cellulose nitrate tubes were centrifuged at 42,000 rpm to separate micelles. As previously described in “MATERIALS AND METHODS,” BAC concentrations in each centrifugal fraction were determined using an HPLC method. The results (Fig. 1) show that the BAC concentrations in the top fractions were lower than the initial concentrations, while those in the bottom fractions were higher than the initial concentrations (200 µg/mL) after 24-h centrifugation. This observation agrees with the results from a previous study (5). Because of gravity effect, micelles were concentrated in the bottom fractions of the tubes after UC. Therefore, it appeared that BAC molecules partitioned into the micelles, reflected by the highest BAC concentration in the sixth fraction (bottom) and the lowest BAC concentration in the first fraction (top) of UC samples. The results also indicated that the centrifugation at tested conditions for 24 h was needed to separate micelles from the solutions. Further centrifugation (48 and 72 h) slightly increased the separation. To confirm that the free BAC concentration is a function of micelle concentrations, the sample solutions containing different concentrations of polysorbate 80 were prepared and analyzed. As shown in Fig. 2, with an increase of polysorbate 80 concentration, there were reduced BAC concentrations in the first fraction and increased BAC concentration in the sixth fraction. This trend clearly indicated that BAC did partition into micelles, and the amount of BAC distribution into micelles depended on the concentration of surfactant in solutions. Therefore, UC method could be potentially used for partial separation of free BAC from various micelle solutions even though the separation using this method was not efficient, which is discussed in the following sections.
Fig. 1.
Centrifugation time effect on the BAC distribution in micelle solutions (0.25% polysorbate 80) after UC
Fig. 2.
Polysobrate 80 concentration effect on BAC distribution in UC
Measurement of Free BAC Using UF
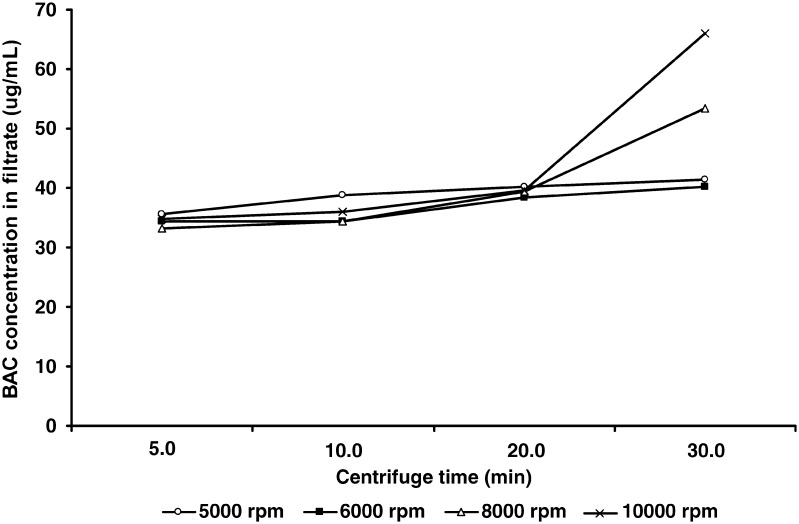
UF is a membrane separation technique used to separate fine particles from solutions or from dissolved molecules in fluids. Surfactants form micelles with lipophilic cores so that BAC partitions into the micelles or possibly form mixed micelles through molecular interaction. Using UF method, the free BAC can be separated from the BAC entrapped in micelles through a membrane where the pore size is small enough to retain the micelles in the donor phase (9). The UF technique applied in the present study involved separating a small portion of the filtrate from the initial micelle solution. It minimally interfered in the micelle equilibrium using an optimized separation process. In a micelle solution containing 0.5% polysorbate 80 and 0.02% BAC, the effects of centrifugation time and speed on the BAC concentration in filtrates were investigated. Results in Fig. 3 showed that the substantially increased BAC concentrations in filtrates were observed at >8,000 rpm for 30 min, which indicated that the equilibrium of the micelle systems was possibly changed by the excessive centrifugation force. On the other hand, it required longer time to obtain sufficient amount of the filtrates for analysis at <5,000 rpm. Therefore, the centrifugation conditions were selected at 5,000 rpm for 5 min based on the minimal influence on the equilibrium and the sufficient volume of filtrates for testing.
Fig. 3.
UF centrifugation speed influenced on BAC concentration in filtrate
To investigate potential adsorption of BAC on the filter membrane, a polysorbate 80 (0.25%) and BAC (0.02%) micelle solution was used as a representative sample. The filtrate was collected and the donor solution was discarded after first UF. The initial sample solution was applied again on the same filter device, and the separation procedures were repeated multiple times. The BAC concentrations in the series of filtrates were determined. As shown in Fig. 4, The BAC concentrations in the second filtrates were higher than those in the first one. However, the concentrations were unchanged from the second filtrate to the seventh filtrate. These results indicated that a potential adsorption of BAC in the membrane occurred in the first pass, but the membrane was saturated after the second pass. Therefore, it is necessary to discard at least the first filtrate in order to obtain accurate free BAC concentrations. In addition, the filter membrane was pretreated with a sample solution to compare non-treated filter for better understanding the effect of the membrane condition. The results showed that there was no significant difference in the BAC concentration of the filtrate between the treated (wet) and non-treated (dry) membrane.
Fig. 4.
Filter membrane condition and filtration time effect on free BAC concentrations
Based on these results, a final experimental protocol for UF method was developed using the Nanosep device with 10K MWCO cellulose filter membrane. The filter device was loaded with a 600-μL sample as a donor phase. The centrifugation was conducted at 5,000 rpm at room temperature for 5 min. As discussed previously, it was necessary to condition the filter at least once by the sample solution before collecting the filtrate. The filter device was weighed before and after ultrafiltration to determine the amount of the filtrates. Each filtrate was analyzed using the HPLC method.
Comparison of UF and UC Processed Samples Using Capillary Electrophoresis
Although free BAC could be separated from micelle solutions using either UC or UF methods, it was desirable to understand whether the separation of free BAC from micelles BAC was complete or not. To determine free BAC concentration in micelle solutions using an HPLC method, the injected samples should be micelle-free. Therefore, it is critical to examine the absence of micelles in the separated samples used for free BAC analysis. Among various methods, CE has been used to characterize micelle systems based on the size and charges of molecules or micelles (10, 11). As a consequence of the partition of the positively charged BAC molecules into the nonionic surfactant micelles, the micelles in the sample solutions are electrically charged and move at different velocities under an applied electric field depending on their charges and sizes (12). As shown in Fig. 5, the migration behaviors of the BAC/polysorbate 80 micellar solution, the polysorbate 80 solution (A in Fig. 5), and BAC aqueous solution (B in Fig. 5) were different in the electropherograms, which made it possible to identify the presence of the micelles. The representative sample solution (0.25% polysorbate 80 and 0.02% BAC) was studied using both UC and UF methods. A distinguishable micelle peak was observed from polysorbate 80-only solution, which also appeared in the donor sample solutions (C in Fig. 5) after ultrafiltration and the first fraction of sample solution (E in Fig. 5) from the UC study. However, there was no polysorbate 80 micelle peak in the filtrates of UF (D in Fig. 5). BAC-only sample did not interfere with the identification of polysorbate 80 micelles. These results strongly suggested that free BAC could be completely separated from the micelle solution using UF method. However, there were measurable micelles in the first fraction of sample solution processed using UC method. Although a previous study indicated that UC could separate micelle–solute (5), the results in the present study demonstrated that the free BAC was not completely separated from the micelle systems using UC method under the applied conditions.
Fig. 5.
Electropherograms of polysorbate 80 solutions
Comparison of UF and UC Processed Samples Using Filtration
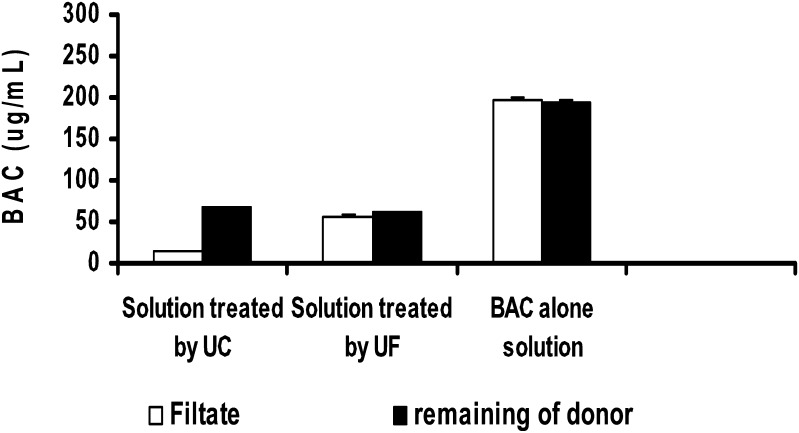
In addition to the test of the micelle separation efficiency using CE method, we further used the multiple filtration method to detect the micelles in the sample solutions processed by UF or UC. Since free BAC molecules can go through the filter, the repeated filtrations of the filtrates which do not contain micelles should not alter the BAC concentrations as long as the first filtrate was discarded. Hence, the 10K MWCO filters were used for the repeated filtration of the UC or UF processed samples, and a BAC-only solution was used as a control. In Fig. 6, there was a significant difference in the BAC concentrations between the remaining donor solution and the filtrate following application of the first fraction of UC-processed sample. This result demonstrated that there were still micelles in the first fraction (top) of the UC-processed samples even though the micelle concentrations were much higher in the sixth fraction (bottom) than those in the first fraction. This is consistent with the CE result which indicated that the separation of micelles using UC was not complete. However, the BAC concentrations in the remaining donor solution and the filtrate of the UF-processed sample were almost identical. In addition, the BAC concentrations remained unchanged after filtration using the BAC-only solution. These results confirmed that UF-processed filtrates did not contain micelles so that free BAC could be completely separated from micelle solution using UF.
Fig. 6.
Comparison of BAC concentrations in the filtrate and remaining donors using UF or UC processed sample solutions
Mass Balance of BAC in UF Method
UF method was further validated by the mass balance of BAC. The sample solution added on the membrane of the filter device was defined as donor, and the solution passing through the filter was defined as filtrate. The mass balance was estimated by calculating the recovery of the BAC from each part of samples according to the following equation:
 |
where CD, CRD, and Cf are the BAC concentration in the initial donor solution, in the remaining donor solution, and in the filtrate, respectively, and where VD, VRD, and Vf are the initial volume of the donor solution, the remaining volume of the donor solution, and the volume of filtrate, respectively. To investigate the effect of surfactant concentration on BAC mass balance, a series of micelle solutions containing 0.01–0.5% of polysorbate 80 was included. Results showed that the average recovery from these solutions was higher than 93% (Table I). It suggested that there was no substantial loss of BAC in the UF process and polysorbate 80 concentrations did not significantly influence the BAC mass balance in the tested solutions.
Table I.
BAC Mass Balance in UF Process
| Polysorbate80 (%) | A RD (µg) | A F (µg) | A D (µg) | Recovery (%) |
|---|---|---|---|---|
| 0 | 53.55 ± 1.93 | 52.26 ± 0.81 | 103.97 ± 4.02 | 101.76 ± 2.02 |
| 0.01 | 72.22 ± 2.66 | 29.77 ± 2.14 | 98.72 ± 3.69 | 103.32 ± 0.94 |
| 0.25 | 83.81 ± 0.25 | 10.37 ± 0.60 | 96.96 ± 0.75 | 97.11 ± 0.21 |
| 1 | 98.10 ± 2.31 | 1.76 ± 0.05 | 101.80 ± 3.41 | 98.09 ± 0.43 |
| 2 | 98.93 ± 1.58 | 0.36 ± 0.17 | 101.02 ± 3.15 | 98.27 ± 0.23 |
| 5 | 96.69 ± .07 | 0 | 102.90 ± 1.91 | 93.95 ± 0.69 |
A D, A RD, and A f are the initial amount of BAC in donor, remaining amount of BAC in donor after UF, and amount of BAC in filtrate (mean value ± SD, n = 3)
Effect of Polysorbate 80 Concentration on BAC Membrane Adsorption
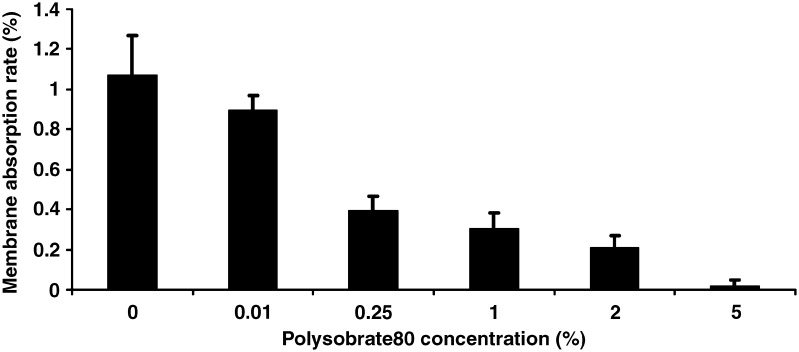
The percent adsorption was calculated using recovered amount of BAC from membrane and the initial amount of BAC loaded. As shown in Fig. 7, the amount of BAC membrane adsorption was less than 1.2% of the total BAC in the solutions. Therefore, the BAC adsorption on the membrane was negligible. Interestingly, the BAC membrane adsorption decreased with increasing polysorbate 80 concentrations. In particular, the adsorption was only 0.01% when the polysorbate 80 concentration was up to 5%. The adsorption of BAC was a function of the partition of BAC between micelle solutions and membranes. More BAC molecules were entrapped into micelles in the solutions containing relatively higher concentrations of polysorbate 80, resulting in the reduced partition into the membrane. Despite that the adsorption of BAC on the filter membrane was not significant, this result clearly demonstrated the influence of surfactant/micelle concentration on the adsorption.
Fig. 7.
Polysorbate 80 effect on BAC membrane absorption in the process of UF
Effect of Surfactants and Buffers on BAC Partition in Micelle Solutions
To understand the effect of different nonionic surfactants on BAC partition in micellar solutions, we also investigated free BAC concentrations in cremophor EL and tyloxapol solutions, which are commonly used in ophthalmic formulations. Using UF method, the effect of surfactants on BAC apparent partition coefficient (Km) in micelle solutions was shown in Table II. Within the concentrations tested, the magnitude of BAC apparent partition coefficient was in the order of polysorbrate 80 > tyloxapol > cremophor EL both in the aqueous solutions and the citric buffer solutions. The increase in surfactant contents resulted in decreasing free BAC concentrations and so increasing BAC apparent partition coefficients in all the micelle solutions. Among the tested nonionic surfactants, free BAC concentration was the highest in cremophor EL solutions and the lowest in polysorbate 80 solutions at the identical concentration (% w/v) of the surfactants, reflecting the surfactant-dependent property of the BAC partitioning. Based on the HLB value, which represents the hydrophilic–lipophilic balance of surfactants, polysorbate 80 (HLB, 15) is more hydrophilic than tyloxapol (HLB, 13) and cremophor EL (HLB, 12–14). BAC is an ionic compound so that it is more likely to partition into the polysorbate 80 micelles than into the others. In addition, the comparison in the present study was conducted under the same percent weight/volume concentrations of the surfactants which do not reflect the effect of molecular weight and aggregation number in a micelle. Interestingly, the results showed the BAC apparent partition coefficients in the three nonionic surfactant systems were higher in citric buffers (10 mM, pH 5.0) than those in non-buffered aqueous solutions. It suggested that ionic strength might be a potential factor influencing BAC partition in micelle systems. BAC partition into micelles could also vary depending on the other components of formulations as a consequence of competitive partition.
Table II.
Effect of Surfactant on BAC Apparent Partition Coefficient (K m) in Micelle Solutions (mean value ± SD, n = 3)
| Surfactant (%) | K m in non-buffered aqueous solution | K m in citric buffer (pH 5.0 10 mM) | ||||
|---|---|---|---|---|---|---|
| Cremophor EL | Tyloxapol | Polysobrate80 | Cremophor EL | Tyloxapol | Polysobrate80 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.01 | 0.12 ± 0.01 | 0.12 ± 0.04 | 0.16 ± 0.02 | 0.48 ± 0.15 | 0.51 ± 0.17 | 0.51 ± 0.14 |
| 0.1 | 0.47 ± 0.04 | 0.72 ± 0.02 | 0.75 ± 0.05 | 1.12 ± 0.10 | 1.49 ± 0.22 | 1.57 ± 0.14 |
| 0.25 | 1.11 ± 0.06 | 1.64 ± 0.04 | 1.78 ± 0.07 | 2.29 ± 0.15 | 2.80 ± 0.11 | 3.45 ± 0.20 |
| 0.5 | 2.19 ± 0.08 | 3.40 ± 0.07 | 3.78 ± 0.08 | 5.25 ± 0.57 | 6.94 ± 0.46 | 8.63 ± 0.81 |
| 1 | 9.82 ± 0.37 | 19.48 ± 0.78 | 28.43 ± 2.44 | 32.98 ± 0.69 | 45.01 ± 15.32 | 64.48 ± 10.15 |
| 2 | 20.46 ± 0.79 | 37.25 ± 3.75 | 125.78 ± 3.33 | 101.70 ± 20.97 | 135.16 ± 63.8 | 230.40 ± 34.76 |
| 5 | 77.20 ± 8.32 | 46.35 ± 19.23 | ∞ | ∞ | ∞ | ∞ |
Correlation Between Polysorbate 80 Concentration and BAC Antimicrobial Effectiveness
As mentioned previously, the hydrophobic cores of the polysorbate 80 micelles provide a favorable pocket for BAC partitioning, and BAC may also tend to interact with polysorbate 80 to form mixed micelles. Thus, the thermodynamic activity of BAC in micelle solutions is likely associated with the free BAC concentrations that affect the antimicrobial activity. A recent published study showed that antibiotics encapsulated in surfactant micelles could affect its antimicrobial efficiency (13). As shown in Table III, several in-house prototype placebo formulations were evaluated for their antimicrobial activity. Results showed that the formulations containing more than 0.3% of polysorbate 80 failed AET testing. BAC partition data showed that the free BAC concentration decreased with the increase of the surfactant concentration in solutions. It suggested that BAC partitioned into the micelle and only free BAC was possibly responsible for its preservative ability in formulations.
Table III.
Antimicrobial Effectiveness Testing Results of BAC in Different Concentrations of Polysorbate 80 Experimental Ophthalmic Formulation
| Formulationa | AETa | Comment |
|---|---|---|
| 0.02% BAC, 63 mM Phosphate buffer at pH 5.5 | P | Met EP criteria and USP requirements at all time points |
| 0.3% polysorbate 80, 0.02% BAC, 63 mM phosphate buffer at pH 5.5 | P | Met EP criteria and USP requirements at all time points |
| 0.4% polysorbate 80, 0.02% BAC, 63 mM phosphate buffer at pH 5.5 | F | Failed to meet EP criteria |
| 0.5% polysorbate 80, 0.02% BAC, 63 mM phosphate buffer at pH 5.5 | F | Failed to meet EP criteria and USP requirements |
aF failed antimicrobial effectiveness testing (AET), P passed AET
CONCLUSIONS
Determination of the free preservative concentration in liquid formulations is critical for the evaluation of preservative antimicrobial effectiveness. In the present study, we have developed and evaluated the UF method for the determination of the free BAC concentration and apparent partition coefficient in micelle solutions. In comparison with UC method, UF is a rapid and accurate method with minimal change of the equilibrium of micelle systems. Free BAC concentration is a function of surfactant type, surfactant concentration, and possible ion strength of solutions, and it could affect its antimicrobial efficiency significantly.
Acknowledgments
The authors would like to thank Thomas Sand for instrument support and Jerry Ryan for microbiological support.
Contributor Information
Jun Liu, Email: firejj217@gmail.com.
Guang Wei Lu, Phone: +1-860-6861598, FAX: +1-860-6867810, Email: guang.w.lu@pfizer.com.
References
- 1.Budavari S. Merck index. 12. Whitehouse Station, NJ: Merck Research Laboratories; 2001. [Google Scholar]
- 2.Fillipi BR, Brant LW, Scamehorn JF, Christian SD. Use of micellar-enhanced ultrafiltration at low surfactant concentrations and with anionic–nonionic surfactant mixtures. J Colloid Interface Sci. 1999;213:68–80. doi: 10.1006/jcis.1999.6092. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard J, Fink WT, Duffy JP. Effect of sorbitol on interaction of phenolic preservatives with polysorbate 80. J Pharm Sci. 1977;66:1470–3. doi: 10.1002/jps.2600661031. [DOI] [PubMed] [Google Scholar]
- 4.Scamehorn JF, Christian SD, Elsayed DA, et al. Removal of divalent metal-cations and their mixtures from aqueous streams using micellar-enhanced ultrafiltration. Sci Technol. 1994;29:809–30. [Google Scholar]
- 5.Park JY, Rippie EG. Micellar distribution equilibria: ultracentrifugal study of apparent partition coefficients. J Pharma Sci. 1977;66:858–61. doi: 10.1002/jps.2600660631. [DOI] [PubMed] [Google Scholar]
- 6.Kirschbaum J. Micellar properties of drugs: determination of molecular weight, size, and molecular interactions of drug micelles using the analytical ultracentrifuge. J Pharma Sci. 1974;63:981–7. doi: 10.1002/jps.2600630648. [DOI] [PubMed] [Google Scholar]
- 7.Holmberg K. Handbook of applied surface and colloid chemistry. New York: Wiley; 2002. [Google Scholar]
- 8.Dudkiewicz-Wilczyńska J, Tau J, Roman I. Application of the HPLC method for benzalkonium chloride determination in aerosol preparations. J Pharm Biomed Anal. 2004;34:909–20. doi: 10.1016/j.jpba.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Norton D, Shamsi SA. Capillary electrochromatography of Triton X-100. Electrophoresis. 2004;25:586–93. doi: 10.1002/elps.200305682. [DOI] [PubMed] [Google Scholar]
- 10.Lin CE. Determination of critical micelle concentration of surfactants by capillary electrophoresis. J Chromatogr A. 2004;1037:467–78. doi: 10.1016/j.chroma.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Heinig K, Vogt C. Determination of surfactants by capillary electrophoresis. Electrophoresis. 1999;20:3311–28. doi: 10.1002/(SICI)1522-2683(19991001)20:15/16<3311::AID-ELPS3311>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Bilek G, Kremser L, Blaas D, Kenndler E. Analysis of liposomes by capillary electrophoresis and their use as carrier in electrokinetic chromatography. J Chromatogr A. 2006;841:38–51. doi: 10.1016/j.jchromb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Gaysinsky S, Taylor TM, Davidson PM, et al. Antimicrobial efficacy of eugenol microemulsions in milk against Listeria monocytogenes and Escherichia coli O157:H7. J Food Prot. 2007;70:2631–7. doi: 10.4315/0362-028x-70.11.2631. [DOI] [PubMed] [Google Scholar]