Abstract
Alginate matrix tablet of diltiazem hydrochloride (DTZ), a water-soluble drug, was prepared using sodium alginate (SAL) and calcium gluconate (CG) by the conventional wet granulation method for sustained release of the drug. The effect of formulation variables like SAL/CG ratio, drug load, microenvironmental pH modulator, and processing variable like compression force on the extent of drug release was examined. The tablets prepared with 1:2 w/w ratio of SAL/CG produced the most sustained release of the drug extending up to 13.5 h. Above and below this ratio, the drug release was faster. The drug load and the hardness of the tablets produced minimal variation in drug release. The addition of alkaline or acidic microenvironmental modulators did not extend the release; instead, these excipients produced somewhat faster release of diltiazem. This study revealed that proper selection of SAL/CG ratio is important to produce alginate matrix tablet by wet granulation method for sustained release of DTZ.
Key words: calcium gluconate, diltiazem hydrochloride, matrix tablet, pH modulator, sodium alginate
INTRODUCTION
In recent years, biopolymers have received increased attention as excipients in pharmaceutical formulations as they are derived from natural sources, do not require organic solvents for processing, are easily available, and are amenable to chemical modifications (1) and thus offer many important functional properties to formulations (2). Sodium alginate (SAL), a hydrophilic biopolymer obtained from brown seaweeds, has been found to be highly promising in this respect because of its high biological safety (3). Chemically, SAL is a polysaccharide composed of varying proportions of d-mannuronic acid (M) and l-guluronic acid (G) residues which are arranged in MM or GG blocks interspersed with MG blocks (4). In addition to its use as a thickening, gel-forming, and colloidal-stabilizing agent in food and beverage industries, it is also used as binder in tablet formulation (5). Its unique property of forming water-insoluble calcium alginate gel through ionotropic gelation with Ca2+ ions in a simple and mild condition has made possible to encapsulate both macromolecular agents (6–8) and low-molecular-weight therapeutic agents (9–11). Although current uses of alginate-based devices are mainly related to encapsulation of various agents, matrix tablets containing SAL as release-retarding agent have drawn more attention as they are easy and economical to prepare using the common tabletting procedures (5,12,13).
Most of the investigations relating to alginate matrix tablets involve the preparation by direct compression of drug, SAL, and a source of Ca2+ ion like calcium gluconate (CG), calcium acetate, or calcium chloride with or without a secondary viscosity modifier like carbopol 934 or hydroxypropyl methylcellulose (HPMC). When such tablets come in contact with aqueous medium, in situ gelation takes place through ionotropic gelation between SAL and Ca2+ ion and diffusion of drug occurs through the gel matrix. However, there are different kinds of reports on the effect of Ca2+ ion on drug release from alginate matrix tablets prepared by the direct compression method.
While Giunchedi et al. (12) reported an increase in ketoprofen release with increase in the concentration of CG in alginate matrix tablets, Nokhodchi and Tailor (14) reported that lower concentration of calcium chloride provided faster release of theophylline particularly in the initial hours and higher concentration retarded the drug release. In both cases, addition of HPMC as a viscosity modifier provided better sustained release profiles of the drugs. Bayomi et al. (13) reported that release of theophylline decreased as the concentration of CG in the tablet, prepared by moisture-activated dry granulation method, was increased in the presence of carbopol as a viscosity modifier.
Besides the dry granulation and direct compression methods, wet granulation is another established and widely used method of tablet manufacture. This method may provide a greater possibility of more efficient ionotropic gelation reaction between SAL and CG than either direct compression or dry granulation method and thereby may result in better sustained release tablet.
The purpose of this study was to prepare sustained release matrix tablets using SAL and CG by the conventional wet granulation method and to evaluate the effect of formulation and processing variables on drug release. Diltiazem hydrochloride (DTZ), a highly water-soluble drug, which is used for the treatment of angina pectoris (15,16), arrhythmias, and hypertension, has been selected as a model drug as it is characterized by a short biological half-life and requires frequent administration (17,18).
MATERIALS AND METHODS
Materials
DTZ was obtained as a gift sample from Stadmed, Kolkata, India. SAL, CG, magnesium stearate, citric acid, sodium bicarbonate, sodium hydroxide, and potassium dihydrogen phosphate were purchased from S.D. Fine-Chem, Mumbai, India. All materials were of laboratory reagent grade and used as received. Double-distilled water was used throughout the experiment.
Preparation of Matrix Tablet
DTZ, SAL, and CG (passed through #60 mesh screen) were mixed in a stainless steel bowl. The powder blend was moistened with the required amount of water and then granulated using #18 mesh screen. The granules were dried at 60°C for 2 h and the moisture content of the granules as measured using the IR Moisture Meter was found to be confined within 0.85% to 1.2%. The dried granules were then passed through #22 mesh screen, mixed with magnesium stearate, and compressed into tablet using a flat-faced 10-mm punch in a ten-station rotary minipress tablet machine (RIMEK, Karnavati Engineering, Gujarat, India). The tablets were prepared under the following conditions:
Keeping the amount of drug (90 mg) constant, the ratio of SAL/CG was varied (tablets F1 to F6).
Keeping the amount (90 mg) of drug and SAL/CG ratio (1:1.5 w/w) constant, hardness (3.5 to 5.5 kgf) of the tablets were varied (tablets F7 to F10).
Using various amounts of SAL and CG, the ratio (1:1.5 w/w) of SAL/CG was kept constant and hardness was maintained at 4.5 kgf, the drug load (60 to 120 mg) was varied (tablets F11 to F13).
Keeping the amount (90 mg) of the drug and SAL/CG ratio (1:1.5 w/w) constant, the amount of NaHCO3 (1% to 4% w/w; tablets F14 to F17) or the amount of citric acid (0.85% to 6.4% w/w; tablets F18 to F21) was varied.
The composition of the tablets is shown in Table I. Each formulation containing about 100 tablets were prepared in duplicate.
Table I.
Composition of Alginate Matrix Tablet Prepared by Wet Granulation Technique
| Formulation code | Amount of drug (mg) | SAL (mg) | CG (mg) | SAL/CG | Magnesium stearate (mg) | Sodium bicarbonate %/(mg) | Citric acid %/(mg) | Hardness (kgf) |
|---|---|---|---|---|---|---|---|---|
| F1 | 90 | 225 | 75 | 3:1 | 4 | – | – | 3.7 |
| F2 | 90 | 200 | 100 | 2:1 | 4 | – | – | 3.8 |
| F3 | 90 | 150 | 150 | 1:1 | 4 | – | – | 3.9 |
| F4 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 4.1 |
| F5 | 90 | 100 | 200 | 1:2 | 4 | – | – | 5.2 |
| F6 | 90 | 75 | 225 | 1:3 | 4 | – | – | 5.5 |
| F7 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 3.5 |
| F8 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 4.5 |
| F9 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 5.5 |
| F10 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 6.5 |
| F11 | 60 | 132 | 198 | 1:1.5 | 4 | – | – | 4.5 |
| F12 | 90 | 120 | 180 | 1:1.5 | 4 | – | – | 4.2 |
| F13 | 120 | 108 | 162 | 1:1.5 | 4 | – | – | 4.4 |
| F14 | 90 | 120 | 180 | 1:1.5 | 4 | 1/(4) | – | 5.3 |
| F15 | 90 | 120 | 180 | 1:1.5 | 4 | 2/(8) | – | 5.4 |
| F16 | 90 | 120 | 180 | 1:1.5 | 4 | 3/(12) | – | 5.4 |
| F17 | 90 | 120 | 180 | 1:1.5 | 4 | 4/(16) | – | 5.6 |
| F18 | 90 | 120 | 180 | 1:1.5 | 4 | – | 0.85/(3.37) | 5.6 |
| F19 | 90 | 120 | 180 | 1:1.5 | 4 | – | 1.68/(6.75) | 5.6 |
| F20 | 90 | 120 | 180 | 1:1.5 | 4 | – | 3.31/(13.5) | 5.3 |
| F21 | 90 | 120 | 180 | 1:1.5 | 4 | – | 6.40/(27) | 5.6 |
SAL Sodium Alginate; CG Calcium Gluconate
Physical Testing of Tablet
Weight variation test (using Precisa Electronic Balance, model XB 600M/C, Switzerland), thickness measurement (using Digimatic Caliper, model CD-6″CS, Mitutoyo Corporation, Japan), hardness test (using Monsanto type hardness tester), and friability test (using Friabilator, Veego, Mumbai, India) were done following the usual methods.
Potency Test of Tablet
Ten tablets were weighted and powdered in a glass mortar. A quantity of the powder equivalent to about 20 mg DTZ was transferred into a 100-ml volumetric flask and 50 ml USP phosphate buffer (PB) solution (pH 6.8) was added. The stoppered flask was shaken for 24 h in a mechanical shaker and volume was made up to the mark with the buffer solution. The mixture was filtered and an aliquot following suitable dilution was analyzed at 237 nm using a spectrophotometer (model UV-2400 PC series, Shimadzu, Japan) for DTZ content and finally potency of the tablet was determined using a calibration curve constructed using PB solution of pH 6.8. The reliability of the above analytical method was judged by conducting recovery analysis at three levels of spiked drug solution in the presence or absence of the polymer and the excipients and for three consecutive days. The recovery averaged 98.93 ± 2.15%.
Morphological Examination of Tablet
A tablet was placed in a Petri dish and immersed in 75 ml 0.1 (N) HCl solution (pH 1.2). The liquid was removed carefully after 2 h with a syringe, and a photograph of the tablet was taken with a digital camera fitted with zoom lens (NIKON COOLPIX L18, Japan). The same tablet was again immersed in 75 ml of USP PB solution (pH 6.8). Liquid was removed after 2 h and a photograph of the tablet was taken.
In Vitro Release Study
In vitro drug release study was carried out in acidic solution 0.1 (N) HCl (pH 1.2) for an initial 2 h followed by in USP PB solution (pH 6.8) using USP II dissolution rate test apparatus (model TDP-06P, Electro Lab, Mumbai, India). One weighed tablet was placed in 900 ml acidic solution (37 ± 1°C) and rotated with paddle at 100 rpm. After 2 h, the acidic solution was removed carefully and replaced by 900 ml buffer solution (37 ± 1°C). Aliquot was withdrawn at different times and replenished immediately with the same volume of fresh solution. The withdrawn samples following suitable dilution were analyzed spectrophotometrically at 236 nm for acidic solution and 237 nm for buffer solution. The amounts of drug released in acidic medium and PB solution were calculated from the calibration curves drawn, respectively, in 0.1 (N) HCl and PB solution (pH 6.8). Each release study was duplicated.
Fourier Transform Infrared Analysis
Fourier transform infrared (FTIR) spectra of pure DTZ, physical mixture, and powdered tablet (F4) were recorded in FTIR spectrophotometer (Perkin-Elmer model RX-1, UK). The samples were mixed with KBr and converted into pellets at 600 kg pressure using a hydraulic press. The spectra were taken in the wavelength region of 4,500–450 cm−1.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) thermograms of DTZ, physical mixture, and powdered tablet (F4) were obtained in the following way: a weighed amount (4.8 to 6.2 mg) of a sample was kept in hermetically sealed aluminum pan and heated at a scan speed of 10°C/min over a temperature range of 30–300°C in a differential scanning calorimeter (Perkin-Elmer model Pyris Diamond TG/DTA, UK) which was calibrated against indium. The chart speed was 10 mm/min. DSC thermograms were also obtained in an atmosphere of air in a differential scanning calorimeter (DTG-60H, Shimadzu, Japan) under similar operating conditions.
X-Ray Diffraction Analysis
The qualitative X-ray diffraction analysis (XRD) studies were performed using an X-ray diffractometer (Miniflex Gonimeter, Japan) .Pure DTZ, physical mixture, and powdered tablet (F4) were scanned from 0° to 60°diffraction angle (2θ) range under the following measurement conditions: source, Kb-filtered Cu-Kα radiation; voltage, 30 kV; current, 15 mA; scan speed, 1°C/min.
Statistical Analysis
Each formulation was prepared in duplicate, and each analysis was duplicated. The effect of formulation variables on drug release was tested for significance level by using analysis of variance (ANOVA, single factor) with the aid of Microsoft® Excel 2003. Difference was considered significant when p < 0.05.
RESULT AND DISCUSSION
DTZ matrix tablets were prepared by wet granulation method using SAL and CG to entrap the drug in calcium alginate matrix formed through cross-linking reaction between SAL and CG in the presence of water. The composition of the tablets is shown in Table I. Physical testing of the tablets revealed the following information: the weight of the tablets was confined within ±5% of the average weight, thickness varied from 3.64 to 3.77 mm with MSD 0.20% (n = 10), and the maximum friability found was 0.81%. The variation in drug content from the labeled amount was less than ±5%.
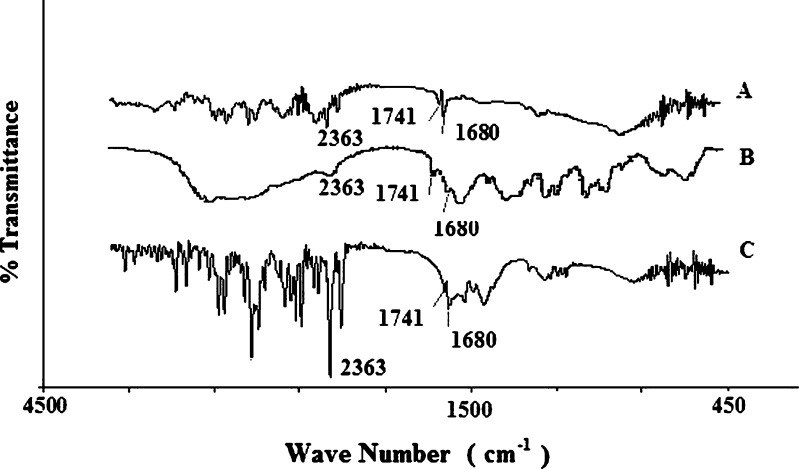
FTIR spectra of DTZ showed the characteristics bands of >C=O lactam, >C=O acetate, and N–H, respectively, at 1,680, 1,741, and 2,363 cm−1. The spectra obtained from the physical mixture of SAL, CG, pure drug, magnesium stearate, and the powdered tablet indicated the presence of the characteristic bands of the drug almost at the same wave numbers (Fig. 1).
Fig. 1.
FTIR spectra of DTZ (A), physical mixture (B), and powdered tablet (C)
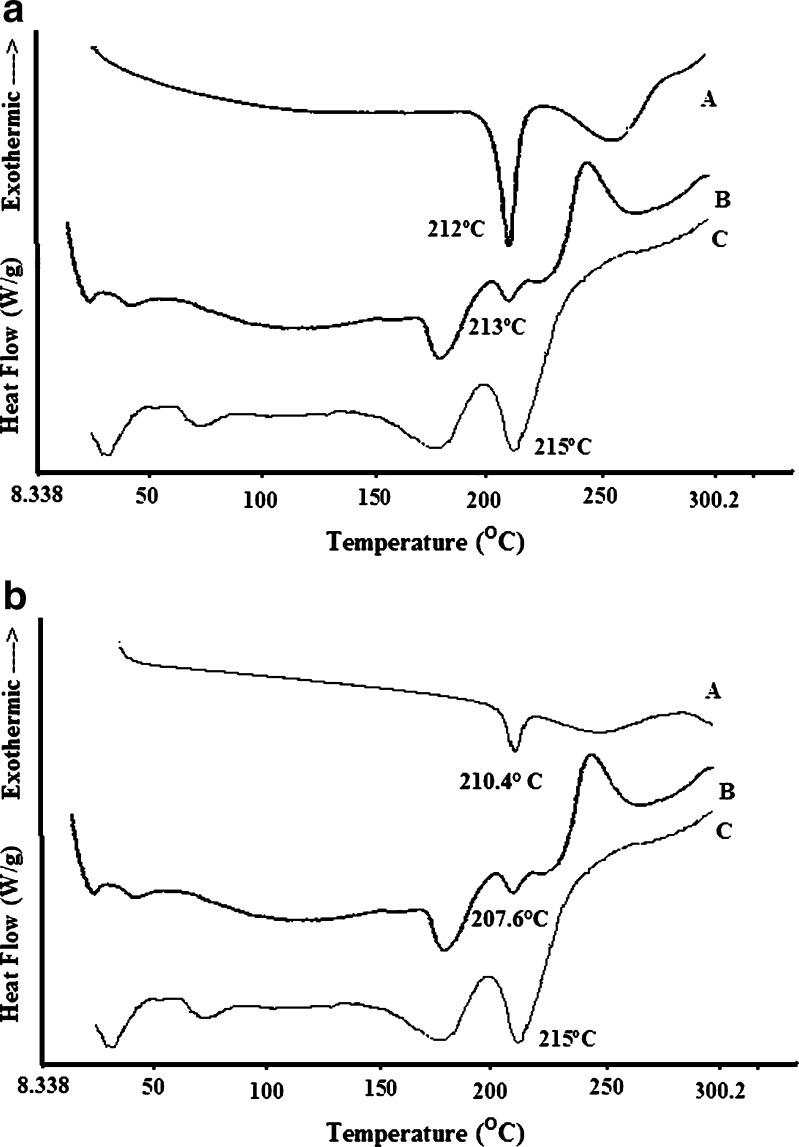
The DSC curves of DTZ, physical mixture, and powdered tablet (F4) obtained under nitrogen atmosphere are shown in Fig. 2a. A sharp endothermic peak corresponding to the melting point of DTZ was found at 212°C. The endothermic peaks of the drug in physical mixture and tablet were found almost at the same temperature (213°C and 215°C, respectively).The physical mixture, however, showed an endothermic peak at 178°C followed by an exothermic peak at 205°C. This could be due to the mass loss of CG. Another exothermic peak appearing at 247°C was due to the degrading of SAL. In addition to the thermal events observed for the physical mixture, the tablet showed another endothermic peak at 68°C which could be due to loss of water associated to the hydrophilic groups of SAL When DSC studies were carried out under atmospheric condition, no significant change in thermal behavior was noted (Fig. 2b). While DTZ showed a melting endothermic peak at 210.4°C, the same peak was seen in the physical mixture and tablet at 208°C and 215°C, respectively. The DSC scan obtained by both methods showed no major peak change, indicating that DTZ was still in crystalline form in the tablet. A small shift of the peak of the drug obtained from the tablet towards high temperature may be attributed to the rigidity induced by the formation of calcium alginate gel matrix in the tablet during wet massing stage.
Fig. 2.
DSC thermograms of DTZ a under nitrogen atmosphere and b under air atmosphere. A DTZ, B physical mixture, and C powdered tablet
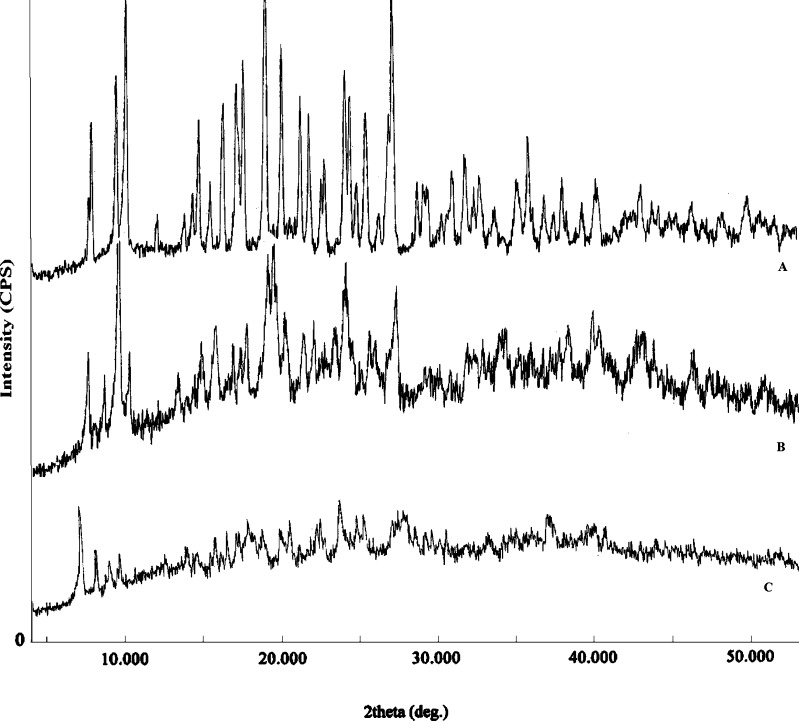
The XRD diffractograms of DTZ, physical mixture, and the powdered tablet are shown in Fig. 3. The XRD pattern of DTZ clearly indicated its crystalline nature. The crystalline nature of the drug was also maintained in the physical mixture and tablet, although the intensity of the peaks obtained from the tablet was reduced.
Fig. 3.
X-ray diffractograms of DTZ (A), physical mixture (B), and powdered tablet (C)
The results of FTIR and DSC studies confirmed the absence of any interaction between drug and excipients and that of the XRD study indicated that no polymorphic transformation of the drug took place during tabletting by wet granulation process.
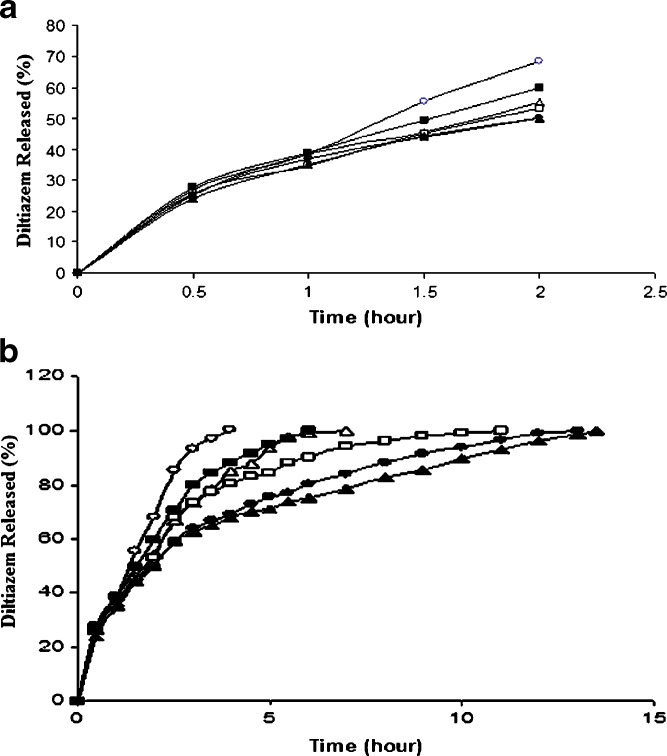
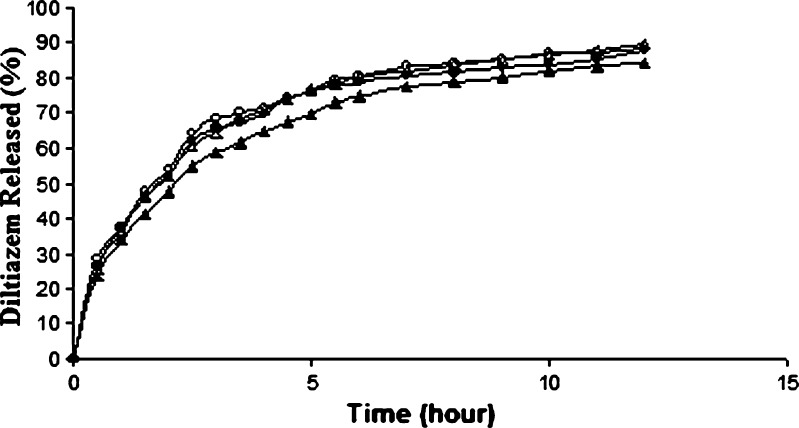
Drug release study which was carried out in acidic medium for the first 2 h showed a rapid release ranging from about 50% to 68% depending on the SAL/CG ratios in the tablets (Fig. 4a). The release of drug from all the tablets appeared to be almost the same up to 1 h and can be accounted for the burst release. Burst release from SAL matrix tablet are quite obvious, particularly in acidic medium, as no diffusion barrier capable of controlling the influx of dissolution medium into the tablet and efflux of drug from the tablet are formed. At the second hour, the termination point of the dissolution study in acidic medium, the release from F1 and F6 tablets were higher and that from F2 to F5 tablets appeared to be almost the same. However, ANOVA (one criteria) test of the amount of drug released at the second hour and t50% (the time required for 50% drug release) from F2 to F5 tablets showed a significant difference (p < 0.05; Table II). For the determination of actual order of drug release, area under the curves (AUCs) up to 2 h were calculated from the release profiles using the trapezoidal rule and the release of drug was found to follow the order: F1>F6>F2>F3>F4>F5. This means that the drug release from F1 tablet, which was prepared with the highest SAL/CG ratio, was the fastest and decreased as the ratio of SAL/CG in the tablets decreased. After a certain ratio of SAL and CG, the release again increased.
Fig. 4.
Release of diltiazem from alginate matrix tablets in a acidic medium (pH 1.2) and b in acidic medium for 2 h followed by in PB solution (pH 6.8). SAL/CG ratio: open circles 3:1 (F1), open triangles 2:1 (F2), open squares 1:1 (F3), filled circles 1:1.5 (F4), filled triangles 1:2 (F5), filled squares 1:3 (F6). Each data point is the mean of four determinations (maximum SEM ±1.9%)
Table II.
Characteristics of Drug Release from Alginate Matrix Tablets Prepared with Different SAL/CG Ratios: 3:1 (F1), 2:1 (F2), 1:1 (F3), 1:1.5 (F4), 1:2 (F5), 1:3 (F6)
| Formulation code | Amount of drug released (%) at 2 h (mean ± SD, n = 4) | t 50% (hour; mean ± SD, n = 4) | AUC (% mg h/ml) up to 2 h in acidic medium (mean ± SD, n = 4) | AUC (% mg h/ml) from 2 h to complete release in PB (mean ± SD, n = 4) |
|---|---|---|---|---|
| F1 | 68.35 ± 0.99 | 1.30 ± 0.03 | 77.29 ± 3.73 | 179.89 ± 4.26 |
| F2 | 55.27 ± 0.95 | 1.72 ± 0.3 | 68.10 ± 1.70 | 379.36 ± 2.06 |
| F3 | 53.22 ± 0.05 | 1.80 ± 0.02 | 66.26 ± 0.43 | 797.02 ± 0.95 |
| F4 | 50.49 ± 0.52 | 1.95 ± 0.01 | 65.44 ± 0.09 | 917.07 ± 0.88 |
| F5 | 51.71 ± 0.01 | 1.86 ± 0.01 | 64.18 ± 0.02 | 927.59 ± 0.84 |
| F6 | 59.79 ± 0.075 | 1.53 ± 0.02 | 72.78 ± 0.15 | 343.19 ± 1.23 |
| ANOVA test with F2 to F5 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
SAL Sodium Alginate; CG Calcium Gluconate; AUC Area Under the Curve
Following the dissolution study in acidic medium for 2 h, the tablets were placed in PB solution of pH 6.8, and the drug release was found to be extended for different periods depending upon the SAL/CG ratio in the tablets (Fig. 4b). While F1 tablet released 100% of the drug in 4 h, the drug release from F5 tablet was the slowest and completed in 13.5 h. Calculation of AUCs from 2 h to complete release indicated that the release of drug from different tablets followed the same order (F1>F6>F2>F3>F4>F5) as that found in acidic medium (Table II).
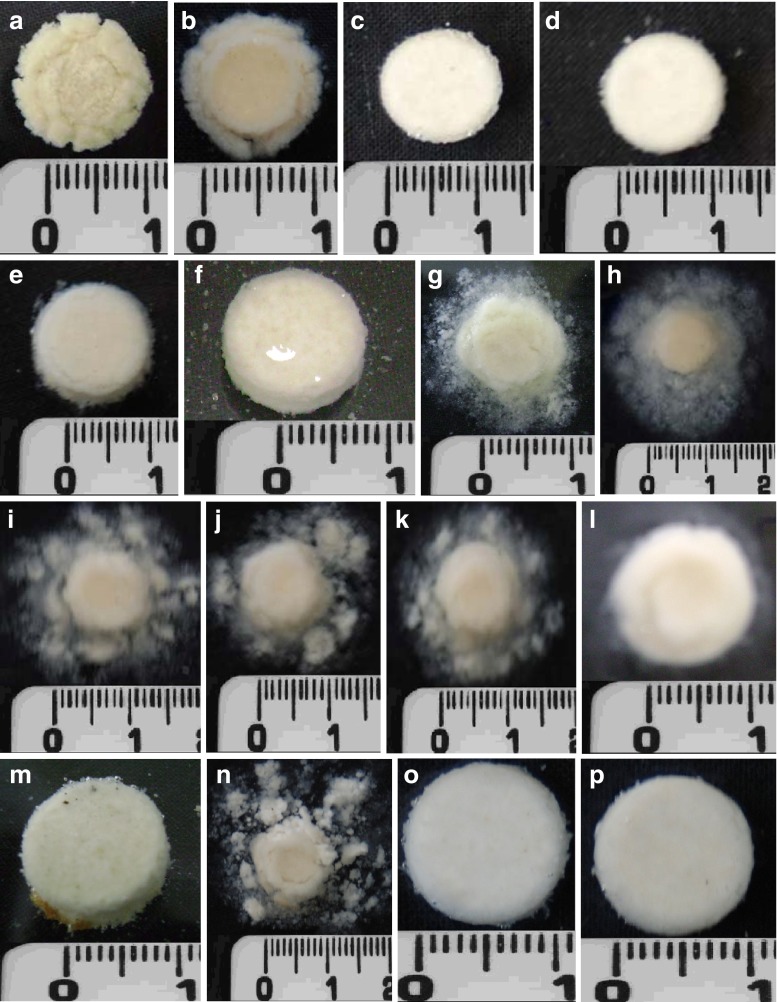
The above results are partly in agreement with the observations of Nokodchi and Tailor (14) for theophylline release from SAL/CaCl2 matrix tablets prepared by the direct compression method. They reported that, at a low concentration of CaCl2, the drug release was faster and, as the concentration of CaCl2 was increased, the drug release decreased. Besides the particle size, viscosity grade, and M/G ratio of SAL (5), the extent of cross-linking that depends on the ratio of SAL/Ca2+ ion and the change of pH of the dissolution medium may considerably influence the drug release from alginate matrix tablets. In contact with acidic solution, SAL is converted into alginic acid which although water-insoluble, swells in water and may act as tablet disintegrant. The amount of free SAL and that of calcium alginate formed during the wet massing stage of tablet preparation were, respectively, the highest and the lowest in F1 tablet. When the tablet was placed in acidic solution, free SAL was converted into alginic acid. Since calcium alginate does not swell appreciably in acidic solution, a large amount of alginic acid made the tablet swell. Figure 5a shows the fate of F1 tablets which were kept in static condition in acidic medium for 2 h. The tablet swelled and cracks/fractures developed throughout the matrix. Under agitation during the dissolution study, the tablet disintegrated rapidly, exposing a large surface area of the tablets to dissolution medium and resulting in rapid a drug release. As the ratio of SAL/CG in the tablets decreased, the amount of free SAL decreased and that of calcium alginate increased. Consequently, swelling and formation of fractures or cracks in the tablets decreased (Fig. 5b–f) and the drug release gradually decreased. The slowest drug release from F5 tablets indicates that all of the SAL was converted into calcium alginate. Although calcium alginate is insensitive to acidic solution (19), it does swell to some extent (11) and thus provides slow release of the embedded drug. The F6 tablet, which was prepared with the lowest SAL/CG ratio, is expected to have SAL completely converted into calcium alginate and to provide the slowest release of the drug. Surprisingly, the F6 tablet provided faster drug release that was next to the F1 tablet. Probably, excess CG acted as a channeling agent to provide faster drug release (12).
Fig. 5.
Photo images of alginate matrix tablets hydrated for 2 h in acidic media (pH 1.2) followed by in PB solution (pH 6.8) for 2 h. a, b, c, d, e, f in acidic media and g, h, i, j, k, l in buffer media. m and n Tablet contains 2% sodium bicarbonate. o and p Tablets contains 1.68% citric acid
Subsequent placement of the alginate matrix tablets in a solution of higher pH may lead to reconversion of alginic acid to SAL (5) that forms gel in presence of water. In addition, calcium alginate, which is insensitive to acidic medium, swells in aqueous medium having higher pH and then undergoes erosion due to breakdown of gel structure. At higher pH, ion exchange takes place between the gel-forming Ca2+ions and Na+ ions of the dissolution medium (20). As the Ca2+ ions are exchanged, electrostatic repulsion between the carboxylate anion of alginic acid accelerates the swelling and erosion of alginate gel (21). However, the degree of swelling and erosion is dependent on the degree of cross-linking between SAL and Ca2+ ions. When larger amount of CG was used in tablet formulation, more Ca2+ ions became available to bind with SAL during the wet massing stage of tablets preparation. As a result, a better and stronger gel was formed. Although at low concentration of Ca2+ ion calcium alginate matrix is formed, the gel is neither strong nor sufficient enough to delay the penetration of dissolution fluid into the tablet matrix. As the concentration of Ca2+ ions increases, stronger gel of calcium alginate is formed that delay the influx of the dissolution medium and the efflux of the dissolved drug out of the matrix. As a result, drug is released in a more sustained manner as the ratio of SAL/CG was decreased.
The physical characteristics of the tablets indicated that, although the compression force was kept constant, the hardness of the tablets increased from 3.7 kgf (for F1 tablet) to 5.5 kgf (for F6 tablet), although the thickness of the tablets was almost the same (3.7 mm). Bayomi et al. (13) also reported that increase in the concentration of CG resulted in an increase in hardness of the granules prepared by moisture-activated process and the tablets prepared with harder granules by direct compression method resulted in decrease in release of theophylline.
Figure 5g, h shows the appearance of F1 and F2 tablets which were kept in acidic solution for 2 h and then the liquid was carefully removed with a hypodermic syringe and replaced with PB solution. The cracks and fractures, which were developed in acidic medium due to the presence of a relatively large amount of free alginic acid, almost disappeared. Instead, a gel layer was found to be formed surrounding the core of the tablets and the gel layer began to erode from the tablet surface. Although not quantitative, the erosion of the tablets tended to decrease as the ratio of SAL/CG in the tablets decreased (Fig. 5i–l).
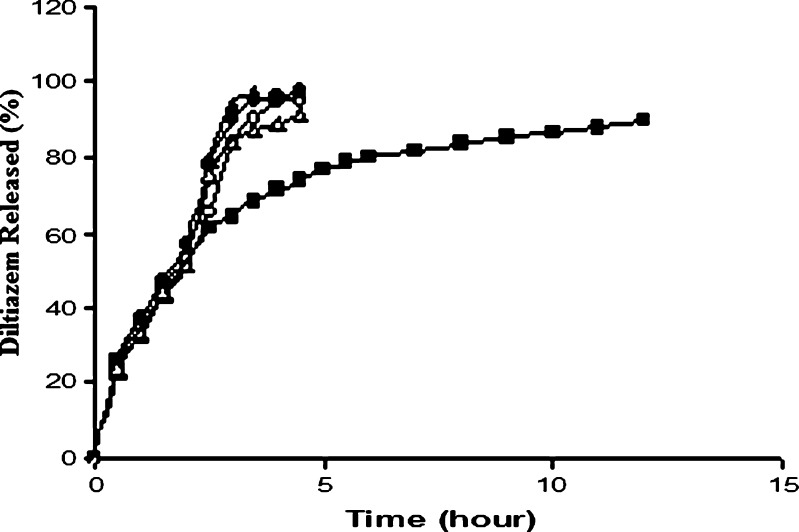
DTZ tablets (F7–F10) having a potency of 90 mg and containing a SAL/CG ratio of 1:1.5 w/w were prepared at various compression force to impart hardness of the tablets from 3.5 to 6.5 kgf, and the drug release profiles are represented in Fig. 6. The release profiles of the drug from the tablets having hardness of 3.5 to 5.5 kgf appeared to be almost overlapping. Although ANOVA test of the AUCs (Table III) revealed a significant difference (p < 0.05; Table III), the effect of compression force on drug release appeared to be minimal. However, the tablet having the highest hardness (6.5 kgf) tended to release the drug more slowly at every time point studied. Increase in compression force should reduce the tablet porosity and, hence, decrease the drug release. Various researchers have studied the effect of compression force on drug release from various matrices (22–24) and reported a minimal effect of compression force on drug release. Liew et al. (5) stated that the formation of a gel barrier around a matrix tablet controls the drug release behavior. Therefore, drug release is expected to be more closely related to the porosity of the hydrated gel layer which is independent of the dry matrix porosity. Significant decrease in drug release from tablet prepared at the highest compression force studied indicates that, above a certain critical hardness, dry matrix porosity does play an important role probably affecting the imbibition of dissolution fluid into the tablet.
Fig. 6.
Effect of compression force on diltiazem release from alginate matrix tablets prepared with different hardness. Open circles 3.5 kgf (F7), open triangles 4.5 kgf (F8), filled circles 5.5 kgf (F9), filled triangles 6.5 kgf (F10). Each data point is the mean of four determinations (maximum SEM ±6.87%)
Table III.
Characteristics of Drug Release from Alginate Matrix Tablets Prepared with Different Hardness and Drug Load
| Formulation code | Hardness (kgf) | AUC (% mg h/ml) 0–12 h (mean ± SD, n = 4) | Formulation code | Drug load (mg) | AUC (% mg h/ml) 0–12 h (mean ± SD, n = 4) |
|---|---|---|---|---|---|
| F7 | 3.5 | 866.51 ± 0.34 | F11 | 60 | 962.23 ± 2.33 |
| F8 | 4.5 | 856.62 ± 5.78 | F12 | 90 | 982.51 ± 0.93 |
| F9 | 5.5 | 844.16 ± 3.28 | F13 | 120 | 987.61 ± 0.53 |
| F10 | 6.5 | 797.25 ± 4.24 | |||
| ANOVA test | p < 0.05 | ANOVA test | p < 0.05 |
AUC Area Under the Curve
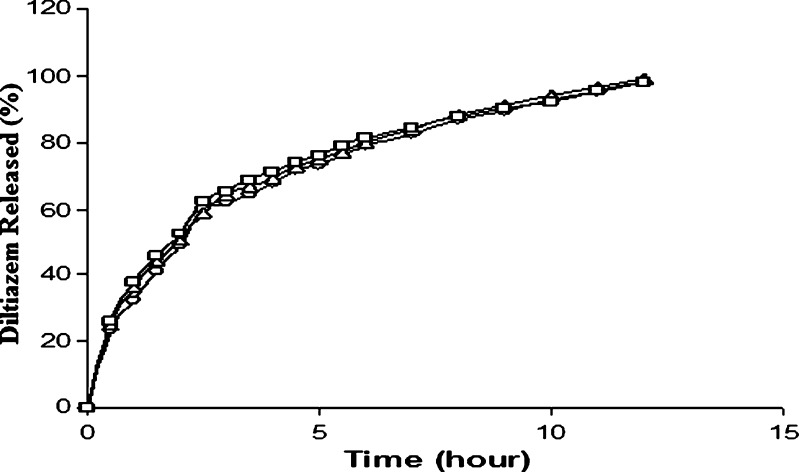
The effect of drug load (90 to 120 mg) on drug release was studied with tablets (F11–F13) prepared using a SAL/CG ratio of 1:1.5 w/w and the same compression force (tablet hardness was varied from 4.2 to 4.5 kgf) to eliminate the effect of gel strength of calcium alginate and hardness of the tablets on drug diffusion. Figure 7 indicates that the tablet having higher potency tended to release the drug marginally faster, although ANOVA test on AUCs (Table III) showed a significant difference (p < 0.05). The higher the drug load in a tablet, the higher the concentration gradient and, therefore, drug diffusion through the gel layer becomes faster, resulting in faster drug release. The marginal increase in drug release with increase in drug load might be due to a slight decrease in hardness (4.5 to 4.4 kgf) of the tablets, although the thicknesses of the tablets were almost the same (3.8 mm).
Fig. 7.
Effect of drug load on diltiazem release from alginate matrix tablets. Open circles 60 mg (F11), open triangles 90 mg (F12), open squares 120 mg (F13). Each data point is the mean of four determinations (maximum SEM ±7.95%)
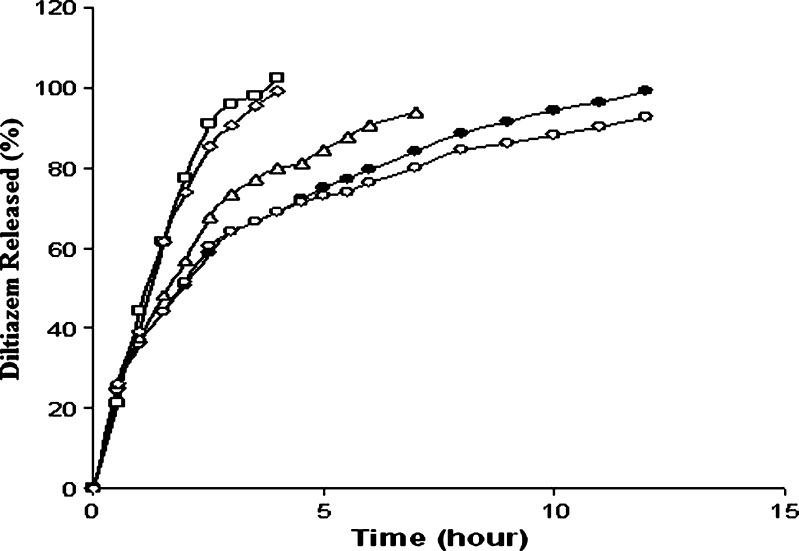
In order to reduce the burst release of the drug in acidic medium from alginate matrix tablets, inclusion of sodium bicarbonate (NaHCO3) as pH modifier has been suggested. While Sriamornsak et al. (3) stated that the addition of NaHCO3 produced a more basic microenvironment that created the most favorable condition for calcium alginate formation, Ching et al. (25) stated that, in addition to pH-modifying effects, carbon dioxide generated from the reaction between NaHCO3 and hydrochloric acid formed a physical gas barrier that resulted in reduction in drug release. DTZ tablets (F14–F17) having a potency of 90 mg were prepared using SAL/CG in a ratio of 1:1.5 w/w and 1–4% w/w of NaHCO3 by wet granulation method keeping the hardness of the tablets constant (5.3–5.6 kgf). Dissolution study showed that release of DTZ in acidic medium was not delayed; instead, 51.4% to 56.6% of the drug was released in 2 h. Subsequently, placement of the tablets in PB solution resulted in a total release of more than 80% in 1 h (Fig. 8). Variation in the concentration of NaHCO3 in the tablets did not produce any appreciable change. This means that addition of NaHCO3 could not prolong the drug release in acidic medium; instead, faster release even in comparison to the release from tablets prepared under identical condition without bicarbonate (tablet F4) was evident. It is possible that, when tablets containing bicarbonate are placed in acidic dissolution medium, acid–base reaction takes place instantaneously, leading to effervescence, which may make the tablets highly porous, encouraging increased influx of the dissolution medium into the tablets. Subsequent placement in PB solution caused rapid erosion of the tablets leading to faster drug release, although the hardness of the tablets containing NaHCO3 were higher (5.6 kgf) than those of the tablet not containing NaHCO3 (F4 tablet, 4.5 kgf hardness). Figure 5m shows that tablet containing NaHCO3 swelled in acidic medium to a great extent and presence of bubbles was also noted. The porous tablet eroded rapidly when placed in PB solution (Fig. 5n).
Fig. 8.
Effect of sodium bicarbonate on diltiazem release from alginate matrix tablets. Filled squares 0% (F9), open circles 1% (F14), open triangles 2% (F15), filled circles 3% (F16), filled triangles 4% (F17). Each data point is the mean of four determinations (maximum SEM ±6.26%)
Alternatively, if the microenvironmental pH inside the tablets is kept acidic, it is expected that solubility of CG in water will increase and hence CG will ionize to a great extent to supply more Ca2+ ions for binding with SAL. This may result in more prolonged drug release. DTZ matrix tablets (F18–F21) containing 90 mg DTZ, SAL and CG in a ratio of 1:1.5 w/w, and citric acid in variable amounts (0.85% to 6.40% w/w) were prepared and the release profiles of the drug from such tablets are shown in Fig. 9. The release of drug up to 5 h from the tablet which was prepared with the lowest amount of citric acid was almost same to that from the tablet prepared under identical condition without citric acid (F4). After 5 h, a marginal decrease in drug release was evident. On the other hand, increase in the concentration of citric acid further accelerated the release of the drug in spite of increase in hardness from 3.9 to 5.6 kgf.
Fig. 9.
Effect of citric acid on diltiazem release from alginate matrix tablets. Filled circles 0% (F4), open squares 0.85% (F18), open circles 1.68% (F19), open triangles 3.31% (F20), open diamonds 6.40% (F21). Each data point is the mean of four determinations (maximum SEM ±8.92%)
The acidic environment produced by citric acid to form denser calcium alginate matrix might have been offset due to the following reasons. Generally, organic acid are incorporated in hydrophilic matrix tablets to increase solubility of weakly basic drugs in alkaline medium. The organic acids decrease the microenvironmental pH and improve the solubility of weak bases and thereby increase the drug release (26,27). Thus, solubility of DTZ, which is a weak base, might have increased by the addition of citric acid, resulting in faster release in PB solution. In addition, organic acids also increase the release of weakly basic drugs by acting as pore formers in tablet matrix (26). Figure 5o, p shows the appearance of the tablets containing 0.85% w/w citric acid in acidic solution and PB solution. No gross change in appearance of the tablet in two media was evident.
CONCLUSION
DTZ tablets were prepared by wet granulation method using SAL and CG in various ratios and using different hardness, drug load, and pH modifiers. FTIR and DSC studies demonstrated the absence of drug–polymer interaction. Powder X-ray diffractometry study showed no change in crystallinity of the drug, although the intensity of the peaks in the diffractogram was found to be reduced. In vitro drug release study in gastrointestinal fluid indicated that the release of drug prepared with the highest SAL/CG ratio was the fastest and decreased as the ratio of SAL/CG in the tablets decreased. However, after a certain ratio of SAL/CG, the release again increased. The effect of compression force on drug release appeared to be minimal. Similarly, increase in drug load did not produce any significant change in drug release. Incorporation of either NaHCO3 or citric acid as pH modifier could not reduce the initial burst release; instead, both the excipients tended to increase the drug release.
Results of this study indicate that sustained release alginate matrix tablet of DTZ, a highly water-soluble drug, can be prepared by wet granulation method simply by optimizing the ratio of SAL and CG. The change in neither the formulation variables nor the process variable were found to better prolong the drug release for extended periods of time except the ratio of SAL/CG that formed calcium alginate gel matrix during the wet massing of the tablet-manufacturing method.
Acknowledgements
One of the authors (S. Mandal) wishes to express thanks to the University Grants Commission (UGC), New Delhi, India for the financial support (UGC Research Fellowship in Science for meritorious students). The authors also offer thanks to Stadmed, Kolkata, India for supplying diltiazem hydrochloride as a gift sample.
References
- 1.Vayas SP, Khar RP. Controlled drug delivery concepts and advances. Delhi: Vallabh Prakashan; 2002. p. 102. [Google Scholar]
- 2.Tapia C, Costa E, Moris M, Sapag-Hagar J, Valenzuela F, Basualto C. Study of the influence of the pH media dissolution, degree of polymerization, and degree of swelling of the polymers on the mechanism of release of diltiazem from matrices based on mixtures of chitosan/alginate. Drug Dev Ind Pharm. 2002;28(2):217–224. doi: 10.1081/DDC-120002455. [DOI] [PubMed] [Google Scholar]
- 3.Sriamornsak P, Thirawong N, Korkerd K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur J Pharm Biopharm. 2007;66:435–450. doi: 10.1016/j.ejpb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Aslani P, Kennedy RA. Studies on diffusion in alginate gels, I. Effects of cross-linking with calcium or zinc ions on diffusion of acetaminophen. J Control Release. 1996;42:75–82. doi: 10.1016/0168-3659(96)01369-7. [DOI] [Google Scholar]
- 5.Liew CV, Chan LW, Ching AL, Heng PWS. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int J Pharm. 2006;309:25–37. doi: 10.1016/j.ijpharm.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Polk A, Amsden B, Yao KD, Peng T, Goosen MFA. Controlled release of albumin from chitosan-alginate microcapsules. J Pharm Sci. 1994;83:178–185. doi: 10.1002/jps.2600830213. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi A, Kawabuchi M, Sugihara M, Sakurai Y, Okano T. Controlled release of macromolecular dextran from calcium-alginate gel beads. Proc Int Symp Control Rel Bioact Mater. 1996;23:737–738. [Google Scholar]
- 8.Bowersock TL, HogenEsch H, Suckow M, Guimond P, Martin S, Borie D, et al. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. doi: 10.1016/S0264-410X(98)00437-X. [DOI] [PubMed] [Google Scholar]
- 9.Cui JH, Goh JS, Park SY, Kim PH, Le BJ. Preparation and physical characterization of alginate microparticles using air atomization method. Drug Dev Ind Pharm. 2001;27:309–319. doi: 10.1081/DDC-100103730. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Rodriguez ML, Holgado MA, Sanchez-Lafuente C, Rabasco AM, Fini A. Alginate/chitosan particulate systems for sodium diclofenac release. Int J Pharm. 2002;232:225–234. doi: 10.1016/S0378-5173(01)00915-2. [DOI] [PubMed] [Google Scholar]
- 11.Halder A, Maiti S, Sa B. Entrapment efficiency and release characteristics of polyethyleneimine-treated or -untreated calcium alginate beads loaded with propranolol-resign complex. Int J Pharm. 2005;302:84–94. doi: 10.1016/j.ijpharm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Giunchedi P, Gavini E, Moretti MDl, Pirisino G. Evaluation of alginate compressed matrixes as prolonged drug delivery systems. AAPS PharmSciTech. 2000;1(3):31–36. doi: 10.1208/pt010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayomi MA, Al-Suwayeh SA, El-Heiw AM. Excipient–excipient interaction in the design of sustained-release theophylline tablets: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2001;27(6):499–506. doi: 10.1081/DDC-100105174. [DOI] [PubMed] [Google Scholar]
- 14.Nokhodchi A, Tailor A. In situ cross-linking of sodium alginate with calcium and aluminum ions to sustained the release of theophylline from polymeric matrices. Il Farmaco. 2004;59:999–1004. doi: 10.1016/j.farmac.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart ER. Physicians' desk reference. 45. Oradell: Medical Economics; 1991. p. 1293. [Google Scholar]
- 16.Goodman LS, Gilman A. The pharmacological basis of therapeutics. 7. New York: Macmillan; 1985. [Google Scholar]
- 17.Chaffman M, Brogden RN. Diltiazem, a review of its pharmacological properties and therapeutics efficacy. Drugs. 1985;29:387–454. doi: 10.2165/00003495-198529050-00001. [DOI] [PubMed] [Google Scholar]
- 18.Maxxo DJ, Obetz CI, Shuster J. Diltiazem hydrochloride. In: Brittain H, editor. Analytical profiles of drug substances and excipients, vol. 23. New York: Academic; 1994. pp. 53–98. [Google Scholar]
- 19.Yotsuyanagi T, Ohkubo T, Ohhashi T, Ikeda K. Calcium induced gelation of alginic acid and pH-sensitive reswelling of dried gels. Chem Pharm Bull. 1987;35:1555–1563. [Google Scholar]
- 20.Tomida H, Mizuo C, Nakamura C, Kiryu S. Imipramine release from Ca-alginate gel beads. Chem Pharm Bull. 1993;41:1475–1477. [Google Scholar]
- 21.Kikuchi A, Kawabuchi M, Sugihara M, Sakurai Y, Okano T. Pulsed dextran release from calcium-alginate gel beads. J Control Release. 1997;47:21–29. doi: 10.1016/S0168-3659(96)01612-4. [DOI] [PubMed] [Google Scholar]
- 22.Timmins P, Delargy AM, Minchom CM, Howard R. Influence of some process variables on product properties for a hydrophilic matrix controlled release tablet. Eur J Pharm Biopharm. 1992;38:113–118. [Google Scholar]
- 23.Bettini R, Colombo P, Massimo G, Catellani PL, Vitali T. Swelling and drug release in hydrogel matrices: polymer viscosity and matrix porosity effects. Eur J Pharm Sci. 1994;2:213–219. doi: 10.1016/0928-0987(94)90025-6. [DOI] [Google Scholar]
- 24.Velasco MV, Ford JL, Rowe P, Rajabi-Siahboomi AR. Influence of drug:hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from hydroxypropylmethylcellulose tablets. J Control Release. 1999;57:75–85. doi: 10.1016/S0168-3659(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 25.Ching AL, Liew CV, Chan LW, Heng PWS. Modifying matrix micro-environmental pH to achieve sustained drug release from highly laminating alginate matrices. Eur J Pharm Sci. 2008;33:361–370. doi: 10.1016/j.ejps.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Streubel A, Siepmann J, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Control Release. 2000;67:101–110. doi: 10.1016/S0168-3659(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 27.Tatavarti AS, Hoag SW. Micro-environmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J Pharm Sci. 2006;95(7):1459–1468. doi: 10.1002/jps.20612. [DOI] [PubMed] [Google Scholar]