Abstract
The aim of this study was to develop novel gel-assisted layer-by-layer (LBL) nanomatrix with high payload of doxorubicin (DOX) and to assess its efficacy against Leishmania donovani. The biodegradable LBL nanomatrix was fabricated using LBL technique using polyions (protamine and sodium alginate) on decomposable core. The developed system was characterized in vitro in terms of layer-by-layer growth and payload efficiency. The efficacy of optimized formulations was evaluated against L. donovani strain in terms of inhibitory concentration (IC50). Uptake studies by infected macrophages were investigated both qualitatively and quantitatively using fluorescence microscopy and flow cytometry. The autogelling property subsequent to core removal inside the nanomatrix resulted in high payload efficiency of DOX (i.e., >70%). The reversal in charge followed the same trend with additional layers, and the magnitude of the charge remained constant up to five complete bilayers of polyions. The DOX can be effectively encapsulated, delivered, and subsequently taken up by L. donovani-infected macrophage cells. The matrix is completely internalized into macrophages showing improved efficacy (IC50 of formulation is almost ≤1.9-fold as compared to plain drug, P < 0.05) against intracellular amastigotes. Having ample of opportunity to manipulate surface architecture, this system demonstrates unique platform as a low cost ideal substitute for visceral leishmaniasis to expensive lipid-based formulations.
Key words: flow cytometry, layer-by-layer, Leishmania donovani, nanomatrix, visceral leishmaniasis
INTRODUCTION
In visceral leishmaniasis (VL), the parasite resides and multiplies within macrophages of the reticuloendothelial system. The disease is associated with several pathophysiological lethal-cascading events without specific chemotherapy. However, the drugs currently in use are highly toxic and have serious adverse effects that limit their clinical application. The most prevalent treatment consists of the administration of antimonials but there is growing evidence that the rate of response to the antimonials are declining due to the emergence of resistance and frequent relapses (1). Cytotoxic drugs like methotrexate and doxorubicin hydrochloride (DOX) are reported to be highly effective against VL but their therapeutic value, however, is strongly limited by cumulative dose-dependent cardiotoxicity (2–4). Herein, we introduce assembled gel-assisted nanomatrix loaded with DOX (layer-by-layer (LBL)-DOX) based on novel LBL technology for antileishmanial therapy to overcome the obstacles of resistance and toxicity as represented in Scheme 1. This biodegradable gel-assisted nanomatrix is aimed to deliver drugs into cell components by complete internalization and to allow direct visualization of intracellular drug distribution. This system is anticipated to provide potential alternative to costly lipid based including marketed formulation for antileishmanial therapy.
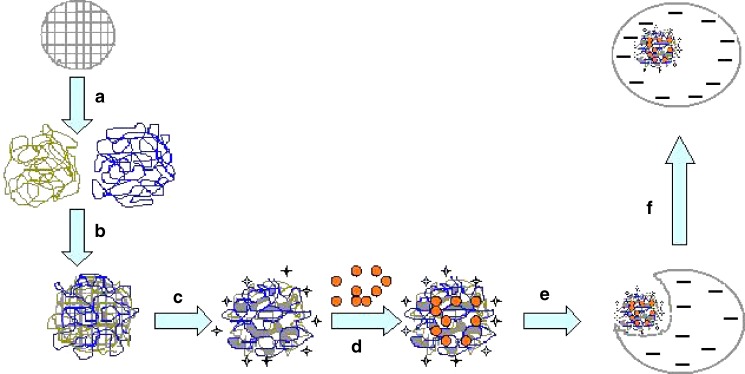
Scheme 1.
Schematic representation of gel-assisted nanomatrix (LBL-DOX) fabrication and its interaction with infected macrophages by endocytosis after 24 h. A, B Deposition of natural polyions [(SA/PRM)5] over preformed porous CaCO3 particles using LBL technique. C Core removal step at lower pH-produced intact gel-like structure inside the nanomatrix. D DOX loading at lower pH. E Adhesion of LBL-DOX on the surface of infected macrophages. F Complete internalization of LBL-DOX inside the cell due to endocytosis
MATERIALS AND METHODS
DOX was obtained as a gift sample from Sun Pharma Advance Research Centre, Vadodara, India. Sodium alginate (SA), protamine sulfate (PRM), and pluronic F-68 (PF-68) were purchased from Sigma, USA.
Parasite Culture
For in vitro infection, Leishmania donovani parasites (MZP 301) drawn from splenic aspirates of VL patients from an endemic region (Muzaffarpur, Bihar, India) were cultured in RPMI-1640 medium (HyClone) supplemented with 0.2% NaHCO3, 2.05 mM l-glutamine, 12 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer (HiMedia, India), 15% (v/v) heat-inactivated fetal bovine serum (FBS; HIFBS; Gibco, Germany), and 50 mg/L gentamicin at 25°C. Metacyclic stage parasites were isolated from 6-day-old parasite cultures that were centrifuged, washed twice with 0.02 M phosphate-buffered saline (PBS; pH 7.2), resuspended in complete RPMI FBS (5%), was added to the parasite solution, and incubated for 30 min at 37°C for opsonization. Incubation was followed by washing with PBS twice before use in in vitro tests.
Fabrication and Characterization of LBL-DOX by LBL Technique
The spherical inorganic template CaCO3 with monodisperse population was synthesized according to the procedure described previously (5). This template was used to fabricate LBL assembly using 0.2% w/v each of SA and PRM in combination [(SA/PRM)5] followed by core removal at low pH to obtain gel-assisted nanomatrix. Finally, 1 mL of nanomatrix suspension (1% w/v) was centrifuged at 20,000 rpm, and supernatant was decanted and added 1 ml DOX solution (500 µg/ml in 0.01 M HCl) with constant stirring at room temperature for 12 h to get a population of LBL-DOX. The payload efficiency in nanomatrix was determined by measuring amount of DOX remaining in the supernatant liquid using reversed phase high-performance liquid chromatography (6). To cross-check the material balance, pellets were incubated in 0.1 M NaOH (pH ≥12) by vortexing for 30 min followed by centrifugation at 20,000 rpm for 10 min, and DOX concentration was directly determined using method described previously. Layer-by-layer growth study was performed by measuring zeta potential of plain CaCO3 core particle and fabricated nanomatrix using Zetasizer nanoZS (Malvern, UK).
CULTURE EXPERIMENT
Inhibitory Effect of LBL-DOX on Intramacrophagic Amastigote
The efficacy of different formulations against L. donovani strain was evaluated in terms of inhibitory concentration (IC50) as per protocol described earlier (7). Briefly, adherent mouse macrophage cell lines J774A.1 were grown in 16-well chamber slides (Nunc, Denmark) at 37°C with 5% CO2. The macrophages were then incubated with late log phase infective promastigotes for 24 h at 37°C at a 1:10 cell/parasite ratio; approximately 80% of the cells were infected. After 12 h, chamber slides were washed to remove nonphagocytosed promastigotes, resupplemented with medium, and kept with different concentration of LBL-DOX and plain DOX separately for 24 h. The infection level was assessed by counting the percentage of infected macrophages and average number of amastigotes in each macrophage. The IC50 (concentration of drug that inhibits 50% of L. donovani amastigotes) was obtained by plotting a graph of the percentage of inhibition at different concentrations of all the observations using Origin 6.1 version software and expressed in microgram per milliliter (Table I).
Table I.
In Vitro Activity of both Formulations on Intramacrophagic Amastigotes of L. donovani
| Formulation code | IC50 (µg/mL) |
|---|---|
| LBL-DOX | 0.16 ± 0.05 |
| Plain DOX | 0.30 ± 0.04 |
The IC50 (concentration of drug that inhibits 50% of L. donovani amastigotes) was obtained by plotting a graph of the percentage of inhibition at different concentrations of all the observations using Origin 6.1 version software and expressed in microgram per millimeter. The results are expressed as ±SD of three separate experiments
LBL-DOX doxorubicin-loaded [(SA/PRM)5] multilayers
Uptake Study Using Flow Cytometry
For uptake study, infected J774A.1 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM)-l-glutamine, supplemented with 10% heat-inactivated FBS and 0.1% antibiotic solution (penicillin/streptomycin), and incubated at 37°C, 5% CO2. Macrophages were washed, collected by centrifugation, counted by Trypan blue exclusion, and 400 µl (2 × 105cells per milliliter) were added per well into Lab-tek II chamber slides (16-well chamber slides, Nunc, Denmark). Cells were incubated for 2–3 h and washed with DMEM to remove nonadherent cells. Then, LBL-DOX and plain DOX were added separately in each well at three different concentrations, i.e., 0.1, 0.5, and 1 µg/ml in DMEM and incubated for 24 h. After incubation, the medium was removed, and cells were detached using EDTA and trypsin. Cell pellets were collected by centrifugation (500×g, 5 min) and resuspended in PBS (pH = 7.4). The LBL-DOX attached to the cell surface was removed following a previous protocol (7). The cell suspensions were quantitated using flow cytometer (FACS Caliber, Becton Dickinson, USA). The data has been represented as mean fluorescence obtained from a population of 10,000 cells. Qualitative uptake of LBL-DOX was observed using high-resolution camera (Leica DFC320,) mounted on Leica DM5000B microscope using excitation and emission wavelength of 480 nm and 550 nm, respectively.
Statistical Analysis
The data are presented as mean ± standard deviation (±SD). The statistical significance of differences in percentage between treated and untreated was analyzed by one-way analysis of variance using GraphPad Prism software.
RESULTS AND DISCUSSION
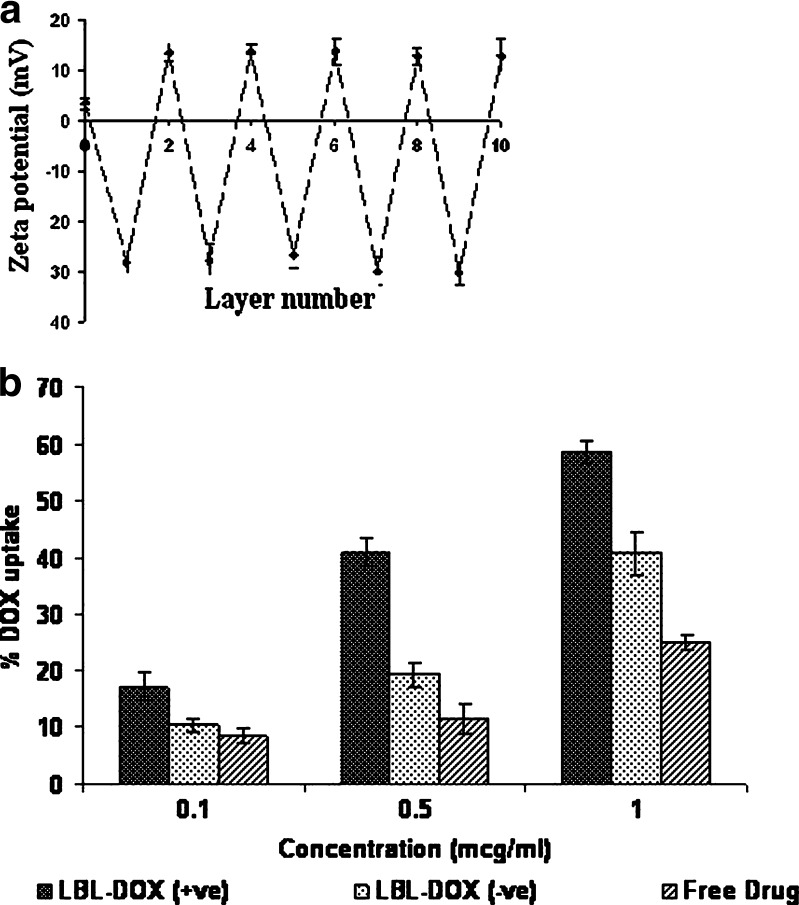
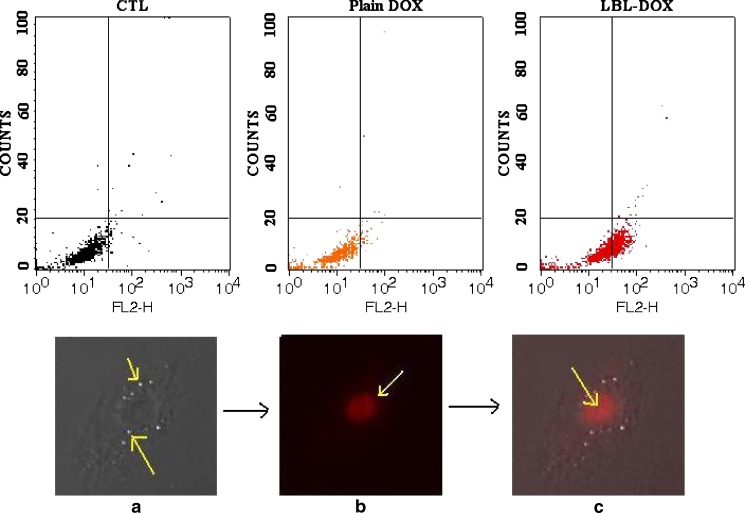
Spherical CaCO3 core particles (3–5 µm) doped with PF-68 with a porous inner structure was prepared as reported previously (5) and used as template to fabricate nanomatrix. Multilayer nanomatrix was fabricated by sequential recharging of SA and PRM [(SA/PRM)5] applying LBL technique followed by core removal at lower pH to achieve gel-embedded nanomatrix (8). This core removal step renders the system with wall thickness in the range of 100–300 nm as determined by subtracting the size of the bared core particle from the coated one (data not shown). Surface electrical potential (zeta potential) at each stage of the layering process are shown in Fig. 1a. The uncoated CaCO3 core particles are positively charged with ζ-potential + 3.8 mV when suspended in milli-Q water. The first SA layer reversed the charge to −26.75 mV. The first PRM coating changed the charge to +13.06 mV. The reversal in charge followed the same trend with additional layers, and the magnitude of the charge remained constant up to five complete bilayers (Fig. 1a). An improvement in payload efficiency of 70.16 ± 2.8% was achieved with LBL-DOX which could be ascribed to two mechanisms, i.e., (1) acidic pH facilitates electrostatic interaction of DOX with anionic gel and (2) concomitant formation of gel inside the matrix. The immobile DOX might be a part of matrix that is tightly associated with the complex formed between sodium alginate and remnant CaCO3 during core removal, thus showing sustained release (7). The LBL-DOX showed significant improvement (P < 0.05) in efficacy against infected macrophages as IC50 of LBL-DOX (0.16 µg/mL) was 1.9-fold lower than that of plain DOX (0.30 µg/mL). This effect could be best described by the role of cationic PRM present as an outer coating which facilitates its interaction with macrophages. The cell viability study of plain LBL nanomatrix demonstrates that more than 90% of the cells were viable when tested against plain J774 macrophages. This data indicates that the observed efficacy against infected macrophages is due to complete internalization of LBL-DOX and killing of parasites. The reason behind the encouraging results of LBL-DOX, especially with regard to intracellular parasites, is probably due to targeted delivery to tissues facilitated by gel-assisted fluidized structure and electrostatic interaction. When analyzed by flow cytometry, across-the-board enhancement in percentage uptake of LBL-DOX (+ve; 58.7 ± 1.2%) was observed at all concentrations, i.e., almost 2.4- and 1.4-fold compared to plain DOX (24.7 ± 1.3%; P < 0.05) and LBL-DOX (−ve; 40.7 ± 2.7%; P < 0.05), respectively, after 24 h (Fig. 1b). The uptake of plain DOX even after 24 h could be due to nonspecific interaction with cell membrane. To rule out the possibility of active uptake of the LBL-DOX merely due to physicochemical interaction, the same experiment was performed at 4°C (data not shown) at which the physiological processes are inhibited, and no interaction was observed. Figure 2 upper panel represents flow cytometry diagram of L. donovani-infected macrophages exposed to LBL-DOX for 24 h. Right and left lower quadrants represent cells that have and have not taken up the formulation, respectively. The improvement would have been facilitated through nonspecific electrostatic interactions of cationic PRM present on LBL surface with negatively charged cell membrane followed by endocytosis. The higher uptake of LBL-DOX could be due to shear stress-induced adhesion to the cells rendered by gel-assisted LBL-DOX. It has been reported that high shear stress rendered by gel can partially assist in uptake by cell membrane (9). We can speculate that lipid raft-mediated uptake is probably the key mechanism to enter the cells. Since, it has been reported by Bruno et al. (10) that cellular uptake of polyelectrolyte capsule is mostly caveolae mediated (a subtype of lipid raft-mediated uptake). The improved antileishmanial activity of LBL-DOX (+ve) for intracellular amastigotes may be due to their preferential uptake by macrophages followed by their cytotoxic action on the parasites in the vacuoles. A qualitative analysis of in vitro phagocytic uptake through epifluorescence indicates (Fig. 2 lower panel) complete internalization of LBL-DOX (+ve) and is phagocytosed by infected macrophages as shown by scattered fluorescence in the cell. LBL-DOX contains an enzymatically or hydrolytically degradable polycations which steadily degrade in infected macrophages subsequent to lipid raft-mediated uptake.
Fig. 1.
a Demonstration of layer-by-layer growth of gel-assisted nanomatrix as a function of zeta potential. Layer 0 indicates ζ-potential of core CaCO3 MP. The data represented as ± SD of three separate experiments (n = 3). b In vitro phagocytic uptake of LBL-DOX (+ve), LBL-DOX (−ve), and plain DOX as a function of concentration through flow cytometry after 24 h. The data represented as ± SD of three separate experiments (n = 3)
Fig. 2.
Upper panel shows flow cytometry diagram of L. donovani-infected macrophages exposed to LBL-DOX for 24 h. Right and left lower quadrants represent cells that have and have not taken up the formulation, respectively. CTL control cells, Plain DOX plain doxorubicin, LBL-DOX doxorubicin loaded gel-assisted nanomatrix [(SA/PRM)5]. Lower panel represents in vitro phagocytic uptake of LBL-DOX after 24 h by amastigote-infected J774A.1 macrophages using fluorescence microscopy. a Represents phase contrast microscopy of infected cell having amastigotes at the surface, indicated by yellow arrow. b Image showing scattered fluorescence of DOX within gel-like structure revealing penetration after core removal. c Infected cell showing complete internalization of LBL-DOX in macrophages indicated by yellow arrow
To our knowledge, this is the first demonstration of system that enables enhanced uptake of LBL system bearing DOX to L. donovani-infected macrophages. In this study, we have demonstrated simple approach to prepare biodegradable and biocompatible LBL system with high payload of DOX. Epifluorescence microscopic observation provides evidences for complete internalization of LBL-DOX in infected macrophages which could be the possible reason for improved efficacy. In the nutshell, the results suggest that developed LBL-DOX could provide controlled and effective delivery of DOX at low cost for effective treatment of visceral leishmaniasis as an alternative to expensive lipid based formulations.
Acknowledgment
Author Dr. P.R. Mishra is thankful to Department of Science and Technology, New Delhi, India for providing financial support under Fast Track Scheme. Girish K Gupta is thankful to Indian Council of Medical Research and Council of Scientific and Industrial Research, New Delhi, India for providing SRF and RA fellowships subsequently. The authors also thankful to Mr. Vishwakarma, SAIF, CDRI, Lucknow, India for performing flow cytometry of the samples. CDRI communication no. 7672
References
- 1.Sundar S, Rai M. Advances in the treatment of leishmaniasis. Curr Opin Infect Dis. 2002;15:593–8. doi: 10.1097/00001432-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Brunete JA. Treatment of experimental visceral leishmaniasis with amphotericin B in stable albumin. Antimicrob Agents Chemother. 2004;48(9):3246–52. doi: 10.1128/AAC.48.9.3246-3252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sett R, Basu N, Ghosh AK, Das PK. Potential of Doxorubicin as anti-leishmanial drug. J Parasitology. 1992;78(2):350–4. doi: 10.2307/3283487. [DOI] [PubMed] [Google Scholar]
- 4.Gille L, Nohl H. Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radic Biol Med. 1997;23:775–82. doi: 10.1016/S0891-5849(97)00025-7. [DOI] [PubMed] [Google Scholar]
- 5.Gupta GK, Jain V, Mishra PR. Nature preceding <http://dx.doi.org/10.1038/npre.2008.2548.1>.
- 6.Hartmann G, Vassileva V, Miller MP. Impact of endotoxin-induced changes in p-glycoprotein expression on disposition of doxorubicin in mice. Drug Metab Dispos. 2005;33(6):820–8. doi: 10.1124/dmd.104.002568. [DOI] [PubMed] [Google Scholar]
- 7.Mazumdar KD, Yadav TP, Prajapati VK, Kumar S, Rai M, Dube A, et al. Antileishmanial activity of nano-amphotericin B deoxycholate. J Antimicrob Chemother. 2002;62(2):376–80. doi: 10.1093/jac/dkn189. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy DB, Sukhorukov GB. Microgel-based engineered nanostructures and their applicability with template-directed layer-by-layer polyelectrolyte assembly in protein encapsulation. Macromol Biosci. 2005;5:451–8. doi: 10.1002/mabi.200400180. [DOI] [PubMed] [Google Scholar]
- 9.Weber LM, He J, Bradely B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled β-cell microenvironments. Acta Biomater. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Bruno GDe, Roosmarijn EV, Anja MG, Sukhorukov GB, Hennink WE, Sanders NN, et al. Intracellularly degradable polyelectrolyte microcapsules. Adv Mater. 2006;18:1005–9. doi: 10.1002/adma.200502128. [DOI] [Google Scholar]