Abstract
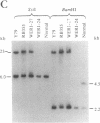
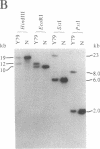
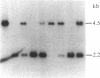
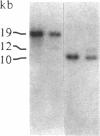
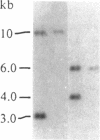
A gene in chromosome region 13q14 has been identified as the human retinoblastoma susceptibility (RB) gene on the basis of altered gene expression found in virtually all retinoblastomas. In order to further characterize the RB gene and its structural alterations, we examined genomic clones of the RB gene isolated from both a normal human genomic library and a library made from DNA of the retinoblastoma cell line Y79. First, a restriction and exon map of the RB gene was constructed by aligning overlapping genomic clones, yielding three contiguous regions ("contigs") of 150 kilobases total length separated by two gaps. At least 20 exons were identified in genomic clones, and these were provisionally numbered. Second, two overlapping genomic clones that demonstrated a DNA deletion of exons 2 through 6 from one RB allele were isolated from the Y79 library. To confirm and extend this result, a unique sequence probe from intron 1 was used to detect similar and possibly identical heterozygous deletions in genomic DNA from three retinoblastoma cell lines, thereby explaining the origins of their shortened RB mRNA transcripts. The same probe detected genomic rearrangements in fibroblasts from two hereditary retinoblastoma patients, indicating that intron 1 includes a frequent site for mutations conferring predisposition to retinoblastoma. Third, this probe also detected a polymorphic site for BamHI with allele frequencies near 0.5/0.5. Identification of commonly mutated regions will contribute significantly to genetic diagnosis in retinoblastoma patients and families.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Harris H. Malignant tumours generated by recessive mutations. Nature. 1986 Oct 16;323(6089):582–583. doi: 10.1038/323582a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance S. K., Smith C. L., Srivastava R., Cantor C. R., Weissman S. M. Megabase-scale mapping of the HLA gene complex by pulsed field gel electrophoresis. Science. 1987 Mar 13;235(4794):1387–1390. doi: 10.1126/science.3029868. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- McFall R. C., Sery T. W., Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977 Apr;37(4):1003–1010. [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shulkin J. D., Sparkes M. C., Moedjono S. Assignment of PGM3 to the long arm of human chromosome 6. Studies using Chinese hamster X human cell hybrids containing a human 6/15 translocation. Cytogenet Cell Genet. 1980;28(1-2):116–120. doi: 10.1159/000131519. [DOI] [PubMed] [Google Scholar]
- Narang S. A., Brousseau R., Hsiung H. M., Michniewicz J. J. Chemical synthesis of deoxyoligonucleotides by the modified triester method. Methods Enzymol. 1980;65(1):610–620. doi: 10.1016/s0076-6879(80)65063-0. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Reid T. W., Albert D. M., Rabson A. S., Russell P., Craft J., Chu E. W., Tralka T. S., Wilcox J. L. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974 Aug;53(2):347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Squire J., Gallie B. L., Phillips R. A. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70(4):291–301. doi: 10.1007/BF00295364. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]