Abstract
Stimulation of β-adrenergic receptors (BAR) by clenbuterol (CLE) increases nerve growth factor (NGF) biosynthesis in the rat cerebral cortex but not in other regions of the brain. We have explored the transcription mechanisms that may account for the cortex-specific activation of the NGF gene. Although the NGF promoter contains an AP-1 element, AP-1-binding activity in the cerebral cortex was not induced by CLE, suggesting that other transcription factors govern the brain area-specific induction of NGF. Because BAR activation increases cAMP levels, we examined the role of CCAAT/enhancer-binding proteins (C/EBP), some of which are known to be cAMP-inducible. In C6–2B glioma cells, whose NGF expression is induced by BAR agonists, (i) CLE increased C/EBPδ-binding activity, (ii) NGF mRNA levels were increased by overexpressing C/EBPδ, and (iii) C/EBPδ increased the activity of an NGF promoter–reporter construct. Moreover, DNase footprinting and deletion analyses identified a C/EBPδ site in the proximal region of the NGF promoter. C/EBPδ appears to be responsible for the BAR-mediated activation of the NGF gene in vivo, since CLE elicited a time-dependent increase in C/EBPδ-binding activity in the cerebral cortex only. Our data suggest that, while AP-1 may regulate basal levels of NGF expression, C/EBPδ is a critical component determining the area-specific expression of NGF in response to BAR stimulation.
The ability to precisely alter the expression of individual genes in response to neurotransmitter receptor activation is believed to result from the interaction of multiple transcriptional regulatory factors with their corresponding DNA-regulatory elements. Activation of β-adrenergic receptors (BAR) increases nerve growth factor (NGF) synthesis in the rat cerebral cortex but not in other brain areas (1, 2). This finding is of particular interest for brain plasticity considering that NGF is a neurotrophic factor required for the development of cholinergic neurons of the basal forebrain (reviewed in ref. 3), which play an important role in learning and memory processes (4, 5). Given the role of NGF in fostering cholinergic plasticity, BAR agonists, by inducing the synthesis and availability of NGF, may be a potential therapeutic approach for the treatment of neurodegenerative diseases affecting the cholinergic system. Activity- and neurotransmitter-dependent regulation of NGF expression have been shown to occur within the central nervous system in an area-specific manner. For instance, excitatory neurotransmitters increase NGF expression mainly in limbic areas (6, 7), glucocorticoid hormones in the hippocampus and cortex (8–10), interleukin-1 in the hippocampus (11), and BAR stimulation in specific layers of the cerebral cortex (2). These observations suggest that the NGF gene may be regulated by multiple cell-specific transcription factors that are activated by diverse stimuli.
The molecular mechanisms underlying the area-specific, BAR-mediated induction of NGF synthesis are still poorly understood. Stimulation of BAR activates cAMP-dependent protein kinase A, which causes a rapid and time-dependent increase in early inducible genes such as c-fos (12, 13) and the associated AP-1-binding activity (14, 15). The NGF promoter contains an AP-1 element important for the expression of NGF (16, 17); thus, AP-1 may be involved in the cortical-specific induction of NGF gene in response to BAR agonists. However, other cAMP-inducible transcription factors could contribute to BAR-mediated induction of NGF expression, including members of the CCAAT/enhancer-binding protein (C/EBP) family of basic region–leucine zipper DNA-binding proteins (18, 19), such as C/EBPβ and C/EBPδ (reviewed in ref. 20). The C/EBP proteins display cell-specific patterns of expression in a number of tissues, including the brain (21, 22), and play a role in synaptic plasticity (23). In this study, we have examined the contribution of AP-1 and C/EBPs in the BAR-mediated increase of NGF expression in the cerebral cortex. We provide evidence that C/EBPδ is one of the transcription factors responsible for the selective induction of NGF expression in the cerebral cortex.
MATERIALS AND METHODS
Cell Cultures.
C6–2B glioma and PC12 cells were grown as described (24). CTX-TNA2 rat astrocytes, established from primary type 1 astrocytes from frontal cortex (25), were grown as monolayer in DMEM supplemented with 10% fetal bovine serum (GIBCO).
Antibodies.
c-fos antiserum (α-c-fos) (26) was kindly provided by M. Iadarola (National Institute on Dental Research, National Institutes of Health, Bethesda, MD). Anti-C/EBPβ (α-C/EBPβ) antibody was obtained from Santa Cruz Biotechnology. Anti-C/EBPδ (α-C/EBPδ) antiserum (C. Cantwell and P.F.J., unpublished data) was raised against the N-terminal portion of murine C/EBPδ and will be described in detail elsewhere.
Nuclear Extracts.
Brain areas were dissected on ice and immediately homogenized in lysis buffer (50 mM Tris⋅HCl, pH 7.5/1.5 mM MgCl2/200 mM sucrose/0.1% Triton X-100/2 mM 2-mercaptoethanol/10 mM NaF/0.5 mM phenylmethylsulfonyl fluoride) and incubated on ice for 5 min. Nuclei were pelleted by centrifugation at 1,600 × g for 5 min at 4°C and washed twice in lysis buffer without Triton X-100. Nuclear proteins were extracted in high salt buffer (420 mM KCl/50 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/10% sucrose/20% glycerol/1 mM EDTA/2 mM DTT/20 mM NaF/0.5 mM phenylmethylsulfonyl fluoride/0.1 μg/ml leupeptin/5 μg/ml antipain/5 μg/ml aprotinin) by shaking at 4°C for 30 min. Debris was pelleted by centrifugation at 14,000 × g for 15 min.
Electrophoretic Mobility-Shift Assay (EMSA).
Nuclear extracts were incubated with a double-stranded oligonucleotide containing a consensus C/EBP-binding site (5′-GATCCTAGATATCCCTGATTGCGCAATAGGCTCAAAGCTG-3′) labeled with [32P]dCTP by the Klenow fragment of DNA polymerase. Binding reactions were carried out for 20 min at room temperature in a 25-μl reaction containing 10 mM Hepes at pH 7.6, 134 mM NaCl, 4% (wt/vol) Ficoll, 5% (vol/vol) glycerol, 1 mM EDTA, 10 mM DTT, 0.25 μg of BSA, 0.06% bromophenol blue, 1 μg of poly(dI-dC), and 0.5 ng of probe. Protein/DNA complexes were separated on 6% PAGE. For supershift assays, nuclear extracts were preincubated with 1 μl antisera at 4°C for 20 min before addition of probe. AP-1-binding activity was analyzed as described previously (15).
RNase Protection Assay.
32P-labeled NGF and basic fibroblast growth factor (FGF2) cRNA probes were generated from plasmid pBSrNGF (27) and pRObFGF103 (28), respectively, as described previously (10, 15). A 404-base 32P-labeled c-fos cRNA probe was synthesized by SP6 RNA polymerase from a PvuII-linearized c-fos clone (13). This polymerase was used to generate a 202-base 32P-labeled c-jun probe from an EcoRI-linearized c-jun plasmid (29). RNase protection assays were performed as described (10, 15).
Plasmids.
PCR was used to amplify a 665-bp fragment of the NGF promoter (30) (−615 to +50) from rat genomic DNA. The PCR primers introduced SalI and SacI restriction sites at the 5′ and 3′ ends, respectively. The product was ligated into luciferase vector pXP2 (31) digested with SalI and SacI to generate the plasmid NGF−615/+50. Oligonucleotides primers consisted of a 5′ “clamp” sequence, the restriction site, and 15–20 bases of homology to the target sequence. PCR (30 cycles: 30 sec at 94°C and 1 min and 30 sec at 60°C) was carried out by using Taq DNA polymerase in a reaction mixture containing 50 mM Tris⋅HCl at pH 8.3, 50 mM KCl, 7 mM MgCl2, 0.1 mM 2-mercaptoethanol, 170 μg/ml BSA, 1 mM dNTPs, and 6% dimethyl sulfoxide. 5′ deletion mutants were generated by PCR amplification using NGF−615/+50 as the template and inserted into pXP2 as SalI–SacI fragments.
Transient Transfection.
Cells were transiently transfected in serum-free Opti-MEM I medium containing 8 μg of DMRIE-C reagent (GIBCO). Transfections included 2 μg of reporter plasmid (NGF−615/+50 or NGF−615/+16), 2 μg of either pMEX, pMEX-C/EBPδ, or pMEX-C/EBPβ (32) expression vectors, and 0.2 μg pRSV β-galactosidase reporter vector as an internal control. After 6 hr cells were washed twice and incubated with growth medium. Cells were harvested after 48 hr, and lysates were analyzed for luciferase activity by using the Enhanced Luciferase Assay Kit (Analytical Luminescence Laboratory, San Diego) and for β-galactosidase activity by using the Luminescent β-galactosidase Genetic Reporter System II (CLONTECH). When β-galactosidase was not used, protein concentration in each lysate was measured (Bio-Rad) to normalize luciferase activity.
DNase I Footprinting.
DNase I protection assays were carried out essentially as described (18). The footprint probe (the noncoding strand of the NGF promoter fragment) was excised from the NGF−615/+50 construct by digestion with SalI–XhoI and end-labeled with polynucleotide kinase and [γ-32P]ATP at the downstream XhoI site. The DNA probe was incubated with 400 ng of bacterially expressed C/EBPδ protein in a mixture containing 50 mM KCl, 25 mM Tris⋅HCl at pH 7.9, 6.25 mM MgCl2, 10% (vol/vol) glycerol, 0.5 mM DTT 0.5 mM EDTA, and 1 μg of poly(dI-dC). Following incubation at 25°C for 2 min, the DNA was digested with DNase I (2 μg/ml) for 1 min at 25°C. The reaction was terminated by addition of DNase stop buffer (1% SDS/20 mM EDTA/200 mM NaCl/100 μg/ml tRNA) containing 200 μg/ml proteinase K. After 1 hr of incubation at 55°C, DNA samples were ethanol-precipitated and loaded onto a 6% polyacrylamide sequencing gel. Maxam–Gilbert sequencing reactions of the probe were loaded in parallel to allow the identification of protected sequences.
RESULTS AND DISCUSSION
Induction of c-fos mRNA by BAR Activation in the Brain.
An AP-1 site in the first intron of the NGF promoter has been recognized as the major DNA element regulating basal and inducible expression of NGF in nonneuronal cells (16, 17). Thus, we initially tested the hypothesis that BAR-mediated induction of cortical NGF expression results from the activation of the AP-1 transcription factor.
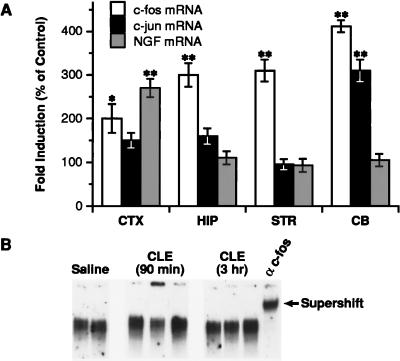
We first analyzed c-fos and c-jun mRNA levels by RNase protection assay in various brain regions of rats treated with clenbuterol (CLE), a lipophilic BAR-2 agonist (33, 34). By 1 hr, CLE evoked a 2- to 3-fold increase in c-fos mRNA levels in the cerebral cortex, hippocampus, and striatum and a 4-fold induction in the cerebellum (Fig. 1A). c-jun mRNA content was enhanced significantly (3-fold) only in the cerebellum (Fig. 1A). By 3 hr, both c-fos and c-jun mRNAs returned to basal levels (data not shown). The patterns of c-fos and c-jun mRNA induction by CLE were clearly distinct from the specific increase in NGF mRNA in the cerebral cortex at 5 hr (Fig. 1A).
Figure 1.
Effect of CLE on c-fos, c-jun, and NGF mRNA and AP-1-binding in rat brain. (A) Male rats were injected with saline or CLE (10 mg/kg; i.p.) and sacrificed 1 hr later for the analysis of c-fos/c-jun mRNAs, and 5 hr later for the analysis of NGF mRNA. Relative levels of c-fos, c-jun, and NGF mRNA were calculated by densitometric analysis of the protected fragment and normalized to the amount of cyclophilin mRNA. CTX, cerebral cortex; HIP, hippocampus; STR, striatum; CB, cerebellum. Data are the mean ± SEM of two separate experiments with three animals per group in each experiment. ∗, P < 0.05, ∗∗, P < 0.01 vs. control (ANOVA and Dunnett’s test). (B) Representative EMSA of AP-1-binding activity in the cerebral cortex. Rats (a total of six each group) were sacrificed 90 min or 3 hr after CLE administration. Four micrograms of nuclear extracts from the cerebral cortex was analyzed for AP-1-binding activity by using a 32P-labeled oligonucleotide probe containing the AP-1NGF sequence. The AP-1-binding specificity was demonstrated by supershift of the complex with α-c-fos.
To further examine the potential role of AP-1, we analyzed nuclear extracts from the cerebral cortex of rats treated with saline or CLE for changes in AP-1-binding activity. EMSA, using an oligonucleotide bearing the AP-1NGF sequence (15), showed no significant changes in AP-1-binding activity at either 90 min or 3 hr after CLE administration (Fig. 1B). CLE also failed to affect AP-1-binding activity in the hippocampus and cerebellum (data not shown). Thus, the lack of correlation between the induction of c-fos/c-jun mRNAs, AP-1-binding activity, and NGF mRNA expression suggests that the AP-1 element may not account for the BAR-mediated increase in NGF transcription in the cerebral cortex.
BAR Activation Increases C/EBPδ-Binding Activity.
The NGF promoter may be sensitive to transcription factors whose expression or activity is regulated by alteration of cAMP concentration. NGF synthesis is increased by BAR stimulation also in C6–2B glioma cells (12, 35, 36), a central nervous system-derived cell line previously used to examine the role of transcription factors in the cAMP-mediated stimulation of NGF transcription (12, 15). Thus, we have used C6–2B cells as an in vitro model to characterize the transcription mechanisms underlying the BAR-mediated increase in NGF mRNA.
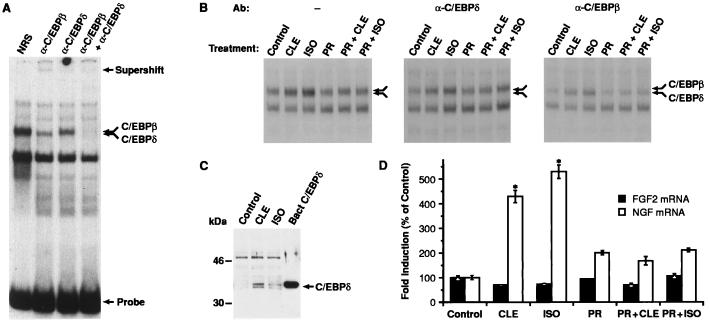
Members of the C/EBP family regulate tissue-specific genes and can be activated in response to elevated cAMP levels (21, 37, 38). To examine whether these proteins regulate BAR-mediated NGF transcription, nuclear extracts from C6–2B cells were analyzed for binding to a consensus C/EBP oligonucleotide. EMSA revealed the presence of two members of the C/EBP family, C/EBPβ and C/EBPδ (Fig. 2A). The upper and lower components of a doublet complex formed with the C/EBP oligonucleotide (double arrow) were supershifted specifically by α-C/EBPβ and α-C/EBPδ antibodies, respectively.
Figure 2.
Analysis of C/EBPδ protein/binding activity in C6–2B glioma cells. (A) Nuclear extracts prepared from C6–2B cells (15) were tested for C/EBP-binding activity by using a 32P-labeled oligonucleotide probe containing the consensus C/EBP site. Samples were preincubated with normal rabbit serum (NRS), α-C/EBPβ, or α-C/EBPδ antisera before addition of probe. The double arrow indicates a doublet corresponding to the C/EBPβ and C/EBPδ complexes. Positions of the supershifted species and free probe are indicated. (B) Cells were exposed for 2 hr to CLE (1 μM) or ISO (1 μM) alone or in combination to PR (1 μM). The double arrow indicates the position of the C/EBPβ-C/EBPδ heterodimers. Preincubation with α-C/EBPδ (Center) or α-C/EBPβ (Right) antisera was carried out to reveal the specific induction of each component of the complex. (C) Example of Western blot analysis of C/EBPδ expression. Ten micrograms of nuclear extracts from CLE- or ISO-treated cells was electrophoresed on a 12% SDS/PAGE gel. Proteins then were transferred to Immobilon P membrane and probed with α-C/EBPδ antibody (1:8,000). Protein/antibody complexes were visualized by using the enhanced chemiluminescence system (ECL, Amersham). Bacterial C/EBPδ was used as positive control. Similar data have been replicated three times. (D) Analysis of NGF mRNA levels in C6–2B cells exposed to CLE or ISO for 3 hr. Data are the mean ± SEM of three separate experiments, with two independent samples per group in each experiment. ∗, P < 0.01 vs. control (ANOVA and Dunnett’s test).
The effect of BAR stimulation on C/EBP-binding activity then was evaluated by exposing C6–2B cells for 2 hr to the BAR agonists CLE or l-isoproterenol (ISO) in the absence or presence of the specific BAR antagonist l-propranolol (PR). Both agonists increased C/EBPδ- and C/EBPβ-binding activity, and their effect was blocked by PR (Fig. 2B Left). The contribution of each component of the C/EBP complex was characterized further by incubating nuclear extracts with α-C/EBPδ (Fig. 2B Center) or α-C/EBPβ (Fig. 2B Right) antibodies before the addition of C/EBP probe. Both agonists increased C/EBPδ- and, to a lesser extent, C/EBPβ-binding activity. Moreover, Western blot analysis of lysates from control or CLE- or ISO-treated cells showed that both BAR agonists increased C/EBPδ protein levels (Fig. 2C), indicating that the BAR-mediated induction of C/EBPδ-binding activity is, at least in part, a result of increased protein expression.
The induction of C/EBPδ protein/binding activity by BAR was associated with a parallel increase in NGF mRNA content. Exposure of C6–2B cells to CLE or ISO for 3 hr resulted in a 4- to 5-fold increase in NGF mRNA levels, which was blocked by PR (Fig. 2D). The specificity of the response was confirmed by the fact that FGF2 mRNA levels remained unchanged in the same samples (Fig. 2D).
C/EBPδ Activates Transcription from the NGF Promoter.
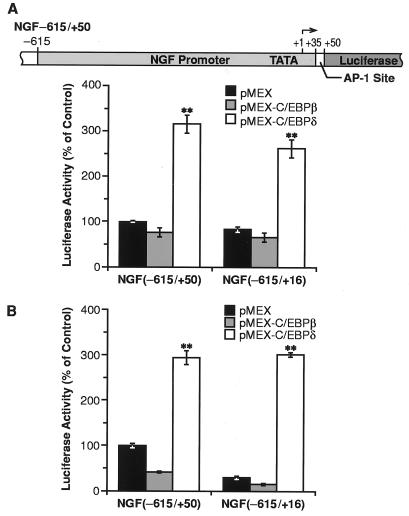
The role of C/EBPδ and/or C/EBPβ in the activation of NGF transcription was evaluated by transiently transfecting C6–2B cells with a luciferase reporter gene fused to a fragment of the NGF gene promoter. The construct NGF−615/+50 (Fig. 3A Upper) contained a 665-bp fragment of the promoter region extending from nucleotide −615 to nucleotide +50 and including the AP-1 site located at position +35 in the first intron of the gene. Cotransfection of the NGF construct with a C/EBPδ expression vector (pMEX-C/EBPδ) elicited a 3-fold increase in luciferase activity, whereas no transactivation was observed when cells were cotransfected with a C/EBPβ expression vector (pMEX-C/EBPβ) (Fig. 3A). Thus, C/EBPδ, but not C/EBPβ, appears to be crucial for the transactivation of the NGF promoter, although C/EBPδ and C/EBPβ display similar DNA-binding and regulatory properties (20). Future studies will address the mechanism by which the NGF-C/EBP site is able to discriminate between C/EBPδ and C/EBPβ.
Figure 3.
Transactivation of NGF promoter by C/EBPδ. (A Upper) Schematic representation of the NGF promoter–luciferase construct. (Lower) Luciferase activity of the NGF promoter constructs in C6–2B cells. Luciferase activity was measured and normalized to the protein content and β-galactosidase activity. Data, expressed as percentage of the control (NGF−615/+50 with pMEX), are the mean ± SEM of 12 independent experiments, each performed in duplicate. (B) Luciferase activity of the NGF promoter constructs in PC12 cells. Cells were transfected and luciferase activity was measured as described above. Data are the mean ± SEM of 12 experiments, each performed in duplicate. ∗∗, P < 0.01 vs. pMEX (ANOVA and Dunnett’s test).
The ability of C/EBPδ to activate the NGF promoter was not affected by deletion of the AP-1 element (NGF−615/+16), showing that transactivation by C/EBPδ is independent of this site (Fig. 3A). In addition, to rule out any anomalous effect resulting from the transformed state of this cell line, we performed the same transfection experiments in CTX-TNA2, an immortalized rat astrocyte cell line established from primary cortical type I astrocytes (25). In these cells, C/EBPδ transactivated the NGF promoter even more efficiently (5-fold) than in C6–2B cells (Fig. 4B).
Figure 4.
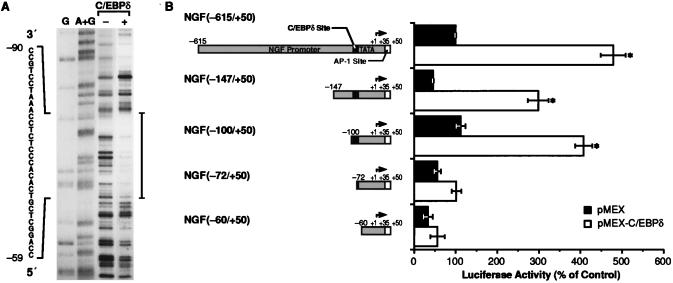
Identification of the C/EBPδ-binding site in the NGF promoter. (A) DNase I footprinting analysis of the NGF promoter with recombinant C/EBPδ. The labeled fragment NGF−615+50 was incubated in the presence (+) or absence (−) of bacterially expressed C/EBPδ and partially digested with DNase I. Lanes G and A+G correspond to Maxam–Gilbert sequencing reactions performed on the same probe. Sequence of the protected area is shown. (B) Transactivation of 5′ deletion mutants of the NGF promoter by C/EBPδ in CTX-TNA2 cells. Each mutant was cotransfected with either pMEX or pMEX-C/EBPδ expression vectors and pRSV β-galactosidase reporter vector. The luciferase activity was measured 48 hr after transfection and normalized to β-galactosidase activity. Data, expressed as percentage of the full-length construct (NGF−615+50 with pMEX), are the mean ± SEM of three separate experiments, with two independent samples in each experiment. ∗, P < 0.01 vs. pMEX (ANOVA and Dunnett’s test).
We also examined the ability of C/EBPδ to transactivate the NGF gene in PC12 cells, a neuronal phenotype that contains C/EBPβ but not C/EBPδ (22, 37). In PC12 cells, we again observed a 3-fold increase in NGF−615/+50 promoter activity when cotransfected with the C/EBPδ expression vector, whereas C/EBPβ had no effect (Fig. 3B). Interestingly, in these cells the basal activity of the promoter decreased substantially when the AP-1 site was deleted, suggesting that AP-1 may regulate NGF transcription when C/EBPδ is not active.
Identification of a C/EBPδ-Binding Site in the NGF Promoter.
To identify the region of the promoter responsible for the transactivation by C/EBPδ, we first performed DNase I footprinting analysis of the fragment −615/+50 by using bacterially expressed C/EBPδ. A large, protected area was generated by C/EBPδ in the proximal region of the promoter between nucleotides −90 and −59 (Fig. 4A). The extent of the footprint (≈30 bp) suggested that the protected sequence may correspond to two adjacent binding sites. Sequence analysis of this segment revealed no canonical C/EBP site, although a sequence with similarity to a cAMP response element was found between nucleotides −70 and −63. Since some cAMP response elements have been shown to bind C/EBP proteins in vitro (39), it is possible that the 3′ half of the footprint results from the interaction of C/EBPδ with this element. Moreover, binding of recombinant C/EBPδ to an oligonucleotide corresponding to the sequence −90/−72 was very weak compared with a consensus C/EBP site (data not shown), consistent with the absence of a canonical C/EBP sequence in this region of the promoter. This is not surprising as a cryptic, low-affinity C/EBP site was also found to mediate transactivation of a liver-specific gene, CYP2D5, by C/EBPβ (40).
To determine whether the 30-bp region mediates transactivation by C/EBPδ, a nested set of 5′ deletion mutants was constructed and transfected into CTX-TNA2 cells in combination with either pMEX or pMEX-C/EBPδ. Although the basal activity of the promoter appeared partially reduced when the region between nucleotides −440 (data not shown) and −147 (Fig. 4B) was deleted, transactivation by C/EBPδ was not affected significantly by deletion of sequences between −615 and −100. However, transactivation was completely abolished when the promoter was truncated to nucleotide −60 (Fig. 4B), thus confirming the results obtained by DNase I footprinting analysis. Furthermore, we found that the ability of C/EBPδ to transactivate the NGF promoter was also inhibited when the promoter was deleted to position −72. These findings identify the major C/EBPδ-responsive site to the region between −100 and −72, consistent with the observation that a C/EBPδ binding site(s) is present in the protected region −90/−59.
An identical pattern of transactivation was observed with the same 5′ deletion mutants truncated at nucleotide +16 (lacking the AP-1 site), except that the basal activity of the promoter was decreased strongly when the region −100/−60 was deleted (data not shown). Therefore, we postulate that cooperativity with other adjacent regulatory elements and AP-1 may be an essential requirement for the cell-specific activation of the NGF promoter by C/EBPδ. Future studies will explore the synergism between C/EBPδ and adjacent cis-regulatory elements in the NGF promoter, including the potential cAMP response element beginning at nucleotide −70.
C/EBPδ Increases NGF mRNA Levels in C6–2B Glioma Cells.
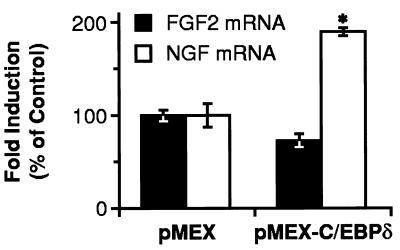
The ability of C/EBPδ to activate NGF expression was examined by analyzing NGF mRNA levels in C6–2B cells transiently transfected with the C/EBPδ expression vector. Overexpression of C/EBPδ increased NGF mRNA content (Fig. 5), while, in the same cells, the levels of FGF2 mRNA, used as a negative control, remained unchanged (Fig. 5), indicating that the increase in NGF mRNA was a specific response to increased C/EBPδ expression.
Figure 5.
C/EBPδ increases NGF mRNA content in C6–2B glioma cells. C6–2B cells were transfected with 30 μg of either pMEX or pMEX-C/EBPδ expression vectors. Relative levels of NGF and FGF2 mRNAs were determined and normalized to the amount of cyclophilin mRNA. Data, expressed as percentage of the control (pMEX), are the mean ± SEM of three separate experiments with two separate samples in each experiment. ∗, P < 0.01 vs. control (ANOVA and Dunnett’s test).
C/EBPδ- and C/EBPβ-Binding Activity Is Induced by CLE Specifically in the Cerebral Cortex.
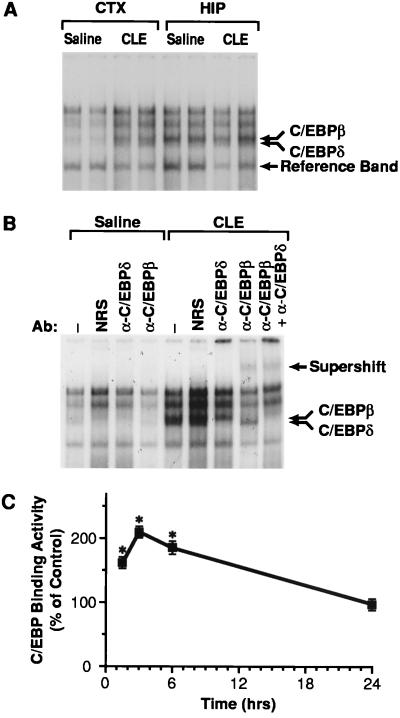
The role of C/EBPδ in the area-specific induction of NGF mRNA was investigated by determining C/EBPδ-binding activity in various brain areas after CLE administration. Rats were treated with saline or CLE for 3 hr and nuclear extracts from the cerebral cortex, hippocampus, and cerebellum were analyzed for binding to the consensus C/EBP oligonucleotide. In saline-treated rats, C/EBPδ- and C/EBPβ-binding activities were readily detectable in the hippocampus but not in the cerebral cortex (Fig. 6A) and cerebellum (data not shown). In CLE-treated rats, C/EBPδ- and C/EBPβ-binding activities increased specifically in the cerebral cortex (Fig. 6A). The identities of the binding species were confirmed by the fact that both upper and lower portions of the C/EBP complex (Fig. 6 A and B, double arrow) were supershifted by α-C/EBPβ and α-C/EBPδ antibodies, respectively (Fig. 6B).
Figure 6.
Analysis of C/EBP-binding activity in the rat brain. (A) Rats were treated with saline or CLE (10 mg/kg; i.p.) and sacrificed 3 hr after injection. Nuclear extracts from the cerebral cortex (CTX) and hippocampus (HIP) were analyzed by EMSA for binding to the 32P-labeled C/EBP consensus site oligonucleotide. In B, nuclear extracts from the cerebral cortex were preincubated in the presence of normal rabbit serum (NRS), α-C/EBPδ, or α-C/EBPβ antisera before addition of probe. The upper arrow indicates the supershift; the double arrow indicates the doublet corresponding to the C/EBPβ and C/EBPδ complexes. (C) Time-course of C/EBP-binding activity in the cerebral cortex. Relative levels of C/EBP-binding activity were quantitated by PhosphorImager analysis of the band corresponding to C/EBPβ and C/EBPδ complexes and normalized to the reference band (see A) to correct for potential artifacts resulting from gel loading. Data are the mean ± SEM of two independent experiments, with three independent samples in each experiment. ∗, P < 0.01 vs. control (ANOVA and Dunnett’s test).
Time-course studies also were performed to characterize the onset of this induction. Densitometric analysis of the band corresponding to C/EBPδ and C/EBPβ complexes indicated that C/EBP-binding activity began to increase at 90 min and peaked at 3 hr (Fig. 6C), well before the increase in NGF mRNA (1). At 6 hr, C/EBP-binding activity was still significantly higher than controls and returned to the basal level within 24 hr (Fig. 6C). By establishing a correlation between increased NGF expression and enhanced C/EBPδ-binding activity in the cortex, these data further support the notion that C/EBPδ might be the cell-specific transcription factor regulating NGF transcription in the cortex in response to BAR stimulation.
CONCLUSION
In the present report, we provide evidence that C/EBPδ may function as a brain region-specific transcription factor regulating NGF transcription. First, stimuli known to induce NGF expression in C6–2B cells also increased C/EBPδ-binding activity. Second, a C/EBPδ-binding site was identified in the NGF promoter by DNase I footprinting analysis and found to possess regulatory function on the promoter activity. Third, NGF mRNA levels were increased significantly in cells overexpressing C/EBPδ. Finally, and most importantly, the cortex-specific induction of NGF mRNA by CLE correlates with a preferential increase in C/EBPδ-binding activity in this region of the brain. In addition, indirect evidence implicates C/EBPδ as a transcription factor regulating NGF gene expression. The high or low levels of C/EBPδ protein in extracts from the hippocampus and cerebellum, respectively, correlate with the constitutive levels of NGF mRNA observed in these regions. These results also are supported by studies of NGF and C/EBPδ mRNA distribution in rodent brain, which show that both NGF (2, 6, 27) and C/EBPδ mRNAs (41) are highest in the hippocampus, Taken together, our results implicate C/EBPδ as regulating basal NGF expression and participating in the brain region-specific induction of NGF transcription by BAR activation.
The NGF promoter was transactivated by C/EBPδ to a similar extent in three different cell lines. However, in PC12 cells the basal activity of the promoter was affected by the deletion of the AP-1 site, although transactivation by C/EBPδ occurred in an AP-1-independent manner. Thus, it appears that transcription of NGF gene is under the control of multiple elements. Because AP-1-binding activity is constitutively and ubiquitously present in the brain, we cannot rule out that both AP-1 and C/EBPδ contribute to the specific expression patterns of NGF in the brain. It is possible that these two proteins function cooperatively to enhance NGF transcription, as it has been observed for other genes such as collagenase-1 in differentiated monocytes (42). However, our data suggest that activation of C/EBPδ is one of the cell-specific events governing the BAR-mediated induction of NGF expression in the cortex, whereas AP-1 may provide an auxiliary, albeit essential, regulatory role.
The potential physiological role of the C/EBPδ-mediated induction of NGF gene remains to be determined. Members of the C/EBP family are activated in response to a variety of cellular stresses or conditions that can cause a permanent cell damage, such as glucose deprivation, hypoxia, and growth arrest resulting from serum or growth factor withdrawal (43–45). C/EBPδ may be part of an adaptive response of the central nervous system to overcome the potential metabolic damage from excessive activation of receptors. Thus, CLE may cause imbalance of glucose availability (46), which, in turn, may lead to potential neuronal damage. We propose that the CLE-mediated activation of C/EBPδ is part of a stress response leading to increased NGF expression, which, in turn, may prevent the potential neuronal hypoglycemic damage.
Acknowledgments
We thank Drs. A. Baird, S. Whittemore, T. Curran, and D. Grayson for the plasmids and Dr. M. Iadarola for c-fos antisera. This work was supported by National Institutes of Health Grants NS 01675 and NS 29664 to I.M., and by National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratories.
ABBREVIATIONS
- BAR
β-adrenergic receptors
- NGF
nerve growth factor
- C/EBP
CCAAT/enhancer-binding protein
- CLE
clenbuterol
- FGF2
basic fibroblast growth factor
- EMSA
electrophoretic mobility-shift assay
- ISO
l-isoproterenol
- PR
l-propranolol
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Follesa P, Mocchetti I. Mol Pharmacol. 1993;43:132–138. [PubMed] [Google Scholar]
- 2.Hayes V Y, Isackson P J, Fabrazzo M, Follesa P, Mocchetti I. Exp Neurol. 1995;132:33–41. doi: 10.1016/0014-4886(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 3.Yuen E C, Howe C J, Li Y, Holtzman D M, Mobley W C. Brain Dev. 1996;18:362–368. doi: 10.1016/0387-7604(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 4.Coyle J T, Price D L, DeLong M R. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 5.Decker M W. Brain Res Rev. 1987;12:423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- 6.Gall C M, Isackson P J. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 7.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabrazzo M, Costa E, Mocchetti I. Mol Pharmacol. 1991;39:144–149. [PubMed] [Google Scholar]
- 9.Barbany G, Persson H. Eur J Neurosci. 1992;4:396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 10.Mocchetti I, Spiga G, Hayes V Y, Isackson P J, Colangelo A. J Neurosci. 1996;16:2141–2148. doi: 10.1523/JNEUROSCI.16-06-02141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spranger M, Lindholm D, Bandtlow C, Heumann R, Gnahn H, Naher-Noe M, Thoenen H. Eur J Neurosci. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 12.Mocchetti I, De Bernardi M A, Szekely A M, Alho H, Brooker G, Costa E. Proc Natl Acad Sci USA. 1989;86:3891–3895. doi: 10.1073/pnas.86.10.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curran T, Franza R B. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 14.de Groot R P, Sassone-Corsi P. Oncogene. 1992;7:2281–2286. [PubMed] [Google Scholar]
- 15.Colangelo A M, Pani L, Mocchetti I. Mol Brain Res. 1996;35:1–10. doi: 10.1016/0169-328x(95)00171-n. [DOI] [PubMed] [Google Scholar]
- 16.D’Mello S R, Heinrich G. Mol Brain Res. 1991;11:255–264. doi: 10.1016/0169-328x(91)90034-u. [DOI] [PubMed] [Google Scholar]
- 17.Hengerer B, Lindholm D, Heumann R, Ruther U, Wagner E F, Thoenen H. Proc Natl Acad Sci USA. 1990;87:3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson P F, Landschulz W H, Graves B J, McKnight S L. Genes Dev. 1987;1:133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P F, McKnight S L. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- 20.Johnson P F, Williams S C. In: Liver Gene Expression. Tronche F, Yaniv M, editors. Austin, TX: Landes; 1994. pp. 231–258. [Google Scholar]
- 21.Cardinaux J R, Magistretti P J. J Neurosci. 1996;16:919–929. doi: 10.1523/JNEUROSCI.16-03-00919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterneck E, Johnson P F. J Neurochem. 1998;70:2424–2430. doi: 10.1046/j.1471-4159.1998.70062424.x. [DOI] [PubMed] [Google Scholar]
- 23.Alberini C M, Ghirardi M, Metz R, Kandel E R. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 24.Colangelo A M, Fink D W, Rabin S J, Mocchetti I. Glia. 1994;12:117–127. doi: 10.1002/glia.440120205. [DOI] [PubMed] [Google Scholar]
- 25.Radany E H, Brenner M, Besnard F, Bigornia V, Bishop J M, Deschepper C F. Proc Natl Acad Sci USA. 1992;89:6467–6471. doi: 10.1073/pnas.89.14.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn J P, Takimoto M, Iadarola M J, Holbrook N, Levens D. J Virol. 1989;63:1737–1742. doi: 10.1128/jvi.63.4.1737-1742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittemore S R, Friedman P L, Larhammar D, Persson H, Gonzales-Carvajal M, Holets V R. J Neurosci Res. 1988;20:403–410. doi: 10.1002/jnr.490200402. [DOI] [PubMed] [Google Scholar]
- 28.Shimasaki S, Emoto N, Koba A, Mercado M, Shibata F, Cooksey K, Baird A, Ling N. Biochem Biophys Res Commun. 1988;157:256–263. doi: 10.1016/s0006-291x(88)80041-x. [DOI] [PubMed] [Google Scholar]
- 29.Szekely A M, Costa E, Grayson D R. Mol Pharmacol. 1990;38:624–633. [PubMed] [Google Scholar]
- 30.Zheng M, Heinrich G. Mol Brain Res. 1988;3:133–140. doi: 10.1016/0169-328x(88)90058-7. [DOI] [PubMed] [Google Scholar]
- 31.Nordeen S K. BioTechniques. 1988;6:454–457. [PubMed] [Google Scholar]
- 32.Williams S C, Cantwell C A, Johnson P F. Genes Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 33.Ordaway G A, O’Donnell J M, Frazer A. J Pharmacol Exp Ther. 1987;241:187–195. [PubMed] [Google Scholar]
- 34.Conway P G, Tejani-Butt S, Brunswick D J. J Pharmacol Exp Ther. 1987;241:755–762. [PubMed] [Google Scholar]
- 35.Schwartz J P, Costa E. Naunyn-Schmiedeberg’s Arch Pharmacol. 1977;300:123–129. doi: 10.1007/BF00505042. [DOI] [PubMed] [Google Scholar]
- 36.Dal Toso R, De Bernardi M A, Brooker G, Costa E, Mocchetti I. J Pharmacol Exp Ther. 1988;246:1190–1193. [PubMed] [Google Scholar]
- 37.Metz R, Ziff E. Genes Dev. 1991;5:1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- 38.Niehof M, Manns M P, Trautwein C. Mol Cell Biol. 1997;17:3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallejo M, Ron D, Miller C P, Habaner J F. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y-H, Williams S C, Baer M, Sterneck E, Gonzales F J, Johnson P F. Mol Cell Biol. 1997;17:2038–2047. doi: 10.1128/mcb.17.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterneck, E., Paylor, R., Kackson-Lewis, V., Libbey, M., Przedborski, S., Tessarolo, L., Crawley, J. N. & Johnson, P. F. (1998) Proc. Natl. Acad. Sci USA 95, in press. [DOI] [PMC free article] [PubMed]
- 42.Doyle G A R, Pierce R A, Parks W C. J Biol Chem. 1997;272:11840–11849. doi: 10.1074/jbc.272.18.11840. [DOI] [PubMed] [Google Scholar]
- 43.Carlson S G, Fawcett T W, Bartlett J D, Bernier M, Holbrook N J. Mol Cell Biol. 1993;13:4736–4744. doi: 10.1128/mcb.13.8.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawcett T W, Eastman H B, Martindale J L, Holbrook N J. J Biol Chem. 1996;271:14285–14289. doi: 10.1074/jbc.271.24.14285. [DOI] [PubMed] [Google Scholar]
- 45.Ubeda M, Wang X-Z, Zinszner H, Wu I, Habener J F, Ron D. Mol Cell Biol. 1996;16:1479–1489. doi: 10.1128/mcb.16.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magistretti P J, Morrison J H. Neuroscience. 1988;24:367–378. doi: 10.1016/0306-4522(88)90338-7. [DOI] [PubMed] [Google Scholar]