Abstract
The aim of the present report was to develop nonionic surfactant vesicles (niosomes) to improve poor and variable oral bioavailability of griseofulvin. Niosomes were prepared by using different nonionic surfactants span 20, span 40, and span 60. The lipid mixture consisted of surfactant, cholesterol, and dicetyl phosphate in the molar ratio of 125:25:1.5, 100:50:1.5, and 75:75:1.5, respectively. The niosomal formulations were prepared by thin film method and ether injection method. The influence of different formulation variables such as surfactant type, surfactant concentration, and cholesterol concentration was optimized for size distribution and entrapment efficiency for both methods. Result indicated that the niosomes prepared by thin film method with span 60 provided higher entrapment efficiency. The niosomal formulation exhibited significantly retarded in vitro release as compared with free drug. The in vivo study revealed that the niosomal dispersion significantly improved the oral bioavailability of griseofulvin in albino rats after a single oral dose. The maximum concentration (Cmax) achieved in case of niosomal formulation was approximately double (2.98 μg/ml) as compared to free drug (1.54 μg/ml). Plasma drug profile also suggested that the developed niosomal system also has the potential of maintaining therapeutic level of griseofulvin for a longer period of time as compared to free griseofulvin. The niosomal formulation showed significant increase in area under the curve0-24 (AUC; 41.56 μg/ml h) as compared to free griseofulvin (22.36 μg/ml h) reflecting sustained release characteristics. In conclusion, the niosomal formulation could be one of the promising delivery system for griseofulvin with improved oral bioavailability and prolonged drug release profiles.
Key words: griseofulvin, niosomes, oral bioavailability, stability, sustained release
INTRODUCTION
In recent years, transporting the drug molecules to the desired site in the biological systems has become a very specific and sophisticated area of pharmaceutical research. The role of the drug delivery system is not only limited to a drug package just meant for convenience and administration but to bring a required change in therapeutic efficacy and safety by carrying the drug molecules to the desired site in the most convenient manner. The oral route is the most common and convenient method of drug administration to the patients, however, oral administration of drugs often lead to degradation due to the highly acidic gastric environment, enzymes of the mucosa or liver, before they enter the systemic circulation. Beside many highly polar drugs, macro molecular drugs may not be absorbed because of their insufficient poor solubility, lipophilicity, and large molecular weight (1–4).
The antifungal agent griseofulvin is also poorly water-soluble drug, and its absorption from oral route is also poor (5,6), as a result, failure in providing effective plasma drug profile on conventional oral administration. The deep-routed micella of fungal infection necessitate antifungal therapy for prolonged period (7). The large dose and frequent administration of griseofulvin may lead to contraindicative manifestation (8). The carrier system serves to protect the drug from prematured degradation and inactivation and protects the host from unwanted immunological or pharmacological effects. A wide variety of carriers can be found in nature which serves to bring specific molecules to specific cells. The conventional micellar systems are known to enhance the solubility of poorly absorbed drugs resulting into improved bioavailability (9,10). Drug delivery system using colloidal particulate carrier, such as liposomes (11) or niosomes (12), has distinct advantages over conventional dosage form and micelles because the particles can act as drug containing reservoirs. Niosomes are now widely studied as an alternative to liposomes because they alleviate the disadvantages associated with liposomes, such as chemical instability, variable purity of phospholipids, and high cost (13,14). Niosomes are nonionic surfactant vesicles and may also be used as a vehicle for poorly absorbable drugs for designing the novel drug delivery system. The niosomal system supposes to enhance bioavailability of poorly bioavailable drugs by crossing the anatomical barrier of gastrointestinal tract via transcytosis of M cells of Peyer's patches at the intestinal lymphatic tissues (15). The present study was aimed at developing and exploring the use of nonionic surfactant vesicles for increasing oral bioavailability of inadequately bioavailable griseofulvin.
MATERIALS AND METHODS
Materials
Griseofulvin was a benevolent gift from Glaxo SmithKline Pharmaceuticals Ltd., Mumbai. Span 20, span 40, span 60, cholesterol, and dicetyl phosphate (DCP) were purchased from Sigma Chemical Co. (St Louis, MO, USA). All other chemicals were of reagent grade and purchased from CDH, India.
Preparation of Niosomes
Thin Film Method
Niosomes formulations were prepared by slight modification in lipid film formation technique reported by Jain et al. (16). The surfactant:cholesterol:DCP were taken in different weight ratio (10 mg total weight of surfactant–lipid mixture) in a round bottom flask and dissolved in 10 ml chloroform, and 20 mg griseofulvin (1:2 ratio of surfactant–lipid mixture:drug) was added in this lipid mixture. The surfactant/lipid/drug film was formed by the evaporation of chloroform using rotavapor (Strike(R) 102), and then, the resulted film was hydrated with 10 ml phosphate-buffered saline (PBS; pH 7.4) aqueous solution for about 45 min. Temperature was maintained at 60 ± 2°C. This was lead to the formation of multilamellar vesicles.
Ether Injection Method
Various niosomal formulations were prepared by ether injection technique reported by Satturwar et al. (17) with slight modifications. Briefly, nonionic surfactant (span 20, 40, and 60) and cholesterol in different ratios were weighed accurately (10 mg total weight of surfactant–lipid mixture) and dissolved in 10 ml of chloroform. Griseofulvin 20 mg (1:2 ratio of surfactant–lipid mixture:drug) was then dissolved in the lipid solution. The resulting solution was taken in a syringe type of infusion pump and injected slowly into 10 ml of aqueous phase (PBS, pH 7.4) held in a beaker maintained at 60 ± 2°C and agitated slowly with the help of magnetic stirrer. As the lipid solution was injected slowly into the aqueous phase, vaporization of chloroform resulted in the formation of niosomes.
Separation of Unentrapped Drug/Purification of Vesicles
The niosomes were purified by size exclusion gel chromatography method reported by Uchegbu et al. (18). Briefly, 0.2 ml niosomal formulation was poured above the Sephadex G50 minicolumn and centrifuged at 200×g for 10 min using PBS 7.4 as an eluent. The purified niosomal formulation was stored (PBS 7.4) in amber color glass vials.
Optimization of Niosomes
Niosomes using different ratio of different surfactants and cholesterol were prepared by thin film method and ether injection method. These preparations were optimized on the basis of size distribution and entrapment efficiency. The different surfactant (span 20, 40, and 60):cholesterol: DCP were taken in the different ratio of 125:25:1.5, 100:50:1.5, and 75:75:1.5. Volume of aqueous phase was 10 ml. Time and temperature for hydration was kept 45 min and 60 ± 2°C, respectively.
Characterization of Formulations
Vesicle Morphology and Size Analysis
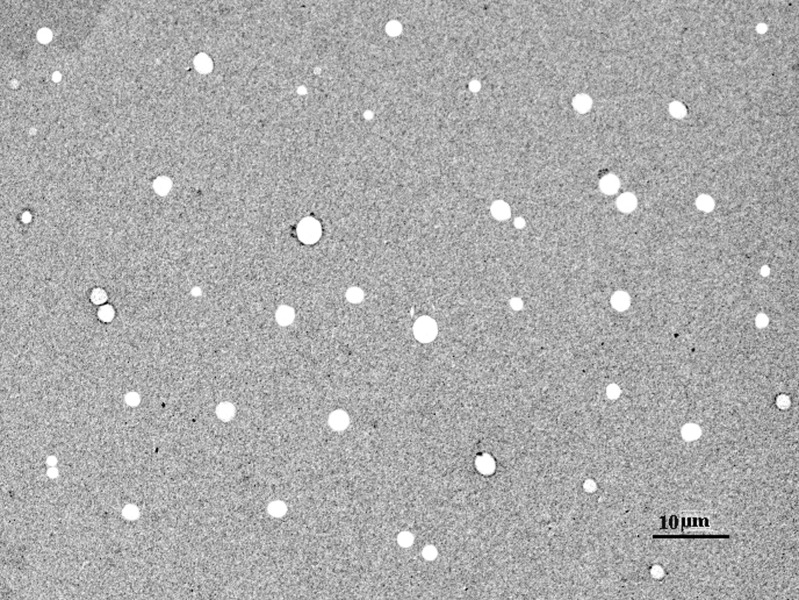
The prepared niosomal formulation was characterized for their morphology using Transmission Electron Microscopy (TEM). Briefly, to an aliquot of a suspension of prepared formulation, sufficient quantity of 1% phosphotungstic acid was added and mixed gently. A drop of the mixture was placed on to the carbon-coated grid and drained off the excess. The grid was allowed to dry, and it was observed under transmission electron microscopy (Philips TEM). Photographs were taken at suitable magnification. The results of TEM are shown in Fig. 1. The vesicles size was determined by dynamic light scattering method (DLS) in a multimodal mode using a computerized inspection system (Malvern Zetamaster, ZEM5002, Malvern, UK). For vesicles size measurement, vesicular suspension was mixed with the appropriate medium (PBS pH 7.4), and the measurements were conducted in triplicate.
Fig. 1.
Transmission electron microscope photograph of span 60 TFM III niosome preparation
Entrapment Efficiency
For determination of entrapment efficiency, the unentrapped drug in niosomal formulation was separated using the Sephadex G-50 minicolumn centrifugation method (18). The separated niosomal suspension (1 ml) was disrupted using 0.1 ml of 0.1% Triton X-100 in distilled water for 5 min. The resulted solution was centrifuged at 3,000 rpm for 5 min. The supernatant was decanted off and suitably diluted with methanol:PBS (pH 7.4; 9:1 v/v ratio). The drug was estimated spectrophotometrically at λmax 291.0 nm against methanol–PBS containing 0.1% Triton X-100 as blank.
In vitro Release Studies
In vitro release studies of griseofulvin from niosomes were performed according method reported by Hu and Rhodes (19). The optimized formulation of span 20, 40, and 60 for entrapment efficiency (TFM-III and EIM-III having composition ratio 75:75:1.5 of surfactant:cholesterol:DCP) was taken for in vitro drug release studies. Dialysis tube containing appropriate volume of griseofulvin-loaded niosomes dispersion was placed into a flask containing 100 ml hydroalcoholic solution (methanol:PBS 9:1 v/v ratio, pH 2) and hydroalcoholic solution (methanol–PBS, pH 7.4). Different pH values were selected for in vitro drug release studies to evaluate effect of pH on drug release and to simulate stomach and blood pH. The niosomal formulations were compared with plain griseofulvin suspension in PBS 7.4 solution (10 mg/ml). The flask was shaken at 50 rpm at 37°C. Aliquots of dialysate were taken at predetermined time and replenished immediately with the same volume of fresh-simulated fluid, and withdrawn sample was assayed spectrophotometrically at 291 nm.
Stability Studies
The prepared formulations were tested for stability by storing them at 4 ± 1°C and at 25 ± 2°C. Formulations were assessed for vesicles size and shape and number of vesicles per cubic millimeter before and after storage for 30 days. Residual drug content was also assessed on 15th and 30th day. Size of the vesicular systems was determined by dynamic light scattering method. The shape of the vesicle was observed under light microscope. The number of vesicles per cubic millimeter was calculated by method described elsewhere (20) using a hemocytometer by applying the following formula:
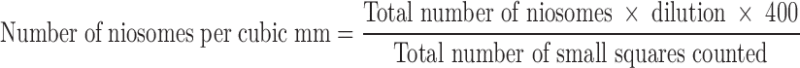 |
Formulations were evaluated for residual drug content by taking samples at 15th and 30th day and estimated by spectrophotometric method, and absorbance of sample was taken at 291.0 nm.
In vivo Studies
Male albino rats (Sprague Dawley strain, 150 ± 5 g) were utilized for in vivo experimental studies. Niosomes formulation S-60 TFM III was selected for in vivo studies on the basis of their promising in vitro performances. All the animal studies were conducted in accordance with the protocol approved by the Institutional Animal Ethical Committee of Dr. H.S. Gour University, Sagar (registration no. 379/01/ab/CPCSEA). The drug was estimated in plasma following administration of niosomally entrapped drug and plain drug suspension to study the alteration in bioavailability of drug brought about on its encapsulation in niosomes.
The animals were divided into three groups, each group containing five animals. The first group was treated as control and was fed with PBS (pH 7.4) by oral route. Second and third groups were treated with a single dose of plain griseofulvin suspension (PBS 7.4) and griseofulvin-entrapped niosomes formulation (equivalent to 100 mg griseofulvin per kilogram of body weight) by oral route. Blood samples were withdrawn at 1, 2, 4, 6, 8, 12, and 24 h after dosing. The blood samples were centrifuged, and 100 μl of plasma was separated and immediately frozen until required for analysis. The plasma samples were deproteinized with 100 μl of acetonitrile containing paraphenylphenol, shaken on a vortex mixture, and centrifuged, and 20 μl of the supernatant was analyzed by high-performance liquid chromatography method reported by Vudathala and Rogers (21). Separation was carried out on reversed-phase C18 column, and the column effluent was monitored using ultraviolet detector at 290 nm. The mobile phase was 45% acetonitrile in 0.1 M acetic acid (pH 3.5) at a flow rate of 1 ml/min.
Statistical Analysis
The results are expressed as mean ± standard deviation (n ± 3), and statistical analysis was performed with SPSS 10.1 for Windows® (SPSS®, Chicago, USA).
RESULTS AND DISCUSSION
Preparation and Characterization of Niosomes
Niosomes were prepared by thin film method and ether injection method. In thin film method, hydration of dried lipid film with aqueous phase resulted in the formation of niosomal dispersion. In ether injection method, lipids were dissolved in ether and injected into hot aqueous solution, and on evaporation of ether, the lipid bilayer encapsulating the aqueous phase was left. In the lipid mixture, spans were used as nonionic surfactant. Since nonionic surfactant, span 60, has the highest phase transition temperature of 50°C (22). In all formulations, hydration was carried out above 50°C, i.e., in ether injection method, aqueous phase was maintained at 60 ± 2°C, and in thin film method, hydration of film was carried out at 60 ± 2°C. Prepared niosomes were optimized on the basis of vesicle size and entrapment efficiency. The optimum surfactant:cholesterol:DCP ratio was found to be 75:75:1.5, i.e., in formulation TFM III and EIM III. Transmission electron microscopy was performed to study vesicle morphology that revealed that niosomes were spherical in shape (Fig. 1). The vesicles size was determined by DLS method in a multimodal mode using a computerized inspection system (Malvern Zetamaster, ZEM5002, Malvern, UK). Mean size of niosomes prepared using span 20 was largest as compared to other formulations. Comparative mean vesicle size of niosomes prepared by different method using different surfactants and varied composition are given in Table I. However, results indicate that there were correlations between the vesicle size and entrapment efficiency as function of the preparation method, surfactant–lipid ratio, and preparation method (Table I). The mean vesicle size decreases consistently from span 20 to 60 but increases with decreasing surfactant ratio/increasing cholesterol ratio (formulations I to III) in both methods (TFM and EIM). The result of entrapment efficiencies (Table II) shows that the entrapment efficiency increases from span 20 to 60 (opposite trend to the size) and also from I to III (same trend as size) within each formulation method. Moreover, vesicle size and entrapment efficiencies were observed higher in TFM formulations as compared to EIM. The difference was not significant between these formulations (p > 0.05).
Table I.
Comparative Average Vesicle Size of Different Griseofulvin Niosomes Formulations
| Formulation code | Composition | Mean vesicle size (μm) | ||
|---|---|---|---|---|
| Span 20 | Span 40 | Span 60 | ||
| TFM-I | 125:25:1.5 | 4.17 ± 0.09 | 4.06 ± 0.07 | 3.99 ± 0.07 |
| TFM-II | 100:50:1.5 | 4.48 ± 0.16 | 4.35 ± 0.15 | 4.18 ± 0.06 |
| TFM-III | 75:75:1.5 | 4.93 ± 0.21 | 4.66 ± 0.24 | 4.37 ± 0.17 |
| EIM-I | 125:25:1.5 | 3.95 ± 0.14 | 3.47 ± 0.12 | 3.16 ± 0.11 |
| EIM-II | 100:50:1.5 | 4.07 ± 0.08 | 3.77 ± 0.06 | 3.5 ± 0.14 |
| EIM-III | 75:75:1.5 | 4.28 ± 0.12 | 3.98 ± 0.14 | 3.69 ± 0.19 |
Values represent mean ± SD (n = 3). The composition ratio indicates the ratio of surfactant:cholesterol:DCP
Table II.
Comparative Percentage of Entrapment Efficiency Study of Formulation
| Formulation code | Composition | Percentage of entrapment efficiency | ||
|---|---|---|---|---|
| Span 20 | Span 40 | Span 60 | ||
| TFM-I | 125:25:1.5 | 61.23 ± 2.71 | 64.62 ± 1.3 | 69.26 ± 2.41 |
| TFM-II | 100:50:1.5 | 62.90 ± 4.83 | 67.39 ± 2.0 | 72.40 ± 2.64 |
| TFM-III | 75:75:1.5 | 65.72 ± 1.92 | 70.55 ± 2.74 | 76.80 ± 1.38 |
| EIM-I | 125:25:1.5 | 42.00 ± 2.87 | 44.90 ± 1.32 | 46.70 ± 1.0 |
| EIM-II | 100:50:1.5 | 44.50 ± 1.53 | 47.10 ± 3.76 | 49.50 ± 1.77 |
| EIM-III | 75:75:1.5 | 46.20 ± 3.11 | 49.90 ± 1.03 | 52.3 ± 2.06 |
Values represent mean ± SD (n = 3). The composition ratio indicates the ratio of surfactant:cholesterol:DCP
Entrapment efficiency is the percentage fraction of the entire drug that entrapped in the niosomes. Niosomes prepared by thin film method exhibited higher entrapment efficiency than those prepared by ether injection method. Among the type of surfactant used, span 60 always showed highest entrapment in niosomes (76.8%) prepared by both of the methods. Entrapment efficiencies of niosomes prepared by different methods using different surfactants and varied composition are presented in Table II.
In vitro Release Studies
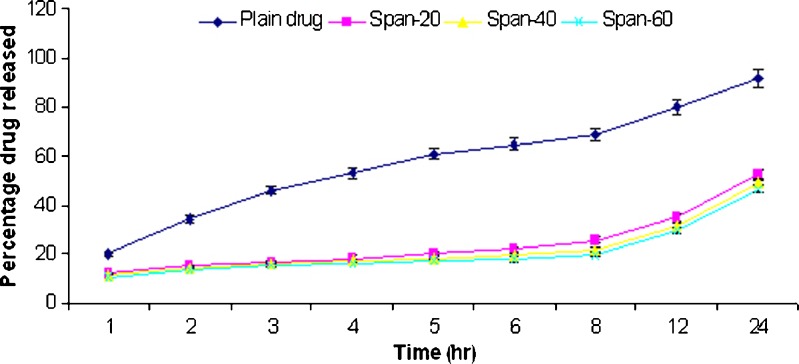
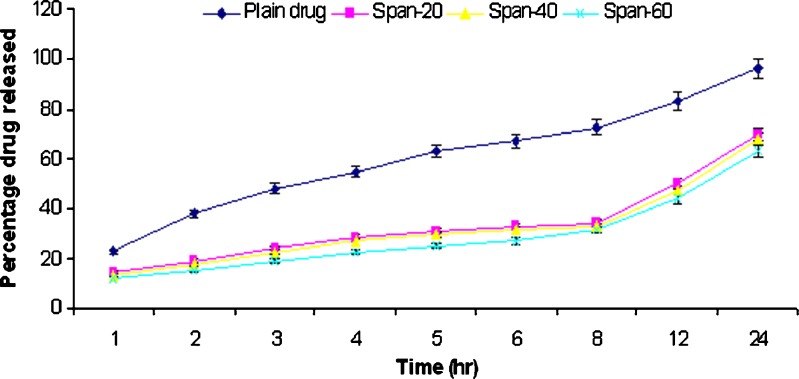
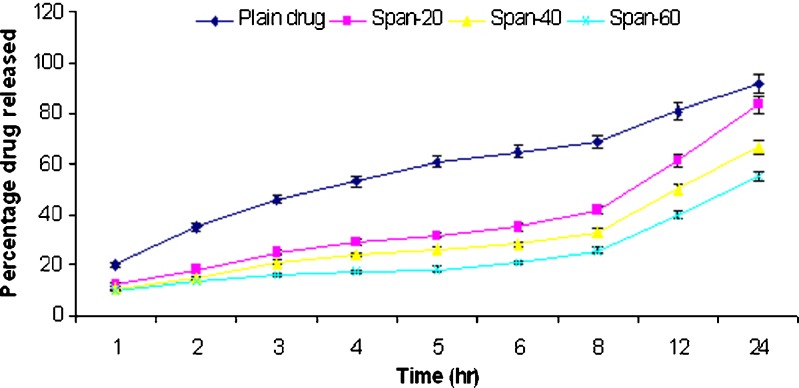
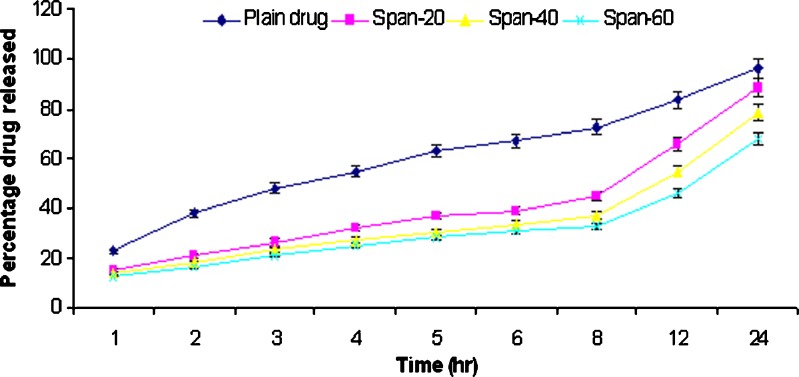
In vitro release studies of griseofulvin from niosomes were performed using dialysis tube containing appropriate volume of griseofulvin-loaded niosomes dispersion placed into a flask containing 100 ml hydroalcoholic solution (methanol–PBS, pH 2) and hydroalcoholic solution (methanol–PBS, pH 7.4). The rate of drug release across the dialysis membrane was slower for drug-loaded vesicles compared to plain drug. The plain drug release in hydroalcoholic solution (methanol–PBS, pH 7.4) was approximately 92% in 24 h. The span 20, 40, and 60 niosomal formulations prepared by thin film method showed release of 52.3%, 48.9%, and 46.8%, respectively, while span 20, 40, and 60 niosomal formulations prepared by ether injection method showed release of 83.5%, 66.8%, and 55.3%, respectively, in 24 h. The plain drug release in hydroalcoholic solution (methanol–PBS, pH 2) was approximately 96.2% in 24 h. The span 20, 40, and 60 niosomal formulations prepared by thin film method showed release of 69.8%, 67.8%, and 63.4%, respectively, while span 20, 40, and 60 niosomal formulations prepared by ether injection method showed release of 88.5%, 78.4%, and 67.8%, respectively, in 24 h. The in vitro release profiles are shown in Figs. 2, 3, 4, and 5.
Fig. 2.
Comparative percentage release rate of different formulations of TFM III in methanol–PBS solution (pH 7.4)
Fig. 3.
Comparative percentage release rate of different formulations of TFM III in methanol–PBS solution (pH 2.0)
Fig. 4.
Comparative percentage release rate of different formulations of EIM III in methanol–PBS solution (pH 7.4)
Fig. 5.
Comparative percentage release rate of different formulations of EIM III in methanol–PBS solution (pH 2.0)
Stability Studies
In the present study, the stability of the vesicles was determined by measuring the vesicle size and shape, number of vesicle per cubic millimeter, and residual drug content before and after 30 days at 4 ± 1°C and 25 ± 2°C. Mean vesicle size was found to increase on storage after 1 month. The increase in vesicle size was more in formulations stored at 25 ± 2°C than at 4 ± 1°C. Results indicate that prepared formulation was relatively stable at 4 ± 1°C, as compared to 25 ± 2°C (Table III). The vesicle size of 5.18 ± 0.26 and 4.32 ± 0.37 μm was recorded at storage temperature of 4 ± 1°C, as compared to initial size of 4.37 ± 0.17 and 3.69 ± 0.19 with S60 TFM-III and S60 EIM-III niosomes formulations, respectively. The increase in size of vesicle was found higher when formulation was stored at 25 ± 2°C as compared to formulation stored at 4 ± 1°C. Effect of storage on number of vesicles per cubic millimeter was evaluated after storage at 4 ± 1°C and 25 ± 2°C for 1 month (Table III). Marked decrease in number of vesicles was observed when stored at 25 ± 2°C. This effect was found to be less prominent in case of the formulations stored at 4 ± 1°C. In comparison of formulation S60 EIM-III, the formulation S60 TFM-III showed better stability as reduction in number of vesicles was found to be less. Storage stability was also evaluated in terms of percent residual drug remaining in the vesicle, considering initially drug content as 100%. At 4 ± 1°C, there was a minimum loss of drug but marked reduction in the residual drug content was found when formulations were stored at 25 ± 2°C (Table IV).
Table III.
Effect of Storage on Mean Vesicle Size and Number of Vesicles per Cubic Millimeter
| Formulation code | Vesicle size (μm) | Number of vesicles | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final at 4 ± 1°C | Final at 25 ± 2°C | Initial | Final (at 4 ± 1°C) | Percent remaining | Final (at 25 ± 2°C) | Percent remaining | |
| S60 TFM-III | 4.37 ± 0.17 | 5.18 ± 0.26 | 6.32 ± 0.11 | 88 | 86 | 97.73 | 73 | 82 |
| S60 EIM-III | 3.69 ± 0.19 | 4.32 ± 0.37 | 5.65 ± 0.41 | 82 | 79 | 96.34 | 62 | 75.61 |
Table IV.
Effect of Storage on Residual Drug Content
| Formulation code | Initial percentage | Percentage of residual drug | |||
|---|---|---|---|---|---|
| At 4 ± 1°C | At 25 ± 2°C | ||||
| 15 days | 30 days | 15 days | 30 days | ||
| S60 TFM-III | 100 | 98.52 ± 2.48 | 95.2 ± 1.56 | 72.5 ± 2.52 | 54.2 ± 2.68 |
| S60 EIM-III | 100 | 97.12 ± 1.68 | 94.3 ± 2.48 | 63.8 ± 1.53 | 47.3 ± 1.68 |
In vivo Studies
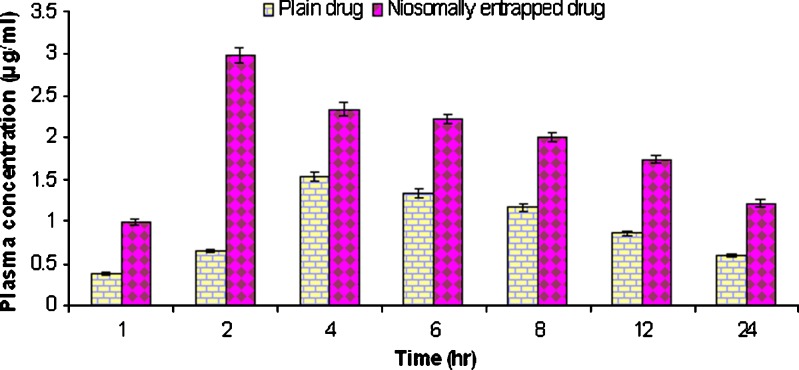
Niosomal formulation exhibited a much faster absorption and reached a peak concentration in plasma (Cmax) in approximately 2 h, whereas plain griseofulvin exhibited slow absorption and reached Cmax in approximately 4 h (Fig. 6). The faster absorption of griseofulvin entrapped niosomal formulation was attributed to transcytosis of M cells of Peyer's patches at the intestinal lymphatic tissues. The niosomal formulation showed better result in comparison to plain griseofulvin. The concentration of griseofulvin in plasma after 1 h (C1 h) was found to be 0.37 μg/ml in case of plain drug as compared to niosome formulation (1 μg/ml). The Cmax achieved by plain griseofulvin was 1.54 μg/ml while Cmax achieved in case of griseofulvin-entrapped niosomes was found to be 2. 98 μg/ml. There was significant (p = 0.0033) difference in concentration of both formulation at 2 h. The retarded release of griseofulvin from niosomes also enhanced AUC0-24 (Table V). There was a significant increase in AUC for niosomal formulation (41.56 μg/ml hr) as compared to plain griseofulvin (22.36 μg/ml hr). Niosomal formulation has shown an increase in the absorption rate of drug as well as an increase in the bioavailability of drug as compared to plain drug. In vivo studies also revealed that niosomal formulation have potential of maintaining therapeutic level of griseofulvin for a longer period of time as compared to plain griseofulvin.
Fig. 6.
Drug plasma concentration profile after oral administration of plain drug and niosomally entrapped drug
Table V.
Pharmacokinetic Data of Plain and Niosomally Entrapped Griseofulvin
| Serial number | Parameters | Plain griseofulvin | Niosomally entrapped griseofulvin |
|---|---|---|---|
| 1. | C1h μg/ml | 0.37 | 1.00 |
| 2. | AUC0-24 μg/ml h | 22.36 | 41.56 |
| 3. | C max μg/ml | 1.54 | 2.98 |
| 4. | t max h | 4 | 2 |
DISCUSSION
Niosomes were prepared by thin film method and ether injection method. Transmission electron microscopy revealed that niosomes were spherical in shape. Niosomes prepared by thin film method exhibited higher entrapment efficiency than those prepared by ether injection method. Among the type of surfactant used, span 60 always showed highest entrapment in niosomes prepared by both of the methods. Physicochemical properties of drug might have well correlated with the hydrophilic–liphophilic balance of span 60. As griseofulvin associates with lipid bilayers, entrapment efficiency depends upon bilayer formation, which might be high in niosomes prepared using span 60 in thin film method. The increase entrapment efficiency might also be result of partly uniform vesicle size and due to well-packed bimolecular film formation. The data indicate almost linear increase in entrapment efficiency on increasing total lipid concentration; higher lipid concentration might have resulted in higher encapsulation volume and thus, increases entrapment efficiency. DCP was included in bilayer composition to impart negative charge because negative charged vesicles have been reported to be more efficient for drug delivery. DCP also provides the stability to the system and prevents the agglomeration and aggregation of vesicles.
In vitro drug release was shown to be retarded by entrapment of griseofulvin in niosomes. Inclusion of cholesterol markedly reduced the reflux of griseofulvin during release phase, which is in accordance with the membrane-stabilizing activity of this lipid (23,24). Span 60 formulation has shown highest retarded release possibly because it bear higher phase transition and higher lipophilicity thus to be less permeable.
During storage, drug leakage and loss in number of vesicles were observed. Lipid vesicles are self assembles of amphiphiles into closed bilayers structures. Hydrated bilayer vesicles, however, are not considered to be thermodynamically stable and are thought to represent a metastable state in that the vesicles possess excess of energy bilayer phospholipids, which can undergo chemical degradation such as oxidation and hydrolysis. Due to this change, vesicular systems maintained in aqueous dispersion may aggregate/fuse, and encapsulated bioactive material may tend to leak out from the bilayer structure during storage. The highest stability profile of S60 TFM-III might be attributed to the membrane-stabilizing effect of cholesterol. The loss of vesicles could be attributed to the disruption/aggregation of vesicles. At 4 ± 1°C, a minimum loss of drug was observed, which might be attributed to the regidization of the vesicles at low temperature that reduced the permeability of the drug through the membrane. Thus, from the results obtained, it can be concluded that prepared vesicular systems are more stable at 4 ± 1°C, as compared to storage at 25 ± 2°C in terms of mean vesicle size, number of vesicle per cubic millimeter, and residual drug content.
In vivo studies revealed that niosomal formulation exhibited a much faster absorption and reached a peak concentration in plasma (Cmax) faster as compared to plain griseofulvin, which exhibited slow absorption. The plain griseofulvin showed higher release in vitro because it passes easily through the pores of dialysis tube. However, when plain drug was administrated orally, it did not attained higher concentration in the blood and showed higher tmax due to poor solubility and absorption profile. On the contrary, niosomal formulation could have absorbed through Peyer's patches. This might be the reason behind the lower in vivo tmax shown by the niosomal formulation. The faster absorption of griseofulvin entrapped niosomal formulation might be attributed to transcytosis of M cells of Peyer's patches at the intestinal lymphatic tissues. The retarded release of griseofulvin from niosomes also enhanced AUC0-24.
CONCLUSION
The in vitro release, in vivo blood level, and pharmacokinetic studies indicated potential of developed griseofulvin-entrapped niosomes formulation for faster absorption and augmenting the bioavailability of griseofulvin. The developed system has also shown potential of maintaining higher level of griseofulvin for a longer period of time as compared to plain griseofulvin, which suggested that this system can also act as a depot formulation inside the body. From the present study, it can be concluded that niosomes can be used efficiently for enhancing bioavailability and for sustained delivery of griseofulvin via oral route.
Acknowledgment
Pratap S. Jadon acknowledges All India Council for Technical Education, New Delhi for junior research fellowship.
References
- 1.Porter CJ, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev. 2001;50:61–80. doi: 10.1016/S0169-409X(01)00151-X. [DOI] [PubMed] [Google Scholar]
- 2.Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25:103–128. doi: 10.1016/S0169-409X(96)00494-2. [DOI] [Google Scholar]
- 3.Shively ML, Thompson DC. Oral bioavailability of vancomycin solid-state emulsions. Int J Pharm. 1995;117:119–122. doi: 10.1016/0378-5173(94)00331-X. [DOI] [Google Scholar]
- 4.Bert A, DeBoer G. Drug absorption enhancement. USA: Howard Academic Publishers; 1994. p. 67. [Google Scholar]
- 5.Arida AI, Al-Tabakha MM, Hamoury HAJ. Improving the high variable bioavailability of griseofulvin by SEDDS. Chem Pharm Bull. 2007;55:1713–1719. doi: 10.1248/cpb.55.1713. [DOI] [PubMed] [Google Scholar]
- 6.Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317:61–68. doi: 10.1016/j.ijpharm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Dangi JS, Vyas SP, Dixit VK. Effect of various lipid-bile salt mixed micelles on transfer of amphotericin-B across the everted rat intestine. Drug Devel Ind Pharm. 1995;21:2021–2027. doi: 10.3109/03639049509065886. [DOI] [Google Scholar]
- 8.Williams DI, Marten RH, Sarkany I. Griseofulvin. Br J Dermatol. 2006;71:434–443. doi: 10.1111/j.1365-2133.1959.tb13380.x. [DOI] [PubMed] [Google Scholar]
- 9.Aungst BJ. Novel formulation strategies for improving and bioavailability with poor membrane permeation. J Pharm Sci. 1993;82:871–879. doi: 10.1002/jps.2600821002. [DOI] [PubMed] [Google Scholar]
- 10.Risovic V, Boyd M, Choo E, Wasan KM. Effects of lipid-based oral formulations on plasma and tissue amphotericin-B concentrations and renal toxicity in male rats. Antimicrob Agents Chemother. 2003;47:3339–3342. doi: 10.1128/AAC.47.10.3339-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narang T, Dogra S, Kaur I, Katare OP, Khurana R. Ciclosporin 1% liposomal gel in limited plaque psoriasis: a prospective randomized double-blind placebo-controlled study in stable plaque psoriasis. Br J Dermatol. 2007;157:53. [Google Scholar]
- 12.Sultana Y, Aqil M, Ali A, Samad A. Advances in the topical ocular drug delivery. Expert Rev Ophthalmol. 2007;2:309–323. doi: 10.1586/17469899.2.2.309. [DOI] [Google Scholar]
- 13.Vora B, Khopade AJ, Jain NK. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release. 1998;54:149–165. doi: 10.1016/S0168-3659(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 14.Nasr M, Mansour S, Mortada ND, Elshamy AA. Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul. 2008;25:499–512. doi: 10.1080/02652040802055411. [DOI] [PubMed] [Google Scholar]
- 15.Rentel CO, Bouwstra JA, Naisbett B, Junginger HE. Niosomes as a novel peroral vaccine delivery system. Int J Pharm. 1999;186:161–167. doi: 10.1016/S0378-5173(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 16.Jain CP, Vyas SP. Preparation and characterization of niosomes containing rifampicin for lung targeting. J Microencapsul. 1995;12:401–407. doi: 10.3109/02652049509087252. [DOI] [PubMed] [Google Scholar]
- 17.Satturwar PM, Fulzele SV, Nande VS, Khandare JN. Formulation and evaluation of ketoconazole niosomes. Indian J Pharm Sci. 2002;64:155–158. [Google Scholar]
- 18.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 19.Hu CJ, Rhodes DG. Proniosomes: a novel drug carrier preparation. Int J Pharm. 1999;185:23–35. doi: 10.1016/S0378-5173(99)00122-2. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesan N, Vyas SP. Polysaccharide coated liposomes for oral immunization—development and characterization. Int J Pharm. 2000;203:169–177. doi: 10.1016/S0378-5173(00)00442-7. [DOI] [PubMed] [Google Scholar]
- 21.Vudathala GK, Rogers JA. Oral bioavailability of griseofulvin from aged griseofulvin : lipid coprecipitates : in vivo studies in rats. J Pharm Sci. 1992;81:1166–1169. doi: 10.1002/jps.2600811207. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (span-20, span-40, span-60 and span-80) and a sorbitan triester (span-85) Int J Pharm. 1994;105:1–6. doi: 10.1016/0378-5173(94)90228-3. [DOI] [Google Scholar]
- 23.Demel RA, DeKruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976;457:109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- 24.Korchowiec B, Paluch M, Corvis Y, Rogalska E. A Langmuir film approach to elucidating interactions in lipid membranes: 1, 2-dipalmitoyl-sn-glycero-3-phosphoethanolamine/cholesterol/metal cation systems. Chem Phys Lipids. 2006;144:127–136. doi: 10.1016/j.chemphyslip.2006.08.005. [DOI] [PubMed] [Google Scholar]