INTRODUCTION
Therapeutic proteins or polypeptides can be formulated into various particulate delivery systems including the polymeric carriers to avoid rapid degradation and to maintain the sustained release for better therapeutic effects (1). Poly(lactic/glycolic) acid (PLGA) micro/nanoparticle is a widely used formulation agent because of its good biodegradability, biocompatibility, and approved clinical usage, which have been available in the market to treat prostate cancer, acromegaly, periodontal disease, and pediatric growth hormone deficiency (2). The sustaining and complete release of proteins in their native or active forms from the PLGA micro/nanoparticles is often a challenging task (3). Proteins are generally formulated into PLGA nanoparticles by water/oil/water double-emulsion–solvent evaporation method, which generates hydrophilic/hydrophobic interface that may result in the unfolding or denaturing of protein molecules (4). Thus, polymeric surfactants are usually used to lower surface tension of protein solutions and to prevent aggregation of protein molecules at hydrophobic surface (1).
Polyvinyl alcohol (PVA) is a widely used polymeric surfactant in the exterior aqueous phase as an emulsifier. However, the safety of PVA still appears to be a concern from the previous literature reports (5,6). Following repeated subcutaneous or intravenous administrations of PVA, various organ lesions and hypertension have been reported in rats, and central nervous system depression and anemia followed by renal damage have also been reported in beagle dogs; whereas, orally administered PVA is relatively harmless in mice or rat (5,6). Therefore, residual PVA was always removed by washing procedures such as repetitive centrifugation (7) or filtration (8), which removes not only the PVA but also the unencapsulated proteins or polypeptides, which might be often valuable or hard to collect. Hence, a few substituting emulsifiers have been studied. For example, poly(ethylene glycol) (PEG) was used successfully as the stabilizer to prepare enzyme-loaded PLGA microparticles (9). Besides, surfactant-free formulation was achieved by nanoprecipitation/solvent-displacement method that would not alter surface properties of PLGA nanoparticles, but this method may suffer the low encapsulation efficiency when low protein concentration solution is to be used (10).
In the present study, we introduced another surfactant-free method to prepare PLGA nanoparticles encapsulating polypeptides by double-emulsion–solvent evaporation method. We used sucrose as the stabilizer of the exterior aqueous phase to prevent aggregation of PLGA nanoparticles during formulation process. Nerve growth factor (NGF), a small secreted protein which can induce the differentiation and survival of particular target neurons, was used as the model protein. As sucrose is a nontoxic material, additional washing procedures are not necessarily needed for NGF-loaded PLGA nanoparticles, and thus, the valuable NGF loss can be minimized. The release of functional NGF molecules from these nanoparticles was verified by either the antibody recognition in enzyme-linked immunosorbent assay (ELISA) method or the induction on the neurite outgrowth of PC-12 cells.
MATERIALS AND METHODS
Materials
Recombinant rat β-NGF (molecular weight 13.2 kDa), mouse anti-beta III tubulin, and NGF ELISA kit were purchased from R&D Systems (Minneapolis, USA). PLGA (50:50, molecular weight 40,000–75,000) and PVA (molecular weight 30,000–70,000) were purchased from Sigma-Aldrich (St. Louis, USA). FITC-conjugated AffiniPure goat anti-mouse IgG was purchased from Jackson immunoresearch (West Grove, USA). RPMI1640 medium was purchased from Gibco (Carlsbad, USA). Fetal bovine serum was from Hyclone (Logan, USA). All other reagents were of analytical grade.
Preparation of NGF-loaded PLGA Nanoparticles
NGF-loaded PLGA nanoparticles in sucrose solution (NGF-PLGA-sucrose) or those in PVA solution (NGF-PLGA-PVA) were prepared by the double-emulsion–solvent evaporation method. The first emulsion was carried out as previously reported with modifications (11). Briefly, 25 μl of 0.5 mg/ml NGF and 0.5 mg/ml BSA solution in 8 mM citrate buffer (pH = 8.0) containing 5% (2-hydroxypropyl)-β-cyclodextrin, which was reported to be able to reduce loss of protein activity in interior water phase (11), was added into 250 μl of PLGA solution in methyl chloride (2 mg/ml) and then sonicated on ice by 5 W with a microtip sonicator (Misonix, Framingdale, USA) for 15 s. The first emulsion was immediately dropped into 1.5 ml of 10% sucrose or 1% PVA solution and sonicated on ice by 5 W for 20 s to form the second emulsion. The organic solvent within the emulsion was removed under a controlled evaporation rate (820 hPa for 10 min, followed by 700, 650, 550, and 350 hPa for 5 min each and decreased to 30 hPa for additional 10 min) by using a rotary evaporator equipped with a vacuum controller (EYELA, Tokyo, Japan). The obtained particle solution was then centrifuged at 3,000 rpm for 10 min to remove residual polymer or aggregates.
Characteristics Analyses of NGF-loaded PLGA Nanoparticles
Hydrodynamic diameter and zeta potential of NGF-PLGA-sucrose and NGF-PLGA-PVE were measured by 90Plus particle sizer and ZetaPALS (Brookhaven, New York, USA) equipped with a 660-nm laser. The measured delay time correlation functions were fitted to NNLS model to calculate particle size distribution. All results were acquired in triplicates. Morphology of NGF-PLGA-sucrose and NGF-PLGA-PVA was observed by transmission electron microscope (TEM; Hitachi, Tokyo, Japan) with acceleration voltage at 80 kV.
In Vitro Release Analyses of NGF
To investigate the release profile of NGF from the PLGA nanoparticles and degradation kinetics of nonformulated NGF in cell culture circumstance, 20 μg/ml NGF-PLGA-sucrose (equivalent 500 ng/ml NGF with 30.8 ng/ml unencapsulated NGF), 20 μg/ml NGF-PLGA-PVA (equivalent 500 ng/ml NGF with 30.1 ng/ml unencapsulated NGF), and 30 ng/ml nonformulated NGF were incubated in 200 μl culture medium. These samples were shaken at 50 rpm at 37°C. At different time points, each 200 μl incubated medium was collected and filtrated by 0.1 μm syringe filter (Millipore MILLEX®VV, Bedford, USA) to remove nanoparticles and collect released or free NGF. The released NGF concentration was then determined by a NGF ELISA kit. The NGF encapsulation efficiency was calculated by the following formula: 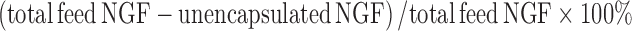 .
.
PC-12 Cell Culture and Induction of Neurite Outgrowth
PC-12 cells were maintained in RPMI1640 medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES, and 1% penicillin streptomycin.
To evaluate biological function of NGF-PLGA-sucrose, cells were seeded onto poly-D-lysine coated glass slide in 24 wells at the density of 4 × 104 cells/well. After incubation overnight, the cells were treated with either the NGF-PLGA-sucrose (equivalent 500 ng/ml NGF with 30.8 ng/ml unencapsulated NGF) or the nonformulated NGF (30 ng/ml) in culture medium. At different time points, the cells were fixed by 4% paraformaldehyde and incubated with mouse anti-beta III tubulin in PBS overnight. The cells were then incubated with FITC-conjugated AffiniPure goat anti-mouse IgG for 1 h, followed by DAPI staining for 5 min. The slides were mounted, and the results were observed by fluorescence microscope (Nikon, Kanagawa, Japan)
RESULTS
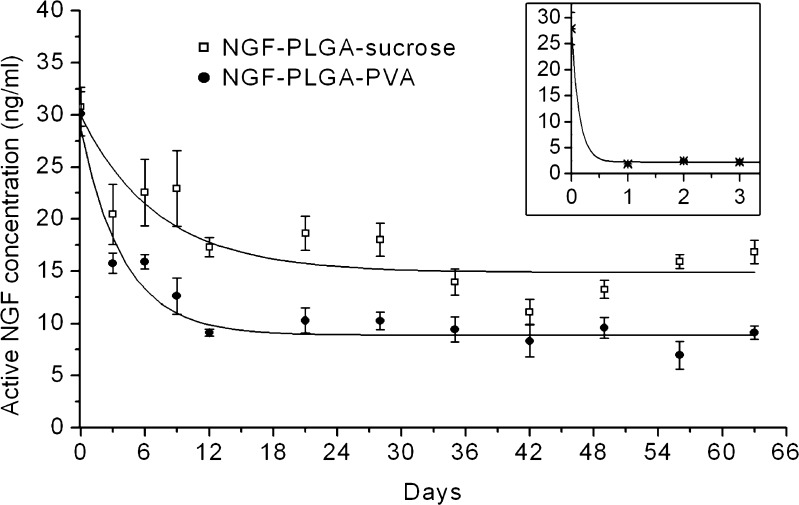
The NGF-PLGA formulated in either PVA or sucrose solution was well-dispersed and spherical in shape (Fig. 1). Characteristics of NGF-PLGA-sucrose and NGF-PLGA-PVA were reported in Table I. NGF-PLGA-sucrose was larger in size and broader in size distribution than NGF-PLGA-PVA. Both NGF-PLGA-sucrose and NGF-PLGA-PVA had high NGF encapsulation efficiency of 94%. Because there was no washing procedure after PLGA nanoparticle preparation, both NGF-PLGA-sucrose and NGF-PLGA-PVA had unencapsulated NGF, whose concentrations were 30.1 ng/ml at day 0 in the release experiment (Fig. 2). NGF, released from both NGF-PLGA-sucrose and NGF-PLGA-PVA was maintained at the concentrations above 20 ng/ml and 12 ng/ml, respectively, within the first 9 days. Afterwards, NGF released from NGF-PLGA-sucrose that can still be maintained at an average concentration of 16 ng/ml for the following two months, which was significantly higher than that (9 ng/ml) of the NGF released from NGF-PLGA-PVA. Since the nonformulated NGF activity degraded rapidly from 28 to 2 ng/ml within one day (Fig. 2 inset), the measured NGF concentration in NGF-PLGA-sucrose and NGF-PLGA-PVA could be considered as released amount from PLGA nanoparticles.
Fig. 1.
TEM images of a NGF-PLGA-sucrose and b NGF-PLGA-PVA. Scale bars, 500 nm
Table I.
Characteristics of NGF-PLGA Formulated in Sucrose or PVA Solution (Mean ± SD, n = 3)
| Mean diameter (nm) | Polydispersity index | Zeta potential (mV) | Encapsulation efficiency (%) | |
|---|---|---|---|---|
| NGF-PLGA-sucrose | 258.2 ± 26.3 | 0.224 | −36.6 ± 1.1 | 93.8 ± 6.0 |
| NGF-PLGA-PVA | 183.2 ± 5.6 | 0.129 | −10.4 ± 1.2 | 94.0 ± 7.1 |
Fig. 2.
In vitro release profiles of NGF from NGF-PLGA-sucrose (square) and NGF-PLGA-PVA (filled circle) in cell culture medium at 37°C. Inset showed degradation kinetics of nonformulated NGF (asterisk) in the same condition
To confirm the biological function of the released NGF molecules, the PC-12 cells were treated with either the NGF-PLGA-sucrose or the nonformulated NGF. NGF is a trophic factor that is commonly used to study the effect in inducing differentiation and neurite outgrowth of PC-12 cells. The neurite outgrowth processes can be seen on day 3 after the treatments, and more extended processes of PC-12 cells can be observed on day 7 (Fig. 3a, b). However, the PC-12 cells treated with the nonformulated NGF began to withdraw their processes on day 9 and became unattached on day 14 (Fig. 3c) due to the fast degradation of NGF. In the NGF-PLGA-sucrose treatment, PC-12 cells remained the same neuron-like morphology (Fig. 3d) till day 14, probably due to sustaining release of functional NGF from the nanoparticles.
Fig. 3.
NGF-PLGA-sucrose prolonged NGF-induced neuron-like morphology of PC-12. Scale bars, 100 μm
DISCUSSION
The negative surface charge of −36.6 mV (Table I) and the TEM image (Fig. 1a) illustrated by a good dispersity and stability of NGF-PLGA-sucrose, which suggested that sucrose could be used as a stabilizer during preparation of PLGA nanoparticles. However, the use of sucrose seemed to result in a larger size distribution probably because it lacks of the amphiphilic property of surfactants, which provide better emulsion stability during formulation process. Since NGF molecules usually degrade rapidly, the maintenance of high NGF concentration (Fig. 2) and function (Fig. 3) suggest that NGF could be well protected and sustained release from PLGA nanoparticles for months. When preparing NGF-PLGA-sucrose, washing procedure was not necessary because the unencapsulated NGF could be reserved to act its function in the beginning. Washing procedure can be skipped in this method because residual NGF is useful, as well as the residual sucrose is safe and would be diluted to a low concentration of about 0.5% while being applied in cell experiment or further application in vivo. Without the washing procedure, the processing of formulation can be shortened, and thus, the possible protein activity loss can be diminished. Moreover, sucrose can serve as a good cryoprotector when the formulation has to be stored at low temperature (12). The surfactant-free method may also reduce the possible side effects from the use of surfactants, which may be concern biocompatibility and biodegradability and reduce the alteration of nanoparticle surface properties from their irreversible incorporation in the nanoparticles (13).
It had been reported that sucrose could increase the viscosity of water-in-oil-in-water multiple emulsion resulting in a better stability of emulsion system (14). It is, thus, possible that high viscosity of sucrose solution stabilized the double-emulsion of NGF-PLGA-sucrose during formulation. After vacuum-assisted solvent evaporation and PLGA solidification, the negative surface charge owing to the carboxyl group of PLGA stabilized the PLGA nanoparticles.
Neurotrophic growth factors have been encapsulated into PLGA nano/micro particles as a delivery system (15,16). Such sustaining release of neurotrophins provide stable or prolonged effective dose, which would be coming from repetitive administrations if the nonformulated neurotrophins are used. Although NGF has been reported to be formulated into PLGA microparticles with optimal encapsulation yields and broad applications (17,18), our submicron formulations may have alternative applications in which the sizes of the particles are important and critical such as the size-dependent cellular uptake efficiency (19).
CONCLUSIONS
We introduced a surfactant-free method for the formulation of PLGA nanoparticles encapsulating proteins or polypeptides. In this method, no washing procedure is used, which not only simplifies the protocol but also prevents the protein loss which is usually accompanied by the washing procedure. This method also has high encapsulation efficiency and sustaining release of functional proteins. In the future, it may be useful for other investigations involving PLGA nanoparticles loading proteins or polypeptides.
Acknowledgments
This research was supported by an intramural grant from the National Health Research Institutes (NHRI-94-NM-PP-05), Taiwan.
References
- 1.Bilati U, Allemann E, Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur J Pharm Biopharm. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D, L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Giteau A, Venier-Julienne MC, Aubert-Pouessel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm. 2008;350:14–26. doi: 10.1016/j.ijpharm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 4.van de Weert M, Hoechstetter J, Hennink WE, Crommelin DJ. The effect of a water/organic solvent interface on the structural stability of lysozyme. J Control Release. 2000;68:351–359. doi: 10.1016/S0168-3659(00)00277-7. [DOI] [PubMed] [Google Scholar]
- 5.Nair B. Final report on the safety assessment of polyvinyl alcohol. Int J Toxicol. 1998;17:67–92. doi: 10.1177/109158189801700505. [DOI] [Google Scholar]
- 6.DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA) Food Chem Toxicol. 2003;41:319–326. doi: 10.1016/S0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 7.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D, L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20:212–220. doi: 10.1023/A:1022219003551. [DOI] [PubMed] [Google Scholar]
- 8.Dalwadi G, Sunderland VB. Purification of PEGylated nanoparticles using tangential flow filtration (TFF) Drug Dev Ind Pharm. 2007;33:1030–1039. doi: 10.1080/03639040601180143. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos IJ, Crespo R, Griebenow K. Poly(ethylene glycol) as stabilizer and emulsifying agent: a novel stabilization approach preventing aggregation and inactivation of proteins upon encapsulation in bioerodible polyester microspheres. J Control Release. 2003;88:135–145. doi: 10.1016/S0168-3659(02)00488-1. [DOI] [PubMed] [Google Scholar]
- 10.Nehilla BJ, Bergkvist M, Popat KC, Desai TA. Purified and surfactant-free coenzyme Q10-loaded biodegradable nanoparticles. Int J Pharm. 2008;348:107–114. doi: 10.1016/j.ijpharm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kang F, Jiang G, Hinderliter A, DeLuca PP, Singh J. Lysozyme stability in primary emulsion for PLGA microsphere preparation: effect of recovery methods and stabilizing excipients. Pharm Res. 2002;19:629–633. doi: 10.1023/A:1015354028908. [DOI] [PubMed] [Google Scholar]
- 12.Jeong YI, Shim YH, Kim C, Lim GT, Choi KC, Yoon C. Effect of cryoprotectants on the reconstitution of surfactant-free nanoparticles of poly(D, L-lactide-co-glycolide) J Microencapsul. 2005;22:593–601. doi: 10.1080/02652040500162659. [DOI] [PubMed] [Google Scholar]
- 13.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/S0168-3659(02)00127-X. [DOI] [PubMed] [Google Scholar]
- 14.Khan AY, Talegaonkar S, Iqbal Z, Ahmed FJ, Khar RK. Multiple emulsions: an overview. Curr Drug Deliv. 2006;3:429–443. doi: 10.2174/156720106778559056. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/S0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang YC, Wu YT, Huang HY, Lin HI, Lo LW, Tzeng SF, Yang CS. Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury. Biomaterials. 2008;29:4546–4553. doi: 10.1016/j.biomaterials.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 17.Hadlock TA, Sheahan T, Cheney ML, Vacanti JP, Sundback CA. Biologic activity of nerve growth factor slowly released from microspheres. J Reconstr Microsurg. 2003;19:179–186. doi: 10.1055/s-2003-39831. [DOI] [PubMed] [Google Scholar]
- 18.Pean JM, Venier-Julienne MC, Filmon R, Sergent M, Phan-Tan-Luu R, Benoit JP. Optimization of HSA and NGF encapsulation yields in PLGA microparticles. Int J Pharm. 1998;166:105–115. doi: 10.1016/S0378-5173(98)00033-7. [DOI] [Google Scholar]
- 19.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/A:1016085108889. [DOI] [PubMed] [Google Scholar]