Abstract
Ofloxacin, available as ophthalmic solution, has two major problems: first, it needs frequent administration every 4 hours or even every 1 hour to treat severe eye infection; second, there is formation of white crystalline deposit on cornea due to its pH-dependent solubility, which is very low at pH of corneal fluid. In order to provide a solution to previous problems, ofloxacin in this study is prepared as topically effective in situ thermosensitive prolonged release liposomal hydrogel. Two preparation procedures were carried out, leading to the formation of multilamellar vesicles (MLVs) and reverse-phase evaporation vesicles (REVs) at pH 7.4. Effects of method of preparation, lipid content, and charge inducers on encapsulation efficiency were studied. For the preparation of in situ thermosensitive hydrogel, chitosan/β-glycerophosphate system was synthesized and used as carrier for ofloxacin liposomes. The effect of addition of liposomes on gelation temperature, gelation time, and rheological behaviors of the hydrogel were evaluated. In vitro transcorneal permeation was also determined. MLVs entrapped greater amount of ofloxacin than REVs liposomes at pH 7.4; drug loading was increased by including charge-inducing agent and by increasing cholesterol content until a certain limit. The gelation time was decreased by the addition of liposomes into the hydrogel. The prepared liposomal hydrogel enhances the transcorneal permeation sevenfold more than the aqueous solution. These results suggested that the in situ thermosensitive ofloxacin liposomal hydrogel ensures steady and prolonged transcorneal permeation, which improves the ocular bioavailability, minimizes the need for frequent administration, and decreases the ocular side effect of ofloxacin.
Key words: chitosan, liposome, ofloxacin, thermosensitive hydrogel, transcorneal permeation
INTRODUCTION
The bioavailability of traditional ocular drug delivery systems, such as eye drops, is very poor (1); this is due to the fact that most of the instilled drug is lost within the first 15–30 s after instillation, and less than 5% of the applied drug penetrates the cornea and reaches intraocular tissues (2,3). Many efforts in ophthalmic drug delivery have been devoted not only to prolong the contact time of the vehicle at ocular surface but also slow down the elimination of the drug and increase its corneal penetration (4).
Fluoroquinolones possess excellent bactericidal activity against most frequently occurring Gram-positive and Gram-negative ocular pathogens (5). Ofloxacin is a second-generation fluoroquinolone derivative, used in external infections of the eye such as acute and subacute conjunctivitis and bacterial keratitis (6). Ofloxacin is available as 0.3% (w/v) ophthalmic solution; its dose is one to two drops every 4 hours or hourly in case of severe infection (7). The marketed eye drop has pH equal to 6.4 (6), and the average pH of tear is 7.2–7.4. Therefore, increasing the pH of ofloxacin solution from 6.4 to 7.2 will increase the unionized fraction of ofloxacin, which exceeds the solubility of drug in water at this pH (less than 4 mg/ml), as a result of a white crystalline deposit on cornea which is one of major side effect of ofloxacin ophthalmic solution (8).
In order to obtain a sustained release, which would be independent of the drug's molecular weight, it was decided to load the selected compound into liposomes and then incorporate the latter in the thermosensitive solution (9–11). The physical barrier provided by the lipid bilayer and the slower diffusion of the liposomes in the hydrogel can significantly decrease the release rate (12).
Introduction of novel delivery systems, including vesicular drug delivery systems like liposomes, has recently been proposed (13–16). Some liposomal drug delivery systems exhibited superior pharmacological properties to those obtained with conventional formulation (17). Activity of the liposomes as a carrier for drugs depends upon various factors such as charge, rigidity, composition of lipid membrane, encapsulation efficiency, and release rate (18–21); they can be modified in particle size, structure, and surface charge to obtain desirable physicochemical properties to suit particular need (22,23). The administration of drugs entrapped in liposomes to the eye was previously studied in several researches; for example, liposome-associated indoxuridine is superior to the solution form of the drug (24). Different liposomal formulations of atropine and atropine sulfate were also studied (25).
Hydrogel has become increasingly important in the biomedical field (26); one of the recent trends in hydrogel research is in situ forming systems for various biomedical application including drug delivery (27,28). In situ forming systems are liquid aqueous solutions before administration, but in gel under physiological conditions, there are several possible mechanisms that lead to in situ gel formation (29), such as pH change, ionic cross-linkage, and temperature modulation. These approaches, which do not require organic solvents, copolymerization agent, or an externally applied trigger for gelation, have gained increasing attention, such as a thermosensitive approach for in situ hydrogel formation (30).
Chitosan is a hydrophilic cationic copolymer produced by the deacetylation of chitin and composed of glucosamine and acetyl glucosamine units (31); it is a biodegradable, biocompatible, and mucoadhesive biopolymer (32–37). Endothermically gelling chitosan solution is prepared by supplementing an aqueous solution of chitosan with β-glycerophosphate (β-GP) salt, an additive that plays three essential roles: (1) to increase the pH into physiological range of 7.0–7.4; (2) to prevent immediate precipitation or gelatin, and (3) to allow for controlled hydrogel formation when an increase in temperature is imposed (38,39). The system remained in the solution at physiological pH and at room temperature and changed into gel upon heating at physiological temperature (40). The objective of the present study was to prepare and characterize topically effective in situ thermosensitive prolonged release ofloxacin liposomal hydrogel in order to provide a solution to the two major problems associated with using conventional ofloxacin ophthalmic solution. Two preparation procedures were carried out, leading to the formation of multilamellar vesicles (MLVs) and reverse-phase evaporation vesicles (REVs) at pH 7.4. The effects of method of preparation, lipid content, and charge inducers on encapsulation efficiency were studied. For the preparation of in situ thermosensitive hydrogel, chitosan/β-GP system was synthesized and used as carrier for ofloxacin liposomes. The effect of the addition of liposomes on gelation temperature, gelation time, and rheological behaviors of the hydrogel were evaluated. In vitro transcorneal permeation was carried to investigate the permeation properties of ofloxacin from in situ thermosensitive liposomal hydrogel formulation.
MATERIALS AND METHODS
Materials
Ofloxacin, egg phosphatidylcholine (PC), β-GP, and cholesterol (CH) were purchased from Sigma Chemical Company (St. Louis, USA). Stearylamine (SA), dihexadecyl hydrogen phosphate (DP), and dipalmitoyl-l-α-phospatidylcholine (DPPC) were purchased from Fluka Chemical Company (Buchs, Switzerland). Chitosan (Mw, 445,200, 90% deacetylated) was purchased from Aldrich chemical Company (Steinheim, Germany). All other reagent were commercially available and of analytical grade.
Methods
Preparation of Multilamellar Liposomes
MLVs containing ofloxacin were prepared using the lipid film hydration technique (41). The desired amount (50 mg) of lipid components of the liposome matrix was solubilized in chloroform (5 ml) in a round-bottom flask in the presence of 5 g of glass beads (mean diameter, 2–3 mm). The organic solvent was evaporated at 30°C on a rotary evaporator (Buchi R-110 Rotavapor, Switzerland) under a nitrogen stream and stored overnight under high vacuum to form a thin lipid film to be deposited on the inner wall of glass and on the glass bead surface. Liposomes were prepared by dispersing the lipid component film of the various liposomal formulations in an isotonic phosphate buffer saline solution pH 7.4 (10 ml) containing ofloxacin (30 mg). This procedure was carried out at 55°C under mechanical stirring. The liposomal suspension was left to mature overnight at 4°C to ensure full lipid hydration. Finally, the liposomal suspension was passed five times through 4.5 µm and ten times through 2.2-µm microporous filter for purification and kept in a refrigerator.
Preparation of REVs Liposomes
Ofloxacin unilamellar (REVs) liposomes were prepared using the reverse-phase evaporation technique (42). The lipid content (50 mg) were accurately weighed into a round-bottom flask and dissolved in chloroform: methanol mixture (1:2 v/v). A thin lipid film was formed on the inner side of the flask by evaporating the organic solvent under vacuum using rotary evaporator at 45°C. The lipid film was redissolved in 10 ml ether and the drug solution (30 mg) in 10 ml acetone, together with 10 ml phosphate buffer saline (PBS, pH 7.4), was added. Sonication was continued for about 15 min until the organic solvent was evaporated. The liposomes were allowed to equilibrate at room temperature, and 10 ml PBS was added to the liposomal suspension and passed five times through 4.5 µm and ten times through 2.2-µm microporous filter for purification and kept in a refrigerator overnight.
Determination of Ofloxacin Encapsulation Efficiency in Liposomes
Free drug was removed from the liposomal suspension by centrifugation for 45 min at 15,000 rpm (Beckman Coulter Inc, Fullerton, CA). The drug content in the supernatant was determined by means of spectrophotometric analysis at 292 nm (43) (UV-1601 PC. Schimadzu, Japan). The encapsulation efficiency (EE%) was calculated as follow:
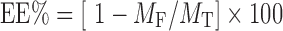 |
1 |
where MF and MT represents the free ofloxacin and total ofloxacin amount, respectively.
EE% was determined to evaluate the effect of method of preparation, lipid composition, and type of charge on the encapsulation of ofloxacin in liposomes.
Size Analysis of Liposomes
The mean particle size of different liposomal formulation was determined using laser diffraction particle size analysis (Malvern Instrument Ltd, Worcestershire, UK), in order to determine the effect of method of preparation and charge on the mean particle size of liposomes.
Preparation of Thermogelling Solution
Clear solution of chitosan were obtained by dissolving 360 mg of chitosan in 18 ml of 2% (v/v) aqueous acetic acid solution, 0.72 g of β-GP in 1 ml deionized water, and the final volume made up to 2 ml with deionized water. The chitosan solution was cooled down to 4°C and continuously stirred while adding drop by drop 2 ml of the β-GP solution; thus, the final 20 ml solution contains 1.8% (w/v) of chitosan and 3.8% (w/v) of β-GP. For the preparation of ofloxacin liposomal thermogelling solution, an accurate amount of ofloxacin liposomes composed of DPPC, CH, and SA in a molar ratio (4:3:1) were added to prepare the hydrogel with a concentration of 0.3% (w/w) of ofloxacin at pH equal to 7.4.
Evaluation in situ Gelation Temperature
The gelation temperature of plain and medicated bases was measured according to the technique described by Gilbert et al. (44). Accordingly, a 2-ml thermogelling chitosan/β-GP solution, either plain or medicated, was transferred to a test tube sealed with a Parafilm and put in a water bath at 4°C. The temperature of the bath was increased in increments of 3°C (or 1°C in the region of sol–gel transition temperature) and left to equilibrate for 15 min at each new setting. The samples were then examined for gelation, which was said to have occurred when the meniscus would no longer more upon tilting through 90o. The maximum temperature tested was 45°C.
Rheological Measurement
The rheological study was carried out on a Bohlin CVO rheometer (Bohlin Instruments, Cranbury, NJ, USA) equipped with a coaxial cylinder C-14 geometry in oscillatory mode immediately after preparation of the chitosan/β-GP solution with or without liposomes. The change in elastic modulus was recorded as a function of time at 37 ± 0.1°C. The frequency was fixed at 1.0 Hz during the measurement; the acquisition rate was set at one point per 13 s.
In Vitro Transcorneal Permeation
The transcorneal permeation experiment was performed with a conventional diffusion chamber. Albino rabbits were humanely killed by intravenous injection of excess sodium phenobarbital, and the whole eyes were enucleated. The method of dissection of cornea was the same as those described previously (45) and conformed to the ethical principles of the Egyptian research institute of ophthalmology on the use of animal. The corneas of both eyes were excised and then mounted on the Franz-type cell diffusion chamber. The receptor compartment was filled with 15 ml of PBS (pH 7.4), while 1 g of hydrogel containing ofloxacin MLVs liposomes with lipid composition of DPPC/CH/SA in molar ratio 4:3:1 or 1 ml of 0.3% ofloxacin solution (composed of pure drug dissolved in distilled water) or 1 ml of ofloxacin liposomal suspension were applied to the epithelial side. The temperature in the diffusion chamber was maintained at 37 ± 0.1°C by thermostatic water bath. The receptor buffer was removed 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 10 h and immediately replaced by previously aerated fresh receptor buffer. The samples were analyzed spectrophotometrically for drug content at 292 nm. Results were expressed as amount permeated and percent permeation as follows:
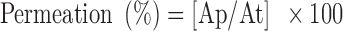 |
2 |
where Ap and At represent amount of ofloxacin permeated in receptor and initial amount of ofloxacin in donor, respectively.
RESULTS AND DISCUSSION
Factors Affecting Encapsulation Efficiency
By inspection of Table I, it is shown that ofloxacin encapsulation efficiency varied with the method of preparation, lipid composition, and type of charge used in preparing liposomes.
Table I.
Encapsulation Efficiency of Ofloxacin in Multilamellar and Revised-Phase Evaporation Liposomes
| Liposomal Lipid Composition | Molar Ratio | Liposomal Charge | MLVs Encapsulation Efficiency ± SD | REVs Encapsulation Efficiency ± SD |
|---|---|---|---|---|
| PC/CH | 4:1 | Neutral | 20.11 ± 0.54 | 13.15 ± 0.23 |
| PC/CH | 4:2 | Neutral | 39.13 ± 1.12 | 21.31 ± 0.45 |
| PC/CH | 4:3 | Neutral | 53.27 ± 0.67 | 35.72 ± 0.52 |
| PC/CH | 4:4 | Neutral | 47.35 ± 0.62 | 28.18 ± 0.28 |
| PC/CH/DP | 4:3:1 | Negative | 57.81 ± 0.49 | 36.61 ± 0.75 |
| PC/CH/SA | 4:3:1 | Positive | 59.01 ± 0.64 | 38.11 ± 0.83 |
| DPPC/CH | 4:1 | Neutral | 23.52 ± 0.13 | 16.13 ± 0.41 |
| DPPC/CH | 4:2 | Neutral | 38.17 ± 0.73 | 27.02 ± 0.72 |
| DPPC/CH | 4:3 | Neutral | 58.32 ± 0.51 | 43.53 ± 0.44 |
| DPPC/CH | 4:4 | Neutral | 49.15 ± 0.91 | 35.70 ± 0.56 |
| DPPC/CH/DP | 4:3:1 | Negative | 62.61 ± 0.86 | 45.21 ± 1.02 |
| DPPC/CH/SA | 4:3:1 | Positive | 65.50 ± 1.07 | 48.33 ± 0.82 |
Each value given in the table was calculated from n = 3 parallels and is given by the mean ± SD
PC egg phosphatidylcholine, CH cholesterol, DP dihexadecyl hydrogen phosphate, SA stearylamine, DPPC dipalmitoyl-l-α-phospatidylcholine
Concerning the effect of the method of preparation of liposome on the encapsulation efficiency of ofloxacin in liposomes, results showed that the EE% had higher values in the case of MLVs liposomes than in REVs liposomes of the same composition and molar ratio. This finding may be because liposomes were prepared at pH 7.4, and ofloxacin has pH dependent solubility, and it has minimum solubility at this pH (4 mg/ml); this will increase the unionized fraction of ofloxacin, which is lipophilic in nature (8). Due to the fact that MLVs contain multiple lamellae capable of loading a higher mass of lipophilic drug than REVs (46), EE% had higher values in the case of MLVs than REVs of the same composition and molar ratio.
Concerning the effect of type of phospholipids on EE%, results showed that the EE% had higher values in the case of DPPC containing liposome than in the case of egg PC containing liposomes of the same molar ratio. This result may be because the encapsulation of ofloxacin depends on the fluidity of the phospholipids bilayer; the lower the fluidity, the greater the EE%. DPPC is a synthetic product that has higher phase transition temperature (42.5 ± 0.25°C) than egg PC (−7°C) (43), so the fluidity of DPPC is less than egg PC; this leads to an increase EE% in the case of liposomes containing DPPC.
Concerning the effect of CH content on EE% of ofloxacin in liposomes, results showed that the EE% increases by increasing the CH content until a certain limit, above which the EE% decreases by increasing CH concentration. The maximum EE% of ofloxacin into liposomes was obtained from liposomes with CH molar ratio (4:3). The one-way analysis of variance showed that a significant difference in EE% between liposomes with different molar ratios of phospholipids/CH and (p < 0.001). The increase in EE% by increasing CH content occur because by increasing the CH concentration in the lipid bilayer, the rigidity of the bilayer increases, resulting in higher stability and reduced permeability of the liposomal membrane (47) and hence greater drug retention (48). Decreasing EE% with increasing CH ratio above a certain limit may be due to the fact that increasing CH above certain concentration can disrupt the regular linear structure of liposomal membrane (49). Thus, the ratio 4:3 (phospholipid/CH) was the most efficient regarding EE%.
Concerning the effect of charge-inducing agent on EE% of ofloxacin in liposomes, results showed that charged liposomes, either positive or negative, have higher EE% than neutral liposomes. This behavior could be due to the presence of the charged phospholipids leading to a slight increase in size of liposomes, thus due to the interlamellar electrostatic repulsion forces that lead to an enhancement of the captured water phase (43). This effect appears in MLVs more than in REVs, which are poorly influenced by the presence of charged phospholipids, owing to the presence of one lamellae per vesicle. Also the EE% of positively charged is more than that of negatively charged liposomes; this could be due to the fact that ofloxacin is a zwitterion, which has 2 pKa (approximately 6 and 8) and isoelectric point at 6.97. Therefore, at pH 7.4, ofloxacin presents in the form of ionized negative charge carboxylic group, so an electrostatic attraction would occur between the drug and positively charged SA; this attraction lead to increasing EE% when compared to negatively charged liposome.
From all the above results, it was cleared that liposomes prepared as MLVs with lipid composition of DPPC/CH/SA in molar ratio (4:3:1) showed higher EE% (65.50 ± 1.07) more than all other liposome formulations.
Size Analysis of Liposomes
Table II shows the results of particle size measurement; it is cleared that the mean particle diameter of MLVs is larger than that of REVs liposomes. This is due to the presence of charge either positive or negative in liposomes, leading to increase in mean particle size more than neutral liposomes, especially for MLVs. These results can be attributed to the inclusion of charge inducer in liposomes, which increases the spacing between the adjacent layers due to repulsion forces, resulting in the formation of large size liposomes in comparison with neutral one (50). Furthermore, the positively charged lipid electrostatically attracts the drug anion, which may be expected to push phospholipids lead groups apart, hence increasing the particle diameter (51). The result shows that the mean particle diameter of neutral MLVs is larger than that of neutral REV liposomes; this is due to the difference in mechanism of vesicular formation, in which the REVs are formed from splicing of two monolayers of inverted micelles collapsing to each other and hence more easily curved, which lead to a decrease in the diameter of REVs less than that of MLVs.
Table II.
Particle Size of Ofloxacin Multilamellar and Reverse-Phase Evaporation Liposomes
| Liposomal Lipid Composition | Molar Ratio | Liposomal Charge | Mean Diameter (nm) for REVs | Mean Diameter (nm) for MLVs |
|---|---|---|---|---|
| DPPC/CH | 4:3 | Neutral | 1,025 | 1,378 |
| DPPC/CH/ DP | 4:3:1 | Negative | 1,210 | 1,735 |
| DPPC/CH/SA | 4:3:1 | Positive | 1,460 | 1,912 |
CH cholesterol, DP dihexadecyl hydrogen phosphate, SA stearylamine, DPPC dipalmitoyl-l-α-phospatidylcholine, REVs reverse-phase evaporation vesicles, MLVs multilamellar vesicles
Gelation Temperature of Chitosan/β-GP Thermogelling Solution
An acceptable ocular thermogelling solution must have a gelation temperature in the range of 30–37°C so as to be in liquid form at room temperature and to form a gel phase instantly in the eye (52). By measuring the gelation temperature of the prepared chitosan/β-GP thermogelling solutions, it was 37°C. These results could be due to the addition of β-GP to chitosan, leading to the formation of thermogelling solution due to the following: (1) increase in the temperature, reduction of chitosan chain polarity and increase in its hydrophobic character (38); (2) electrostatic attraction between the ammonium group of chitosan and phosphate group of β-GP, which is induced thermally (53); and (3) hydrogen bonding between the chitosan chain as a consequence of reduced electrostatic repulsion after neutralization of the chitosan solution with β-GP (12). All previous reasons make chitosan solution form gel at 37°C.
The addition of ofloxacin liposomes to chitosan/β-GP solution leads to a decrease in the gelling temperature from 37°C to 36°C. This decrease could be due to the fact that chitosan has a strong potential for chemical interaction toward hydrophobic materials because of its remaining acetyl groups (12), so hydrophobic interaction are expected to occur between chitosan and liposomes, which possibly promote bridging and thus decreased the energy barrier required to initiate gelation, leading to a decrease in gelation temperature.
Rheological Measurement
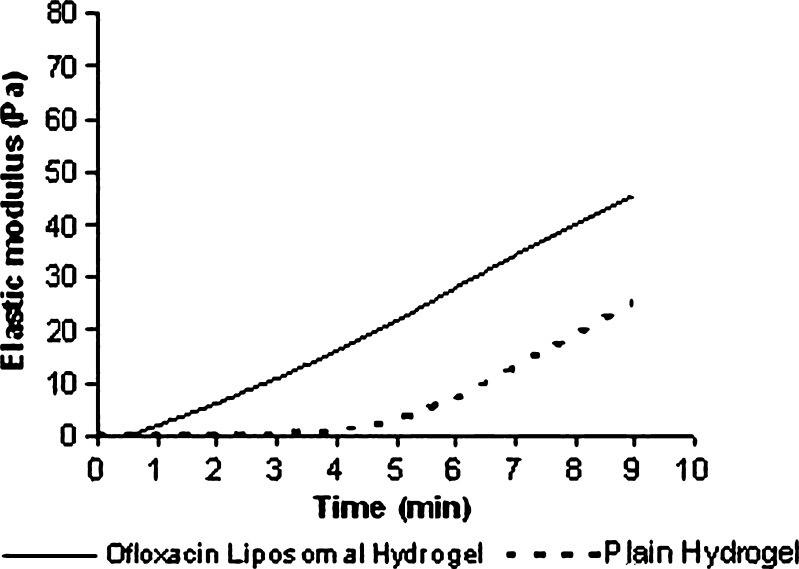
Figure 1 shows the rheological behavior of a chitosan/β-GP solution with and without ofloxacin liposomes at 37°C. The increase in elastic modulus over time clearly indicates that the liquid solution is turning into a solid-like gel. It was cleared that the addition of liposomes to chitosan/β-GP solution leads to a decrease in the gelation lag time from 5 to 1 min. These results could be due to the fact that ofloxacin liposome decreased the gelation temperature to 36°C due to hydrophobic interaction between liposomes and chitosan, thus decreasing the energy barrier required to initiate gelation, which causes reduction in gelation lag time from 5 to 1 min.
Fig. 1.
Elastic modulus as a function of time at 37 ± 0.1°C for plain chitosan/β-GP solution and chitosan/β-GP solution containing ofloxacin liposomes. The frequency of oscillation is 1.0 Hz, and the acquisition rate is 1 point every 13 s
In Vitro Transcorneal Permeation
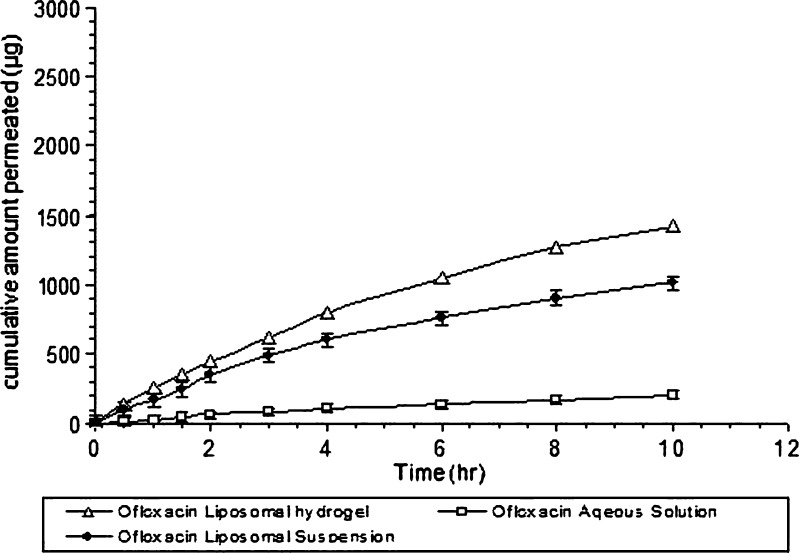
Figure 2 shows the transcorneal permeation profile of ofloxacin from its 0.3% aqueous solution, liposomal suspension, and ofloxacin liposomal thermosensitive hydrogel containing 0.3% (w/w) ofloxacin. The extent of drug permeated after 10 h from liposomal hydrogel, and aqueous solution was significantly different. The cumulative amount of ofloxacin permeating from liposomal hydrogel after 10 h was 1,425 ± 57.03 µg, and percentage permeated was 47.5%, while the cumulative amount permeating from ofloxacin liposomal suspension was 1,015 ± 30.24 µg, and the percentage permeated was 33.8%. However, in the case of ofloxacin aqueous solution, the cumulative amount permeated was 205±11.4 µg, and percentage permeated was 6.8% only.
Fig. 2.
Transcorneal permeation profile of ofloxacin from liposomal hydrogel and liposomal suspension in comparison to its solution (mean ± SD, n = 3)
This means that liposomal suspension showed fivefold higher permeation percent than aqueous solution. This may be explained by the fact that an electrostatic attraction force occurs between positively charged liposomes and negatively charged corneal membrane; we speculate then that liposomes adsorbed to the corneal surface and transfer their membrane associated drug directly to the corneal epithelial cell membrane, thereby facilitating drug passage across the cornea (54,55). Another reason is due to lower solubility of ofloxacin at pH 7.4; at this pH, ofloxacin presents as unionized form. As a result, it gives a cloudy solution (56). Therefore, ofloxacin becomes insoluble, which leads to decrease permeation; in order for the drug to be permeated, it must present first in soluble form either in aqueous or in lipid solution. This leads to a decrease in the percent permeated from aqueous ofloxacin solution in comparison with aqueous ofloxacin liposomal suspension in which the drug remains solubilized either in aqueous or lipid bilayers of the liposomes.
In the case of liposomal thermosensitive hydrogel, the percent permeated was sevenfold more than that of the aqueous solution; this may be explained by the following: (1) encapsulation of ofloxacin in liposomes, which leads to enhance transcorneal permeation as mentioned above; (2) the fact that mucoadhesive properties of the hydrogel base ensure an intimate contact between liposomes and corneal membrane, which prolong the retention of the formulation at site of administration, which is beneficial for enhancing permeation (57,58); and (3) at pH 7.4, approximately 17% of amino group of chitosan are still protonated (56). This cationic character of chitosan allows for the adherence of this thermally gelling solution to the corneal membrane and opens the tight junction between the epithelial cells. Because of these tight junctions, it is highly hydrated and contains fixed negative charges (38).
CONCLUSION
Based on these studies, it can be concluded that the formulation of ofloxacin as liposomal in situ thermosensitive hydrogel will provide the maximum in vitro ocular bioavailability through albino rabbit cornea. Preparation of ofloxacin liposomes as MLVs, addition of CH, use of more rigid phospholipids, and use of positively charge inducer increase ofloxacin encapsulation efficiency into liposomes. Dispersion of liposomes in thermogelling solution prepared from chitosan and β-GP at pH 7.4 correct the major two drawbacks of conventionally used ofloxacin aqueous solution, which were the formation of white crystalline deposit on cornea and high need for frequent administration. This liposomal in situ thermosensitive hydrogel ensures steady, prolonged release and higher transcorneal permeation of ofloxacin and that the formulation improves patient compliance.
References
- 1.Budai L, Hajdu M, Budai M, Grof P, Beni S, Noszal B, Antal I, et al. Gels and liposomes in optimized ocular drug delivery: studies on ciprofloxacin formulations. Int J Pharm. 2007;343:34–40. doi: 10.1016/j.ijpharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Kaur IP, kanwar M. Ocular preparations: the formulation approach. Drug Devel Ind Pharm. 2002;28:473–93. doi: 10.1081/DDC-120003445. [DOI] [PubMed] [Google Scholar]
- 3.VanSantvliet L, Ludwig A. The influence of penetration enhancers on the volume instilled of eye drops. Eur J Pharm Biopharm. 1998;45:189–98. doi: 10.1016/S0939-6411(97)00114-8. [DOI] [PubMed] [Google Scholar]
- 4.Alia AB, Hanan M, Riad K, Hala M, Mohamed D. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res. 2008;31(8):1040–9. doi: 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- 5.Adenis JP, Colin J, Verin P, Saint-Blancat P, Malet F. Ciprofloxacin ophthalmic solution versus rifamycin ophthalmic solution for the treatment of conjunctivitis and blepharitis. Eur J Ophthalmol. 1995;5:82–7. doi: 10.1177/112067219500500203. [DOI] [PubMed] [Google Scholar]
- 6.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release. 2001;73:205–11. doi: 10.1016/S0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JEF. Martindale, the extra pharmacopoeia. 29. London: The Pharmaceutical Press; 1989. pp. 276–77. [Google Scholar]
- 8.Claerhout I, Kestelyn Ph, Meire F, Remon J, Decaestecker T, Van Bocxlaer J. Corneal deposits after the topical use of ofloxacin in two children with verna keratoconjunctivitis. Br J Ophthalmol. 2003;87(5):646. doi: 10.1136/bjo.87.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochot A, Fattal E, Gulik A, Couarraze J, Couvreur P. Liposomes dispersed within a thermosensitive gel: a new dosage form for ocular delivery of oligonucleotides. Pharm Res. 1998;15:1364–9. doi: 10.1023/A:1011989202488. [DOI] [PubMed] [Google Scholar]
- 10.Paavola A, Kilpelainen I, Yliruusi J, Rosenberg P. Controlled release injectable liposomal gel of ibuprofen for epidural analgesia. Int J Pharm. 2000;199:85–93. doi: 10.1016/S0378-5173(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 11.Weiner AL, Carpenter-Green SS, Soehngen EC, Lenk RP, Popescu MC. Liposome–collagen gel matrix: a novel sustained drug delivery system. J Pharm Sci. 1985;74:922–5. doi: 10.1002/jps.2600740903. [DOI] [PubMed] [Google Scholar]
- 12.Ruel-Garie E, Leclairb G, Hildgenb P, Guptac A, Lerouxa B. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release. 2002;82:373–83. doi: 10.1016/S0168-3659(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 13.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–52. doi: 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 14.Gregoriadis G, Florence AT. Liposomes in drug delivery: clinical, diagnostic and ophthalmic potential. Drugs. 1993;45:15–28. doi: 10.2165/00003495-199345010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Farkas E, Schubert R, Zelk’o R. Effect of cholesterol on the characteristics of vesicular gels containing chlorhexidine. Int J Pharm. 2004;278:63–70. doi: 10.1016/j.ijpharm.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Foradada M, Estelrich J. Encapsulation of thioguanine in liposomes. Int J Pharm. 1995;124:261–9. doi: 10.1016/0378-5173(95)00097-3. [DOI] [Google Scholar]
- 18.Choudhari KB, Labhasetwar V, Dorle AK. Liposomes as a carrier for oral administration of insulin: effect of formulation factors. J Microencapsul. 1994;11:319–25. doi: 10.3109/02652049409040461. [DOI] [PubMed] [Google Scholar]
- 19.Galovic Rengel R, Barisic K, Pavelic Z, Zanic Grubisic T, Cepelak I, Filipovic-Grcic J. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur J Pharm Sci. 2002;15:441–8. doi: 10.1016/S0928-0987(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 20.Karathanasis E, Ayyagari AL, Bhavane R, Bellamkonda RV, Annapragada AV. Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery. J Control Release. 2005;103:159–75. doi: 10.1016/j.jconrel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298:198–205. doi: 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Law SL, Hung HY. Properties of acyclovir-containing liposomes for potential ocular delivery. Int J Pharm. 1998;161:253–9. doi: 10.1016/S0378-5173(97)00362-1. [DOI] [Google Scholar]
- 23.El-Samaligy MS, Nagia NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308:140–8. doi: 10.1016/j.ijpharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Smolin G, Okumoto M, Feiler S, Condon D. Idoxuridine liposome therapy for herpes simplex heratitis. Am J Ophthalmol. 1981;91:220–5. doi: 10.1016/0002-9394(81)90177-x. [DOI] [PubMed] [Google Scholar]
- 25.Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery. III. Pharmacodynamic and biodisposition studies of atropine. Int J Pharm. 1989;55:105–13. doi: 10.1016/0378-5173(89)90030-6. [DOI] [Google Scholar]
- 26.Tang Y, Du Y, Xian-Wen H, Shi X, Kennedy JF. Rheological characterization of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohydr Polym. 2007;67:491–9. doi: 10.1016/j.carbpol.2006.06.015. [DOI] [Google Scholar]
- 27.Gariepy ER, Chenite A, Chaput C, Guirguis S, Leroux JC. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int J Pharm. 2000;203:89–98. doi: 10.1016/S0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 28.Hsiue GH, Chang RW, Wang CH, Lee SH. Development of in situ thermosensitive drug vehicles for glaucoma therapy. Biomaterials. 2003;24:2423–30. doi: 10.1016/S0142-9612(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 29.Gariepy ER, Leroux JC. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Bioph. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Jeong B, Kim SW, Baeb YH. Thermosensitive sol–gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/S0169-409X(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 31.Jaepyoung C, Marie-Claude H, Andre B, Carreau PJ. Chitosan and glycerophosphate concentration dependence of solution behavior and gel point using small amplitude oscillatory rheometry. Food Hydrocoll. 2006;20:936–45. doi: 10.1016/j.foodhyd.2005.10.015. [DOI] [Google Scholar]
- 32.Struszczyk H, Wawro D, Niekraszewicz A. Biodegradability of chitosan fibres. In: Braine CJ, Sandford PA, Zikakis JP, editors. Advances in chitin and chitosan. London: Elsevier Applied Science; 1991. p. 580. [Google Scholar]
- 33.Chandy T, Sharma CP. Chitosan as a biomaterial. Biomaterial Artificial Cell and Arttificial Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 34.Hiaro S, Seino H, Akiyama Y, Nonaka I. Chitosan: a biocompatible material for oral and intravenous administration. In: Gebelein CG, Dunn RL, editors. Progress in biomedical polymers. New York: Plenum Press; 1990. p. 283. [Google Scholar]
- 35.He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. doi: 10.1016/S0378-5173(98)00027-1. [DOI] [Google Scholar]
- 36.Henriksen I, Green KL, Smart JD, Smistad G, Karlsen J. Bioadhesion of hydrated chitosans: an in vitro study. Int J Pharm. 1996;145:231–40. doi: 10.1016/S0378-5173(96)04776-X. [DOI] [Google Scholar]
- 37.Lehr C-M, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and other natural polymers. Int J Pharm. 1992;78:43–8. doi: 10.1016/0378-5173(92)90353-4. [DOI] [Google Scholar]
- 38.Chenite A, Buschmann M, Wang D, Chaput C, Kandani N. Rheological characterization of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr Polym. 2001;46:39–47. doi: 10.1016/S0144-8617(00)00281-2. [DOI] [Google Scholar]
- 39.Alberty RA, Silbey RJ. Physical chemistry. 2. New York: Wiley; 1996. [Google Scholar]
- 40.Jaepyoung C, Marie H, Andre B, Pierre J. Carreau, Effect of urea on solution behavior and heat-induced gelation of chitosan-b-glycerophosphate. Carbohydr Polym. 2006;63:507–18. doi: 10.1016/j.carbpol.2005.10.013. [DOI] [Google Scholar]
- 41.Furneri P, Fresta M, Puglisi G, Tempera G. Ofloxacin-loaded liposomes: in vitro activity and drug accumulation in bacteria. Antimicrob Agents Chemother. 2000;44:2458–64. doi: 10.1128/AAC.44.9.2458-2464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szoka F, Papahodjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA. 1978;75:4194–8. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puglisi G, Fresta M, Mazzone G, Furneri P, Tempera G. Formulation parameters of fluoroquinolone-loaded liposomes and in vitro antimicrobial activity. Int J Pharm. 1995;118:65–76. doi: 10.1016/0378-5173(94)00340-B. [DOI] [Google Scholar]
- 44.Gilbert JC, Richardson JL, Davies MC, Palin KJ, Hadgraft J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. J Control Release. 1987;5:113–8. doi: 10.1016/0168-3659(87)90002-2. [DOI] [Google Scholar]
- 45.Malhotra M, Majumdar DK. In vitro transcorneal permeation of ketorolac tromethamine from buffered and unbuffered aqueous ocular drops. Indian J Exp Biol. 1997;35:941–7. [PubMed] [Google Scholar]
- 46.Morilla MJ, Benavides P, Lopez MO, Bakas L, Romero EL. Development and in vitro characterization of a benznidazole liposomal formulation. Int J Pharm. 2002;249:89–99. doi: 10.1016/S0378-5173(02)00453-2. [DOI] [PubMed] [Google Scholar]
- 47.Perugini P, Pavanetto F. Liposomes containing boronophenylalanine for boron neutron capture therapy. J Microencapsul. 1998;15:473–83. doi: 10.3109/02652049809006874. [DOI] [PubMed] [Google Scholar]
- 48.New RRC. Liposomes: a practical approach. Oxford: Oxford University Press; 1990. [Google Scholar]
- 49.Gulati M, Grover M, Singh M, Singh S. Study of azathioprine encapsulation into liposomes. J Microencapsul. 1998;15:485–94. doi: 10.3109/02652049809006875. [DOI] [PubMed] [Google Scholar]
- 50.Rania MH, Samar M, Nahed DM, Ahmed SG. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8(1):1–12. doi: 10.1208/pt0802027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruner SM. Materials properties of liposomal bilayers. In: Ostro MJ, editor. Liposomes from biophysics to therapeutics. New York: Marcel Dekker; 1987. pp. 1–38. [Google Scholar]
- 52.Choi HK, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in-situ gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 53.Ruel E, Chenite A, Chaput C, Guirguis S, Leroux J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int J Pharm. 2000;203:89–98. doi: 10.1016/S0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 54.Singh K, Mezei M. Liposomal ophthalmic drug delivery system. I. Triamcinolone acetonide. Int J Pharm. 1983;16:339–44. doi: 10.1016/0378-5173(83)90152-7. [DOI] [Google Scholar]
- 55.Lee VH, Urrea PT, Smith RE, Schazlin D. Therapeutic review: ocular drug bioavailability from topically applied liposomes. Surv Ophthalmol. 1985;29:335–48. doi: 10.1016/0039-6257(85)90109-2. [DOI] [PubMed] [Google Scholar]
- 56.Ahuja M, Singh G, Majumdar DK. Effect of formulation parameters on corneal permeability of ofloxacin. Sci Pharm. 2008;76:505–14. doi: 10.3797/scipharm.0804-22. [DOI] [Google Scholar]
- 57.Deepika A, Indu PK. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290:155–9. doi: 10.1016/j.ijpharm.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–69. doi: 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]