Abstract
The nanoemulsions composed of citronella oil, hairy basil oil, and vetiver oil with mean droplet sizes ranging from 150 to 220 nm were prepared and investigated both in vitro and in vivo. Larger emulsion droplets (195–220 nm) shifted toward a smaller size (150–160 nm) after high-pressure homogenization and resulted in higher release rate. We proposed that thin films obtained from the nanoemulsions with smaller droplet size would have higher integrity, thus increasing the vaporization of essential oils and subsequently prolonging the mosquito repellant activity. The release rates were fitted with Avrami’s equations and n values were in the same range of 0.6 to 1.0, implying that the release of encapsulated limonene was controlled by the diffusion mechanism from the emulsion droplet. By using high-pressure homogenization together with optimum concentrations of 5% (w/w) hairy basil oil, 5% (w/w) vetiver oil (5%), and 10% (w/w) citronella oil could improve physical stability and prolong mosquito protection time to 4.7 h due to the combination of these three essential oils as well as small droplet size of nanoemulsion.
Key words: citronella oil, hairy basil oil, mosquito repellent, nanoemulsion, vetiver oil
INTRODUCTION
Hemorrhagic fever transmitted by Aedes aegypti is one of leading epidemic diseases in Thailand. Prevention of mosquito bites is still the best way to control mosquito-borne diseases by using insect repellent along with avoiding infested habitats or wearing protective clothing (1,2). Mosquito repellent chemicals have been popularly used and they can be obtained either from natural or synthetic chemical compounds. N, N-diethyl-m-toluamide (DEET) is one of the effective synthetic substances, which has been widely used as the prevalent topical insect repellent over past decade due to its outstanding mosquito biting protection times (3,4). However, it has presented some adverse effects such as dermal toxicity, seizure, or acute manic psychosis. Therefore, natural repellents such as herbal essential oils have been employed as alternative compounds for repelling mosquitoes and other insects (5–8). There are reports on the insect repellency from mint (Teucrium leucocladum), citronella (Cymbopogon nardus), basil (Ocimum genus and their cultivars), thyme (Thymus vulgaris L.), neem (Azadirachta indica A. Juss), and lemongrass (Cymbopogon citratus; 9–14). Trongtokit et al. have assessed repellent activity of 38 Thai essential oils and found that an effective time of repellency strongly depended on the concentrations, experiment designs, and mosquito species (14). It is obvious that repellent effects of natural oil are mostly shorter than the synthetic one like DEET. However, prolonged protection effects have been reported from 20% of the oil from turmeric, citronella, and hairy basil, especially with the addition of 5% vanillin as fixative in which they each can repel three species of mosquitoes for up to 6 h (15).
Although an attempt to develop essential oil for pesticides and insecticides has been made in a variety of water-soluble formulations such as nanoemulsion incorporated with β-cypermethrin (16) and essential-oil-loaded microcapsules for pest control (17), there is so far no report on nanoemulsion formulation for mosquito repellency. Nanoemulsion can be prepared by a low-energy emulsification method, which has been recently developed according to the phase behavior and properties, to promote the formation of ultra-small droplets (18,19). These low-energy techniques include self-emulsification, phase transition, and phase inversion temperature methods (16). However, the most common approach, which is high-energy emulsification by using high shear stirring, high-pressure homogenizers and ultrasound generators, are widely used due to its ease for large scale production and low cost (19). Moreover, its high stability, transparency, and low viscosity make them an attractive system for many applications such as pharmaceuticals (20), cosmetics (18), and food (21–24). In this study, essential-oil-loaded nanoemulsion system was developed. The aims of the study is to investigate an effect of high-energy emulsification and composition of three essential oils, citronella (C. nardus), hairy basil (Ocimum americanum), and vetiver (Vetiveria zizanioides), on physicochemical characteristics (droplet size, zeta potential (ZP), stability, and release kinetics) as well as in vivo mosquito repellency. The relationship between release kinetic parameter and the mosquito protection time of the nanoemulsions was also studied.
MATERIALS AND METHODS
Materials
Citronella oil (C. nardus), hairy basil oil (O. americanum), and vetiver oil (V. zizanioides) were obtained by steam distillation from Thai-China Flavors and Fragrances Industry (TCFF) Co., Ltd (Bangkok, Thailand). Glycerol (AnalaR®) was obtained from BDH (Poole, England). Montanov®82 was obtained from Adinop Co., Ltd. (Bangkok, Thailand). Dulbecco’s modified Eagle’s medium was obtained from Sigma-Aldrich (USA). Fetal bovine serum was purchased from Biochrom AG (Germany). l-glutamine, penicilin G sodium, streptomicin sulfate, and amphotericin B were obtained from Invitrogen Corp. (USA). 3-(4, 5-dimethyldiazol-2-yl)-2, 5 diphenyl tetrazolium bromide (MTT) was from Sigma-Aldrich, Inc. The water used for all experiments was purified water obtained from a MilliQ Plus (Millipore, Schwalbach, Germany). All other reagents used were commercially available and were of analytical grade.
Analysis of Essential Oils
Constituents of essential oils were analyzed by using headspace/gas chromatography–mass spectrometry (GC-MS) analysis. Citronella oil, hairy basil oil, and vetiver oil were incubated at 80°C for 10 min whereas for vetiver oil, incubation time was reduced to 50°C. GC/MS analyses were carried out using Thermo Electron Corporation gas chromatograph (FOCUS PolarisQ, Thermo Fisher Scientific Inc. USA), equipped with a capillary column ZB-5 ms (Phenomenex®, Torrance, USA) with 30 m × 0.25 mm i.d. × 0.25 mm film thickness coated with 5% phenyl and 95% dimethylpolysiloxane. Helium gas was used as the carrier at a flow rate of 1 ml/min. The oven temperature program was 60°C (5 min) then 3°C/min to 170°C (40 min). The 1.5 ml of samples was injected with 1:10 split ratio. Injector and detector temperatures were 80°C and 275°C, respectively. Mass spectra were recorded over 40–400 amu range at 1 scan/s with ionization energy 70 eV and ion source temperature 240°C. Component identification was carried out by comparing the obtained MS data with library on Wiley by NIST MS search version 2.0.
Preparation of Nanoemulsions
Aqueous dispersions of nanoemulsion were composed of 20% (w/w) of essential oil, 75% (w/w) of glycerol, and emulsifier (Montanov 82) at 5%. The oil compositions were presented in Table I. The oil mixture was dissolved in melted emulsifier at 45°C. To obtain nanoemulsion, oil phase was dispersed in the hot aqueous phase under stirring condition at 200 rpm, 50°C for 5 min before high-speed stirring using an Ultra-Turrax T25 (IKA-WERKE, Germany) at 16,500 rpm for 3 min to obtain pre-emulsion, namely nanoemulsion prepared without high-pressure homogenization. The pre-emulsion was then passed through a high-pressure homogenizer (EmulsiFlex-C3, Avestin, Canada) for five cycles at pressure of 1,500 bars. After homogenization the produced oil in water nanoemulsion was cooled down to room temperature to obtain the nanoemulsion prepared with high-pressure homogenization. After production, all formulations were stored at 25°C.
Table I.
Compositions of Essential Oils in Nanoemulsion Formulations
| Sample | Oil concentration (% w/w) | ||
|---|---|---|---|
| Citronella oil | Hairy basil oil | Vetiver oil | |
| F1 | 20 | – | – |
| F2 | 15 | 5 | – |
| F3 | 15 | – | 5 |
| F4 | 15 | 2.5 | 2.5 |
| F5 | 10 | 10 | – |
| F6 | 10 | – | 10 |
| F7 | 10 | 5 | 5 |
Measurement of Emulsion Droplet Size and Zeta Potential
Measurement of droplet size, polydispersity index (PI), and zeta potential of the nanoparticles was performed using photon correlation spectroscopy (PCS) (NanoZS4700 nanoseries, Malvern Instruments, UK). For size measurement, all formulations were diluted with 1 mL of 0.22-µm filtered deionized water to eliminate the effect of viscosity caused by the ingredients. The refractive index of essential oils and water were set at 1.46 and 1.33, respectively. The zeta potential measurements were performed in distilled water which was adjusted to a conductivity of 50 µS/cm with 0.90% (w/v) sodium chloride solution. Droplet size, PI, and zeta potential were reported as the average of three measurements at 25°C. For stability studies, all samples were kept at 25°C and taken at the beginning of storage and subsequently in 2 months of storage prior to characterization.
In Vitro Release Time-Course of Nanoemulsions
The experiment was performed to evaluate the amount of essential oil release from each formulation. The nanoemulsion was loaded at 1 ml per cell in separated closed container by using modified horizontal Franz diffusion cell (14 ml), in which top of the cell was tightly sealed. The temperature of sample and container was accurately controlled at 32°C to mimic human skin. The samples from each nanoemulsion formulation were separately put in each container at specified time. At fixed time intervals, the containers were removed and added with terpinene-4-ol (TCFF Co., Ltd) as internal standard. The sample amount was analyzed by headspace GC-MS technique as mentioned above. The data presented are average values of two separate experiments. The relative amounts of individual components are based on peak areas obtained. d-limonene was selected as the major marker of citronella oil. For standard curve preparation, d-limonene was diluted with methanol to varying concentrations of 0.1, 0.5, 1, 5, and 10 ppm. Standard curve was performed by peak area ratio of marker to terpinene-4-ol as internal standard versus each marker concentration.
The release kinetics of nanoemulsion in various formulations were investigated by fitting the release data into Avrami’s equation (25), which can be expressed using Eq. 1 as followed:
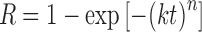 |
1 |
Where R is the release content of marker in essential oil, t is the storage time, k is the release rate constant, and n is a parameter representing the release mechanism.
Mosquito Repellent Efficiency Assay
Mosquito repellent effect of citronella oil formulations was evaluated using the human-bait technique based on standard test of the World Health Organization (15,26). All chemicals used in the formulations are generally regarded as safe materials for human use. This study was approved and conducted by Department of Medical Sciences, Ministry of Public Health, Thailand. The tests were carried out in a 6 × 6 × 3 m room, at 25–29°C and relative humidity of 60–80%. An area 3 × 10 cm on each forearm of three human volunteers (n = 3) was marked out with a permanent marker. Approximately 0.1 ml of nanoemulsion was applied to the marked area of one forearm of each volunteer. During the test, the forearm was covered by a paper sleeve with a hole corresponding to the marked area. Each volunteer put the test forearm in a mosquito cage (40 × 40 × 40 cm3), containing 250 female A. aegypti mosquitoes (3–5 days old), for the first 3 min of every half-hour exposure. If at least two mosquitoes landed on or bit the hand, the repellency test was then continued. The test continued until at least two bites occurred in a 3-min period, or until a bite occurred and was followed by a confirmatory bite (second bite) in the following exposure period. The time between application of the repellents and the second successive bite was recorded as the protection time. Testing period lasted up to 7 h.
Cell Cytotoxicity Study
Normal human foreskin fibroblast (NHF) cells were plated in 90 µl of minimum essential medium supplemented with 10% fetal bovine serum at a density of 8,000 cells per well in 96-well plates. When the cultures reached confluence (typically, 48 h after plating), all tested formulations at varying concentrations were added at 10 µl/well. After 16 h post-incubation, 25 µl of 5 mg/ml MTT was added to each well and then incubated for 4 h. Then all media were removed and 100 µL of dimethyl sulfoxide was added. Plates were incubated for 30 min at 37°C and the absorbance was measured at 550 nm using a microplate reader. Percent cell viability were then calculated and compared with control.
Statistical Analysis
Values were expressed as mean ± standard deviation (SD). Statistical significance of differences was examined using one-way analysis of variance (ANOVA) by LSD post hoc test. A probability value (p) of less than 0.05 was considered to be significantly different.
RESULTS AND DISCUSSION
Essential Oil and Characterization
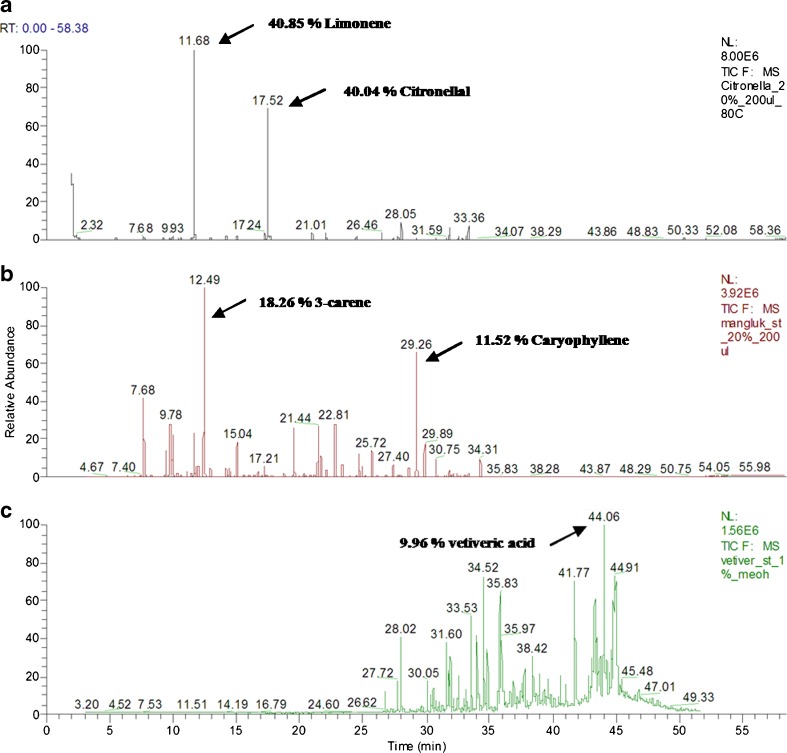
GC-MS was employed to evaluate individual chromatograms of citronella, hairy basil, and vetiver oil (Fig. 1). The main compositions of citronella oil were 40.85% of limonene and 40.04% of citronellal (Fig. 1a) whereas those of hairy basil oil were 18.26% of 3-carene and 11.52% of caryophyllene (Fig. 1b). A main constituent of vetiver oil was 9.96% of vetiveric acid (Fig. 1c).
Fig. 1.
GC-MS chromatograms of citronella oil a, hairy basil oil b, and vetiver oil c
Droplet Size and Zeta Potential
The emulsions were formulated according to the compositions presented in Table I. The nanoemulsions were prepared by two conditions; without and with high-pressure homogenization. Table II demonstrated droplet size of the nanoemulsions determined by PCS. Mean droplet sizes of the nanoemulsions prepared with high-pressure homogenization were in range of 150–160 nm while the sizes from those prepared without the high-pressure homogenization were significantly larger in a range of 195–220 nm (p < 0.05). However, their PI values were not much different in which these were lower than 0.1, suggesting a narrow size distribution. No significant difference in droplet size among the formulations prepared either with or without high-pressure homogenization was found (p > 0.05). However, high-pressure homogenization provides the nanoemulsions with much smaller droplet sizes. This could be due to the shear forces and turbulence, both of which are pressure dependent, produced during homogenization (21). This result was in agreement with a previous study of Yuan et al. (24) where an increase in homogenization cycle resulted in significant decreases in both the droplet size and the range of particle distribution of β-carotene nanoemulsions. Tan and Nakajima also reported a shift of droplet size distribution of nanodispersions to smaller droplet size with narrowed distribution pattern, as the homogenization pressure and cycle increased (23). Zeta potential was employed as a useful tool to predict the physical stability of colloidal systems. The ZPs of nanoemulsions prepared with high-pressure homogenization varied from −45 to −61 mV while those of the formulations prepared without pressure homogenization varied from −27 to −54 mV. Nanoemulsions with high-pressure homogenization tended to exhibit higher ZP, suggesting electrochemical stability after the emulsification homogenization. The change of zeta potential between the formulations should be resulted from their composition. Furthermore, with the same formulation the values of zeta potential were increased after the high-pressure homogenization except for F4 and F6 where insignificant changes were found.
Table II.
Mean Particle Sizes, Polydispersity Index (PI), Zeta Potential and A. aegypti Protection Time of Essential-Oil-Loaded Nanoemulsion Prepared with and without High-Pressure Homogenization
| Formulation | Without high-pressure homogenization | With high-pressure homogenization | ||||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | PI | Zeta potential (mV) | Protection time (h) | Size (nm) | PI | Zeta potential (mV) | Protection time (h) | |
| F1 | 199.7 ± 3.1 | 0.092 ± 0.028 | −53.9 ± 0.3 | 1.5 ± 0.5 | 161.3 ± 1.5 | 0.055 ± 0.022 | −60.3 ± 0.4 | 3.5 ± 0.5 |
| F2 | 204.0 ± 2.0 | 0.068 ± 0.006 | −49.3 ± 1.1 | 2.0 ± 0.0 | 156.3 ± 1.5 | 0.090 ± 0.017 | −59.8 ± 2.7 | 2.7 ± 0.6 |
| F3 | 205.0 ± 2.6 | 0.059 ± 0.017 | −50.7 ± 0.7 | 2.5 ± 0.5 | 148.7 ± 0.6 | 0.078 ± 0.026 | −55.4 ± 13.0 | 3.3 ± 0.3 |
| F4 | 197.3 ± 1.2 | 0.074 ± 0.014 | −53.7 ± 1.4 | 2.5 ± 0.5 | 158.7 ± 2.1 | 0.072 ± 0.013 | −51.0 ± 3.3 | 4.2 ± 0.3 |
| F5 | 220.7 ± 5.0 | 0.050 ± 0.016 | −26.0 ± 1.9 | 3.3 ± 0.3 | 154.3 ± 1.2 | 0.078 ± 0.008 | −59.4 ± 1.6 | 4.2 ± 0.3 |
| F6 | 212.7 ± 0.6 | 0.074 ± 0.011 | −49.3 ± 2.0 | 3.2 ± 0.6 | 151.7 ± 1.5 | 0.087 ± 0.009 | −45.7 ± 1.1 | 4.2 ± 0.6 |
| F7 | 200.0 ± 1.0 | 0.091 ± 0.029 | −27.2 ± 1.2 | 3.8 ± 0.3 | 153.7 ± 1.2 | 0.060 ± 0.009 | −51.2 ± 0.6 | 4.7 ± 0.3 |
Nanoemulsions Stability
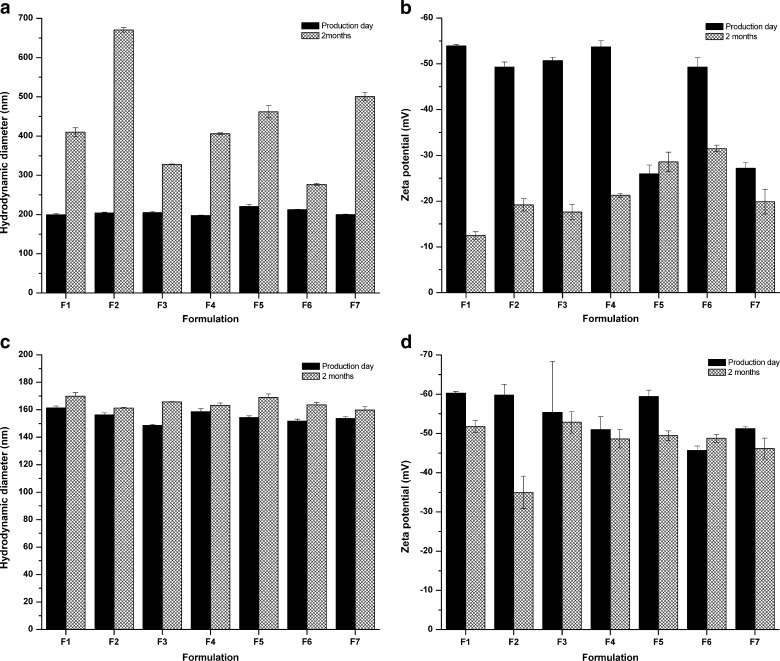
All formulations were kept at 25°C and monthly determined for 2 months of storage by droplet sizes and ZP (Fig. 2). Although there was no phase separation after storage, a dramatically increase in droplet sizes of all nanoemulsion formulations prepared without high-pressure homogenization (p < 0.05) were found (Fig. 2a). This is in contrast to a slight increase found from formulations prepared with high-pressure homogenization (Fig. 2c). A significant decrease in ZP was found in the formulations prepared without high-pressure homogenization except F5 (Fig. 2b) while ZP values of those prepared with high-pressure homogenization tend to decrease but at the lesser extent (Fig. 2d). Regarding ZP values, when absolute value of ZP is higher than 30 mV for colloidal formulation, the particles are likely to be electrochemically stable under the investigated condition (27). ZP values of nanoemulsion without high-pressure homogenization were all lower than |−30| mV whereas those of with high pressure remained higher than 30 mV. According to both results, high shear force could provide much more stable nanoemulsions. Therefore, the results accord well with the finding of Yuan et al. where physical stability of the β-carotene nanoemulsions increased with high-pressure homogenization on both parameter, pressure (up to 100 MPa) and homogenization cycle (up to three cycles), although, chemically, significant degradation occurred during storage (24). The oil in water nanoemulsion loaded with β-cypermethrin has been previously prepared without high-pressure homogenization process and its stability has been observed (16). Although there was no phase separation after several weeks, an increase in droplet size was noted with time and the mechanism of the instability has been attributed to Ostwald ripening.
Fig. 2.
Particle sizes a and c and zeta potential b and d of essential-oil-loaded nanoemulsions prepared without high-pressure homogenization a and b and with high-pressure homogenization c and d
Release Time-Courses of Essential-Oil-Loaded Nanoemulsions
According to the GC-MS chromatograms, the main components of citronella oil were limonene and citronellal. Limonene was chosen as a major marker for this study due to its reported mosquito repellent activity (28). Remaining amounts of limonene from nanoemulsions were measured at 32°C versus storage time. To evaluate the release rate constant of limonene in the nanoemulsion, Avrami’s equation as well as the Weibull distribution function which was successfully applied to describe the shelf life failure (29) was applied to the release time-courses of the encapsulated limonene as reported in the previous work (25).
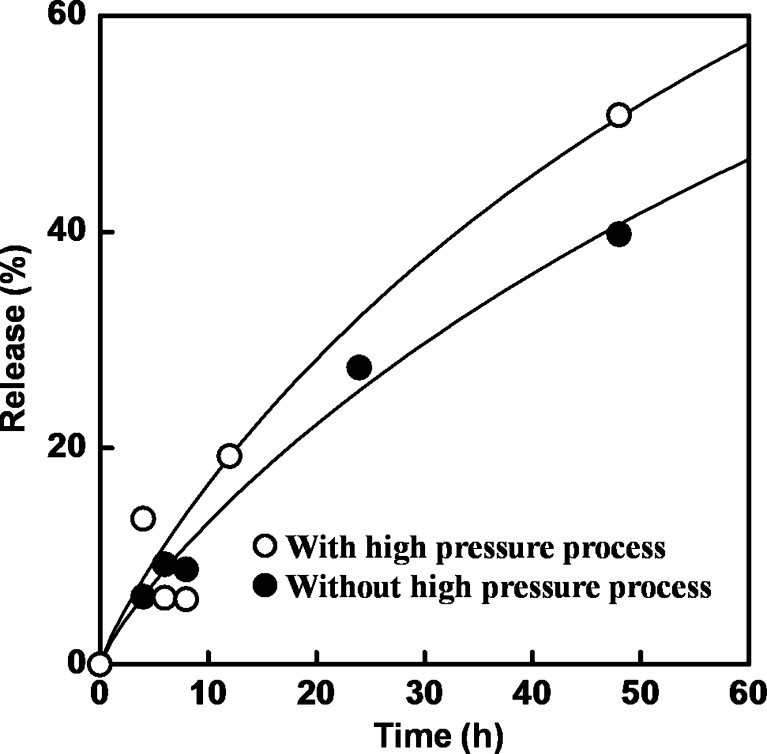
Release kinetic parameters of all formulations were calculated and shown in Table III. The effect of high-pressure homogenization on the release profile is shown in Fig. 3 where the k values of formulations prepared with high-pressure homogenization tended to be higher than those prepared without the homogenization, suggesting high amount of released essential oil from the nanoemulsion. It is possible that the faster release of essential oil from the formulation prepared with high-pressure homogenization is most likely due to smaller oil droplet size and increased surface area of the droplets, resulting in an accelerated dissolution of the essential oils into the carrier solution and diffusion through the liquid to evaporate. Avrami’s parameter n can relate to the release mechanism where n = 1 represents the first-order release mechanism, and n < 1 (theoretically n = 0.54) means that the molecular diffusion of limonene is rate limiting. For n > 1, the release shows a rapid release which might occur from the breaking of emulsion droplet. From the results, the n values for both homogenization were almost in the same range of 0.6 to 1.0, implying that the release of encapsulated limonene was controlled by the diffusion mechanism from the emulsion droplet (22,25).
Table III.
Avrami’s Release Kinetic Parameters, Release Rate Constant (k) and n of Essential-Oil-Loaded Nanoemulsion
| Formulation | Without high-pressure homogenization | With high-pressure homogenization | ||
|---|---|---|---|---|
| k (×10−2 h−1) | n | k (×10−2 h−1) | n | |
| F1 | 1.0 ± 0.1 | 0.84 ± 0.06 | 1.4 ± 0.2 | 0.86 ± 0.13 |
| F2 | 0.9 ± 0.3 | 1.06 ± 0.24 | 1.2 ± 0.3 | 0.76 ± 0.11 |
| F3 | 1.3 ± 0.4 | 0.66 ± 0.14 | 1.9 ± 0.3 | 0.64 ± 0.07 |
| F4 | 0.9 ± 0.1 | 0.82 ± 0.08 | 1.2 ± 0.1 | 1.05 ± 0.12 |
| F5 | 1.2 ± 0.2 | 0.64 ± 0.06 | 1.3 ± 0.2 | 0.93 ± 0.15 |
| F6 | 1.6 ± 0.3 | 0.83 ± 0.12 | 1.5 ± 0.2 | 1.02 ± 0.15 |
| F7 | 1.1 ± 0.5 | 0.76 ± 0.21 | 1.9 ± 0.1 | 0.98 ± 0.06 |
Fig. 3.
Release time-courses of limonene in essential-oil-loaded nanoemulsions (F1) prepared with and without high-pressure homogenization
Mosquito Repellent Efficiency
Mosquito repellent activity of citronella oil, hairy basil oil, and vetiver oil has been reported against A. aegypti, Anopheles dirus, and Culex quinquefasciatus (9,13,15). The mosquito repellent efficiency of the obtained nanoemulsions containing varying combination of these essential oils was then tested in vivo. A. aegypti was used as a representative mosquito in this experiment due to its importance as a major carrier to cause dengue fever in tropical countries. Table II demonstrated that protection time of the essential-oil-loaded nanoemulsions. Mosquito protection times of the formulations prepared with high-pressure homogenization were significantly longer than those prepared without the homogenization. This repellant effect of the obtained nanoemulsions could be attributed to major difference in oil droplet size. Small nanoscale of droplet size of nanoemulsion prepared with high-pressure homogenization would play an important role on their efficacy.
During in vivo test, the nanoemulsions were applied on 3 × 10 cm2 marked skin area, it is possible to speculate that oil nanodroplets could be well-formed and spread on skin surface as thin film. This may result in pronounced mosquito protection time. Hexagonal packaging of lipid nanoparticles in a monolayer was proposed (30,31) where the difference in thin film characteristics between 2 mm lipid microparticles compared to 200 nm of solid lipid nanoparticles was found. In this study, the mosquito repellent properties were from the evaporation of aroma from essential oils. Therefore, the thin film obtained from the nanoemulsions with smaller oil droplets would have higher integrity and more surface area exposed to the air than to the skin; then the evaporation would increase (32).
The longer of mosquito repellant activity of nanoemulsions could be explained by the higher release rate of the nanoemulsion comparing to the larger size emulsion (Table II and Fig. 3). The higher release rate correlated with the higher concentration of aroma vapor above the skin, which is possibly equal to the effective aroma amount for mosquito repellant activity. However, for the nanoemulsion with large droplet size lower release rate can be found, which may then resulted in a lower concentration of aroma above the skin. Therefore, it is possible that this aroma concentration might not reach the effective concentration for mosquito repellant activity, resulting in short protection time. It should be noted that these results were not agreed with our previous work (33). We have reported the low release rate of nanoemulsion with large droplet size that resulted in prolonged mosquito repellant activity compared to the nanoemulsion with small droplet size. A different finding could be from the obtained release parameter (k values) from this study, which was significantly lower than that at 10 times of magnitude. Therefore, the higher extent of aroma vapor on the skin in both types of nanoemulsion (large and small droplet size) from the previous study was likely to occur and above effective concentration for mosquito repellant activity.
The formulations can be divided into three groups, F1, F2 to F4, and F5 to F7, with 20%, 15%, and 10% of citronella oil as major composition, respectively. F1, which contain 20% of citronella oil, demonstrated the shortest protection time (1.5 and 3.5 h for nanoemulsions prepared without and with high-pressure homogenization, respectively). The protection times were significantly increased with the presence of 10% hairy basil oil (F5) or 10% vetiver oil (F6), or both oils at 2.5% (F4) and 5% (F7) loadings. In addition, the longest protection time (4.7 h) can be obtained when combination of hairy basil oil (5%) and vetiver oil (5%) were used with citronella oil (10%) and with high-pressure homogenization. The obtained results revealed that the enhanced mosquito repellent activity was attributable to a combination of these three essential oils and also the small droplet size of the nanoemulsion. Tawatsin et al. (15) has reported that volatile oils extracted from turmeric, citronella grass, and hairy basil can repel A. Aegypti under cage conditions up to 3 h but with the addition of 5% vanillin as a fixative its repellency can be up to 6 h. However, DEET (N,N-diethyl-3-methylbenzamide), the most common chemical repellent currently available, with the addition of 5% vanillin gave protection against the three mosquito species for at least 8 h. The A. aegypti mosquito repellent activity of 38 essential oils from plants has been reported by Trongtokit et al. (13). When the tested oils were applied at a 10% or 50% concentration, none of them prevented mosquito bites for as long as 2 h, but the undiluted oils of C. nardus (citronella), Pogostemon cablin (patchuli), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen) were found as the most effective and provided 2 h of complete repellency.
The Relation Between the Release Characteristics and Mosquito Repellent Efficiency
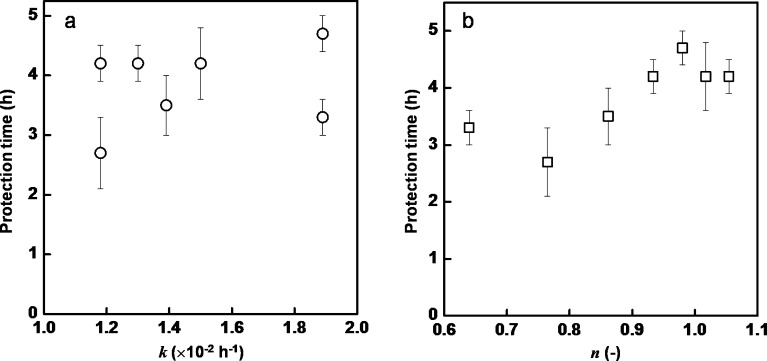
The relation between the protection time and release characteristics were shown in Fig. 4a and b for the release rate constant (k) and release mechanism (n), respectively. The release rate constant did not relate to the protection time. On the other hand, the protection time tended to increase with the increasing of n values with the range of 0.5–1.0. It was indicated that in the diffusion controlled release mechanism, the protection time could be roughly estimated from the n value (25).
Fig. 4.
Relation between the release characteristic and mosquito repellent time of essential-oil-loaded nanoemulsions; release rate constant a and release mechanism b
Cell Cytotoxicity
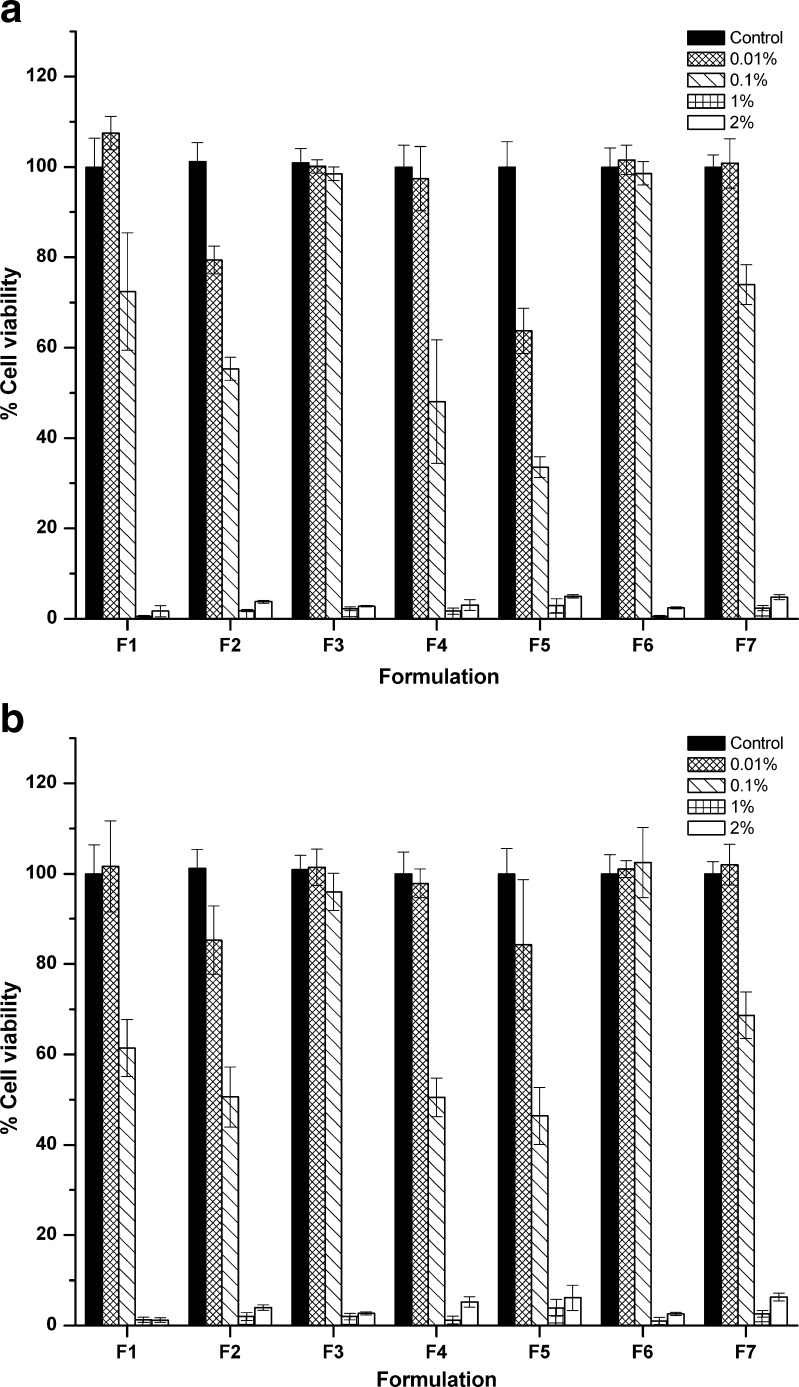
Nanoemulsions were evaluated in term of cytotoxicity on normal human foreskin fibroblast cells by MTT assay. The formulations demonstrated cytotoxic effect against the NHF cells with dose-dependent pattern. The percentage of cell viability was not different between formulations of either nanoemulsion prepared with or without high-pressure homogenization. Mostly, 100% cell viability was found at 0.01% (v/v) concentration (Fig. 5a and b) whereas a decrease in cell viability (40–80%) was found starting from 0.1% (v/v) concentration onwards. The toxicity of the nanoemulsion to NHF cell could be due to a presence of vetiver oil and hairy basil oil in the formulations, in which their anti-proliferative activity has been reported in the human mouth epidermal carcinoma (KB) and murine leukemia (P388) cell line (34). Since the expected concentration when applied on human skin would be approximately 100% (v/v), therefore toxicity was likely to occur toward human skin. However, according to mosquito repellent test there was no report on any irritation or rash from volunteers. Further studies on formulation development to decrease cell toxicity, irritation test, and satisfaction of volunteer are then required for safety purpose.
Fig. 5.
Cytotoxicity of essential-oil-loaded nanoemulsion formulations prepared without high-pressure homogenization a and with high-pressure homogenization b at varying dilutions
CONCLUSIONS
The nanoemulsion droplet size and its composition influenced the release of essential oil. The results indicate that there are advantages to creating a smaller nanoemulsion by using high-pressure homogenization for the insoluble essential oil, although larger nanoemulsions are much easier to prepare and are less expensive. The smaller droplet size nanoemulsion shows the better physical stability, higher release rate, and longer mosquito repellant activity, possibly due to higher thin film integrity on human skin. The highest mosquito protection time (4.7 h) against A. aegypti can be obtained at the optimal composition of 10% (w/w) citronella oil, 5% (w/w) hairy basil oil, and 5% (w/w) vetiver oil in nanoemulsion. No cytotoxicity against NHF cells was found up to 0.01% (v/v) concentration of nanoemulsion.
Acknowledgements
This research was financially supported by National Nanotechnology Center (NANOTEC), Thailand (Research grant number B21 CR0167 10RDCR01). The authors are grateful for GC-MS support by Traditional Thai Medicine Development Center, The Institute of Traditional Thai Medicine Department for Development of Traditional and Alternative Medicine, Thailand; and for mosquito repellent test by Department of Medical Sciences, Ministry of Public Health, Thailand.
References
- 1.Curtis CF. Personal protection methods against vectors of disease. Rev Med Vet Entomol. 1992;80:543–553. [Google Scholar]
- 2.Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 3.Walker TW, Robert LL, Copeland RA, Githeko AK, Wirtz RA, Githure JI, et al. Field evaluation of arthropod repellents, DEET and a piperidine compound, AI3–37220, against Anopheles funestus and Anopheles arabiensis in western Kenya. J Am Mosq Control Assoc. 1996;12:172–176. [PubMed] [Google Scholar]
- 4.Thavara U, Tawatsin A, Chompoosri J, Suwonkerd W, Chansang U, Asavadachanukorn P. Laboratory and field evaluations of the insect repellent 3535 (Ethyl butylacetylaminopropionate) and deet against mosquito vectors in Thailand. J Am Mosq Control Assoc. 2001;17:190–195. [PubMed] [Google Scholar]
- 5.De Paula JP, Gomes-Careiro MR, Paumgartten FJR. Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil. J Ethnopharmacol. 2003;88:253–260. doi: 10.1016/S0378-8741(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 6.Traboulsi AF, El-Haj S, Tueni M, Taoubi K, Nader NA, Mrad A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae) Pest Manage Sci. 2005;61:597–604. doi: 10.1002/ps.1017. [DOI] [PubMed] [Google Scholar]
- 7.Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour Technol. 2008;99:8788–8795. doi: 10.1016/j.biortech.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SS, Chua MT, Chang ED, Huang CG, Chen WJ, Chang ST. Variation in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresour Technol. 2009;100:465–470. doi: 10.1016/j.biortech.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 9.Chokechaijaroenporn O, Bunyapraphatsara N, Kongchuensin S. Mosquito repellent activities of ocimum volatile oils. Phytomedicine. 1994;1:135–139. doi: 10.1016/S0944-7113(11)80031-0. [DOI] [PubMed] [Google Scholar]
- 10.Boonyabancha S, Suphapathom K, Srisurapat A. Repellent effect of volatile oils on Aedes aegypti. Bull Dept Med Sci. 1997;39:61–66. [Google Scholar]
- 11.Barnard DR. Repellency of essential oils to mosquitoes (Diptera:Culicidae) J Med Entomol. 1999;36:625–629. doi: 10.1093/jmedent/36.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kang C, Lee J, Kim Y, Han H, Yun HK. Evaluation of repellency effect of two natural aroma mosquito repellent compounds, citronella and citronellal. Entomological Research. 2005;35:117–120. doi: 10.1111/j.1748-5967.2005.tb00146.x. [DOI] [Google Scholar]
- 13.Trongtokit S, Rongsriyam Y, Komalamisra N, Apiwathnasorn C. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res. 2005;19:303–309. doi: 10.1002/ptr.1637. [DOI] [PubMed] [Google Scholar]
- 14.Jaenson TG, Garboui S, Palsson K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2006;43:731–736. doi: 10.1603/0022-2585(2006)43[731:ROOOLE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Tawatsin A, Wratten SD, Scott RR, Thavara U, Techadamrongsin Y. Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol. 2001;26:76–82. [PubMed] [Google Scholar]
- 16.Wang L, Li X, Zhang G, Dong J, Eastoe J. Oil-in-water nanoemulsions for pesticide formulations. J Colloid Interface Sci. 2007;314:230–235. doi: 10.1016/j.jcis.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 17.Moretti MDL, Sanna-Passino G, Demontis S, Bazzoni E. Essential oil formulations useful as a new tool for insect pest control. AAPS PharmSciTech. 2002;13:1–11. doi: 10.1208/pt030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonneville-Aubrun O, Simonnet JT, L'Alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108–109:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- 20.Wu HL, Ramachandran C, Weiner ND, Roessler BJ. Topical transport of hydrophilic compounds using water-in-oil nanoemulsions. Int J Pharm. 2001;220:63–75. doi: 10.1016/S0378-5173(01)00671-8. [DOI] [PubMed] [Google Scholar]
- 21.Floury J, Desrumaux A, Lardières J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Inn Food Sci Emerging Tech. 2000;1:127–134. doi: 10.1016/S1466-8564(00)00012-6. [DOI] [Google Scholar]
- 22.Soottitantawat A, Yoshii H, Furuta T, Ohkawara M, Linko P. Microencapsulation by spray drying: influence of emulsion size on the retention of volatile compounds. J Food Sci. 2003;68:2256–2262. doi: 10.1111/j.1365-2621.2003.tb05756.x. [DOI] [Google Scholar]
- 23.Tan CP, Nakajima M. β-Carotene nanodispersions: preparation, characterization and stability evaluation. Food Chem. 2005;92:661–671. doi: 10.1016/j.foodchem.2004.08.044. [DOI] [Google Scholar]
- 24.Yuan Y, Gao Y, Zhao J, Mao L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int. 2008;41:61–68. doi: 10.1016/j.foodres.2007.09.006. [DOI] [Google Scholar]
- 25.Soottitantawat A, Yoshii H, Furuta T, Ohgawara M, Forssell P, Partanen R, et al. Effect of water activity on the release characteristics and oxidative stability of D-limonene encapsulated by spray drying. J Agric Food Chem. 2004;52:1269–1276. doi: 10.1021/jf035226a. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Report of the WHO informal consultation on the evaluation and testing of insecticides. CTD/WHOPES/IC/96.1, Control of Tropical Diseases Division. World Health Organization, Geneva, 1996, pp. 32–36, 50–52, 69.
- 27.Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 28.Hebeish A, Moustafa MG, Fouda IA, Hamdy SM, Sawy EL, Abdel-Mohdy FA. Preparation of durable insect repellent cotton fabric: limonene as insecticide. Carb Polymers. 2008;74:268–273. doi: 10.1016/j.carbpol.2008.02.013. [DOI] [Google Scholar]
- 29.Gacular MC, Jr, Kubala JJ. Statistical models for shelf life failures. J Food Sci. 1975;40:404–409. doi: 10.1111/j.1365-2621.1975.tb02212.x. [DOI] [Google Scholar]
- 30.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 31.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 32.Kasting GB, Bhatt VD, Speaker TJ. Microencapsulation decreases the skin absorption of N, N-diethyl-m-toluamide (DEET) Toxicol in Vitro. 2008;22:548–552. doi: 10.1016/j.tiv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Sakulku U, Nuchuchua O, Uawongyart N, Puttipipatkhachorn S, Soottitantawat A, Ruktanonchai U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int J Pharm. 2009;372:105–111. doi: 10.1016/j.ijpharm.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]