Abstract
A method to achieve controlled ice nucleation during the freeze-drying process using an ice fog technique was demonstrated in an earlier report. However, the time required for nucleation was about 5 min, even though only one shelf was used, which resulted in Ostwald ripening (annealing) in some of the vials that nucleated earlier than the others. As a result, the ice structure was not optimally uniform in all the vials. The objective of the present study is to introduce a simple variation of the ice fog method whereby a reduced pressure in the chamber is utilized to allow more rapid and uniform freezing which is also potentially easier to scale up. Experiments were conducted on a lab scale freeze dryer with sucrose as model compound at different concentration, product load, and fill volume. Product resistance during primary drying was measured using manometric temperature measurement. Specific surface area of the freeze-dried cake was also determined. No difference was observed either in average product resistance or specific surface area for the different experimental conditions studied, indicating that with use of the reduced pressure ice fog technique, the solutions nucleated at very nearly the same temperature (−10°C). The striking feature of the “Reduced Pressure Ice Fog Technique” is the rapid ice nucleation (less than a minute) under conditions where the earlier procedure required about 5 min; hence, effects of variable Ostwald ripening were not an issue.
Key words: controlled ice nucleation, freeze-drying, ice fog, reduced pressure, scale-up
INTRODUCTION
Freeze-drying is widely carried out in the biopharmaceutical industry to increase the shelf life of an otherwise unstable drug product. Freeze-drying is carried out in three steps: freezing, primary drying, and secondary drying. During the freezing step, most of the water is converted into ice at temperatures in the vicinity of −40°C. Then, the system is evacuated using vacuum pumps, and the shelf temperature is raised to facilitate ice sublimation during primary drying. In the secondary drying stage, the shelf temperature is further raised to efficiently remove unfrozen water by desorption to provide a low residual water content, typically less than 1%. Reproducibility of the freezing process is the subject of this research, in particular control of the degree of super-cooling.
The degree of super-cooling is defined as the difference between the equilibrium freezing point and the ice nucleation temperature (Tn), which is the temperature at which ice crystals are first formed in the solution. The nucleation temperature governs the number of ice nuclei formed during the freezing step, which in turn affects the product resistance and temperature during the drying stage and, therefore, the drying time. The ice nucleation process is spontaneous and stochastic in nature and depends on the solution properties, process conditions, the surface characteristics of the container, and the presence of particulate matter that may serve as heterogeneous nucleation sites. During primary drying, water vapor flows through the void channels previously filled with ice. A higher degree of super-cooling results in more ice nuclei, meaning smaller ice crystals since the total amount of ice is fixed. Thus, greater super-cooling results in smaller pore size, greater resistance to vapor flow, and longer primary drying time. Smaller pore size not only results in higher resistance to flow of water vapor during primary drying but also means greater surface area and hence faster desorption during secondary drying. Thus, the degree of super-cooling impacts both primary and secondary drying. Optimization of the primary drying step is critical as it is the longest step in the freeze-drying process. The importance of the freezing step and its subsequent effect on primary drying in a macroscopic sample has been reported wherein the mass transfer coefficient for water vapor transport was shown to depend on the cooling conditions imposed on the sample (1). Further, it has been reported that primary drying time increases by 1% for every 1°C increase in degree of super-cooling (2). Yet, according to another report, the primary drying time increased by ∼4% for each degree of additional super-cooling (3). Thus, it would appear that the effects of variation in degree of super-cooling are large and potentially variable depending on the exact circumstances.
Freezing differences not only result in variation within the batch but also may cause batch to batch variations. Further, variation in degree of super-cooling also imposes a challenge during freeze-drying process scale-up. At laboratory scale, the product nucleates at much higher temperature (i.e., lower degree of super-cooling) than in production scale due to the differences in level of particulate matter. In the usual laboratory environment, levels of particulate matter are much higher than in the class 100 environment of a production environment. Thus, a freezing process developed in the laboratory will likely not be representative of freezing in production. Nevertheless, there is only one report in the literature documenting the difference in ice nucleation temperature between laboratory and manufacturing and demonstrating the resulting difference in product temperature and drying time (2). Here, the degree of super-cooling in the production scale was about 10°C higher than in the laboratory, resulting in about a 10% longer drying time (2). However, the impact of a 10°C increase in degree of super-cooling is apparently variable, as another study (3) showed a 40% increase in primary drying time when the degree of super-cooling increased by 10°C. Clearly, these differences make it difficult to scale up a laboratory process when a fixed primary drying time is used. Therefore, it is very important to have a small variation in ice nucleation temperature to allow control of product resistance and drying rate.
Annealing is commonly used during the freezing step to overcome the ice nucleation heterogeneity. Once ice nucleates in the samples, and the system is mostly frozen, usually at about −40°C, the shelf temperature is raised to a temperature above the glass transition temperature, Tg′, of the formulation but below the onset of ice melt. Due to the enhanced molecular mobility, larger ice crystals are formed at the expense of smaller ones, a phenomenon referred to as Ostwald ripening. Thus, annealing can eliminate, or at least minimize, the differences in pore size and drying rate caused by different degrees of super-cooling. However, annealing takes additional time and is not an option for systems susceptible to phase separation, buffer crystallization, interfacial degradation, or degradation when kept above Tg′; hence, the need arises for an alternative technique to achieve homogeneity in pore size and drying rate.
The concept of nucleating by an ice fog was proposed by T. W. Rowe in 1990 (4) and applied to freeze-drying for the first time in 2004 (5). In this technique, the product temperature is reduced to the desired ice nucleation temperature, nitrogen gas is passed through copper coils immersed in liquid nitrogen and then introduced into the product chamber, thereby producing a dense ice fog. The ice fog is forced into the vials, seeding the crystallization of ice. In the previous report, a tube with uniformly punched holes was clamped above the shelf to achieve a uniform distribution of ice fog (5). However, in this variation of the technique, the chamber was maintained at atmospheric pressure, and it was found that even with only using one shelf of vials, the time required for nucleation was about 5 min, which resulted in some Ostwald ripening or annealing in some of the vials that nucleated earlier than the other vials. As a result, the ice crystal structure was not optimally uniform in all the vials because of variable time allowed for Ostwald ripening. The objective of the present study is to introduce a simple variation of the ice fog method whereby a reduced pressure in the chamber is utilized to allow more rapid and uniform freezing, which is also potentially easier to scale up. We find that the ice fog technique used with reduced pressure in the chamber achieves rapid ice nucleation (<1 min) and uniform ice crystal structure in all the vials within the batch.
MATERIALS AND METHOD
Crystalline sucrose (catalog# S9378-1KG, purity 99.5%) was obtained from Sigma Aldrich Co. (St. Louis, MO). Freeze-drying was carried out in 5 mL tubing vials with 20 mm finish Flurotec® stoppers from West Pharmaceutical Co. (Lionville, PA). The vials as well as the stoppers were used as received without any further treatment.
Freeze-Drying
Experiments were conducted on a lab scale freeze dryer, Lyostar II (SP Industries, NY), using sucrose at different concentrations (5% and 10%), variable product load (one vs three shelves), and two fill volumes (2 and 4 mL) as shown in Table I. Aqueous solutions were filtered through a 0.22-μm membrane filter. Vials were filled with 2 or 4 mL of solution and loaded on to the temperature-controlled shelves of the freeze dryer. Thermocouples (28 gauge copper/constantan) were placed in both edge and center vials, at the bottom center of the vials. When the vials reached the desired nucleation temperature, the chamber pressure was reduced to the predetermined set point, the valve separating condenser and chamber was closed, and cold nitrogen gas was passed into the chamber to nucleate the samples at that temperature. The shelf temperature was then lowered to −50°C at a ramp rate of 3°C/min to complete the freezing process. Primary drying was carried out at a shelf temperature of −30°C, and a chamber pressure of 100 mTorr. The end of primary drying was measured by a sharp drop in the Pirani gauge pressure (6). The Pirani vacuum gauge works on the principle of measuring the thermal conductivity of the gas in the drying chamber and is calibrated for nitrogen. However, water vapor has a thermal conductivity about 60% higher than nitrogen. Thus, during primary drying, when the gas in the chamber is essentially 100% water vapor, the Pirani gauge reads 60% higher than the actual pressure, which is measured by the MKS Baratron gauge. A sharp drop in Pirani response indicates that the gas composition is changing from mostly water vapor to mostly nitrogen, which indicates that primary drying is complete. Secondary drying was carried out at a 40°C shelf temperature and a chamber pressure of 100 mTorr.
Table I.
Solute Concentration, Fill Volume, and Load Condition for the Freeze-Drying Experiments
| Sucrose conc. (w/v) | Fill volume (mL) | # of shelves used |
|---|---|---|
| 10%a | 4 | 1 |
| 10% | 2 | 1 |
| 5% | 4 | 1 |
| 5% | 4 | 3 |
| 5% | 2 | 1 |
aRepeated for characterization of reproducibility
Reduced Pressure Ice Fog Technique
The combination of ice fog with reduced pressure is referred to as the Reduced Pressure Ice Fog Technique. When the desired ice nucleation temperature was reached (−10°C), the vacuum pump was turned on to reach the optimized chamber pressure (48–50 Torr, measured using a Pirani vacuum gauge). The chamber was then isolated by closing the valve connecting the chamber and the condenser. To execute the ice fog procedure, nitrogen gas was passed through copper coils immersed in liquid nitrogen and introduced into the chamber through one of the inlet ports available on the top of the chamber. As the cold nitrogen gas enters the chamber, the chamber pressure begins to increase, ice forms, and the ice fog is forced into the vials. As the chamber pressure approaches atmospheric pressure, the isolation valve to the condenser was quickly opened, and the ice fog was pumped into the condenser.
The ice nucleation temperature of −10°C was selected as it represents the maximum super-cooling that can routinely be achieved in a laboratory dryer under full-load conditions. Using a much lower nucleation temperature is difficult due to higher level of particulate matter in the laboratory (i.e., the vials nucleate even before the ice fog technique is executed).
Specific Surface Area Measurement
The specific surface area of the freeze-dried solid was measured using a BET-specific surface area analyzer Flowsorb II (Micromeritics Instrument Corporation, Norcross, GA). The samples were degassed for at least 5 h at room temperature by flowing a mixture of helium and krypton through the sample. The instrument was calibrated using 100% krypton under room conditions. After the sample was dried, single point adsorption of krypton was carried out by dipping the sample holder in liquid nitrogen to reduce the sample temperature to liquid nitrogen temperature (∼77 K). For desorption, the sample holder was immersed in water bath at room temperature. Reproducibility in specific surface area measurement was better than ±5%.
Karl Fischer Residual Moisture Analysis
Residual moisture was measured using a Karl Fischer Residual Moisture Analyzer (Metrohm, KF756). Samples were suspended in 2 mL of “dry” methanol and 0.5 mL of solution was injected into the titration cell. Methanol was dried over molecular sieves with effective pore size of 3 Å. The amount of water in dry methanol was obtained from a blank reading, which was subtracted from the sample reading. Thus, knowing the amount of water content and the weight of the sample the % residual (w/w) water content in the sample could be determined. The reproducibility in the moisture content measurement was ±0.1%.
Dry Layer Resistance Measurement
Dry layer resistance measurements were made using the manometric temperature measurement (MTM) procedure (7,8). The procedure consists of closing the valve connecting the chamber and the condenser for 25 s and recording the chamber pressure vs time data. The MTM equation was fitted to the pressure rise data to obtain product temperature and dry layer resistance, resistance being determined essentially from how fast the pressure initially increases.
RESULTS AND DISCUSSION
The reduced pressure ice fog technique was used to nucleate sucrose solutions under different conditions (Table I). A series of experiments were carried out to determine the optimum chamber pressure. Random ice nucleation was observed in the chamber pressure range of 600 to 200 Torr (i.e., a few vials would nucleate while the majority of the vials did not nucleate), largely because the ice fog was not distributed homogenously throughout the chamber. As the chamber pressure was further reduced below 200 Torr, more uniformity was observed in ice nucleation; however, until pressures in the range of 50 Torr were approached, the technique needed repetition (at least four times) to nucleate all vials at roughly the same time. At a chamber pressure of 48–50 Torr, the ice fog was both dense and homogeneous (visual observation) and caused rapid ice nucleation in all vials. Hence, a chamber pressure of 48–50 Torr was used in all subsequent experiments. During the pressure increase as well as during the opening of the isolation valve, cold nitrogen gas uniformly distributes throughout the chamber and eventually enters all vials resulting in rapid ice nucleation. The striking feature of the “Reduced Pressure Ice Fog Technique” is the time required for ice nucleation, which was less than a minute when one shelf of vials was used, and hence effects of Ostwald ripening, which existed in the method used in a previous report was not an issue.
Effect of Fill Depth
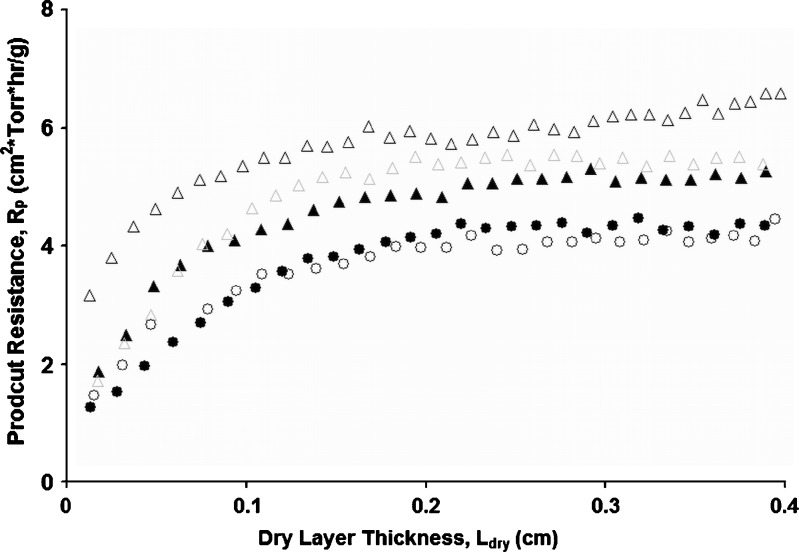
Product resistance data for 5% and 10% sucrose solutions at 2 mL (fill depth ∼0.75 cm) and 4 mL (fill depth of ∼1.5 cm) fill volumes are shown in Fig. 1. Reproducibility of product resistance data (average of three runs, data not shown) is within ±10–15%. For a given solute concentration, product resistance at a given dry layer thickness was similar at different fill volumes, indicating that the reduced pressure ice fog technique provided similar pore structure in the dried cake regardless of the fill volume. Even for a 4-ml fill volume, which corresponds to 1.5 cm fill depth, rapid ice nucleation was achieved uniformly throughout the vial for all the vials on the shelf. Average product resistance over the same dry layer thickness of 0.5 cm, 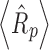 , is the same within experimental error for both 2 and 4 mL fill volumes at a given sucrose concentration (i.e., 5%). However, for 10% sucrose solutions, the
, is the same within experimental error for both 2 and 4 mL fill volumes at a given sucrose concentration (i.e., 5%). However, for 10% sucrose solutions, the 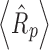 values are slightly higher (Table II). Thus, as expected, average product resistance increases with increase in solute concentration (9). Residual water was <1% in each case. The MTM product temperature (corrected for the temperature difference across the frozen layer) is in good agreement with temperature measured using thermocouples (Table II). At the same fill depth, 10% sucrose has slightly higher product temperature (due to slightly higher product resistance) than 5% sucrose.
values are slightly higher (Table II). Thus, as expected, average product resistance increases with increase in solute concentration (9). Residual water was <1% in each case. The MTM product temperature (corrected for the temperature difference across the frozen layer) is in good agreement with temperature measured using thermocouples (Table II). At the same fill depth, 10% sucrose has slightly higher product temperature (due to slightly higher product resistance) than 5% sucrose.
Fig. 1.
Product resistance as a function of dry layer thickness for 5% and 10% sucrose under same load condition (i.e., one shelf) with 2- and 4-mL fill volume. Closed and open triangles are for 4- and 2-mL fills, respectively, of 10% sucrose, and closed and open circles are for 4- and 2-mL fills, respectively, of 5% sucrose. The difference between the 4- and 2-mL data for 10% sucrose is just outside the usual 10–15% reproducibility and may not be significant
Table II.
Product Temperature and Average Dry Layer Resistance (over 0.5 cm Dry Layer Thickness) During Primary Drying for Different Fill Volume
| Experimental conditions | MTM (°C) | TC center (°C) | <R p> |
|---|---|---|---|
| 10% Sucrose, 4 mL fill | −35.4 | −34.3 ± 0.1 | 4.2 |
| 10% Sucrose, 2 mL fill | −35.7 | −34.5 ± 0.3 | 4.6 |
| 5% Sucrose, 4 mL fill | −35.8 | −35.3 ± 0.1 | 3.1 |
| 5% Sucrose, 2 mL fill | −36.2 | −36.5 ± 0.4 | 3.3 |
Reproducibility of product resistance data is within ±10–15%. Load condition = one shelf. Product temperature reported is the average during the steady state primary drying. MTM product temperature is at the bottom center of the vial (i.e., MTM product temperature at the interface is corrected for the small gradient across the ice).
<R p > = mean dry layer resistance over 0–0.5 cm of dry layer, in cm2 Torr h/g
Effect of Load
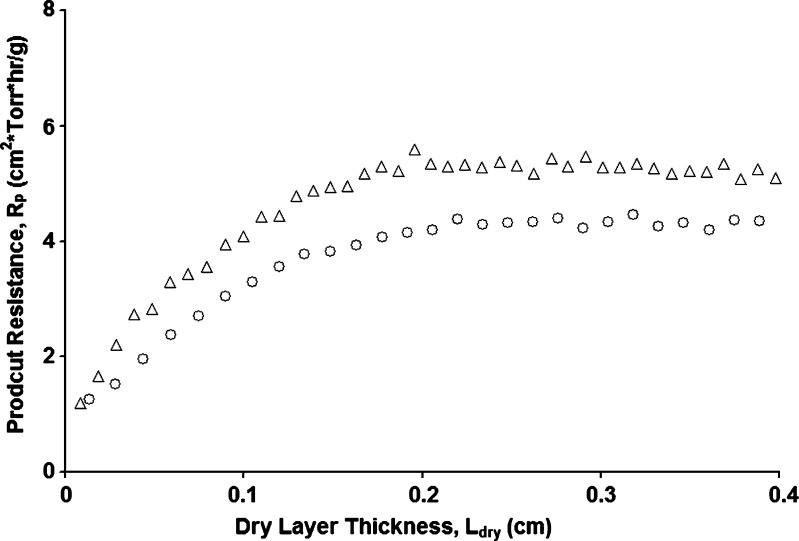
Under full-load condition (i.e., when all the three shelves were loaded with the product vials), equilibration of all the vials on all the shelves at the desired ice nucleation temperature of −10°C proved somewhat difficult. The temperatures for two center vials and one edge vial on each shelf were monitored with a thermocouple. The edge vials required longer to reach the desired nucleation temperature than the center vials and a few vials that could be seen from the front door nucleated even before the ice fog was introduced. While this would not be expected to be a problem in the clean environment of manufacturing, premature nucleation was a problem in our laboratory environment. To circumvent the problem of some vials taking too long to reach the desired nucleation temperature of −10°C, the shelf temperature was set to −12°C. With this practice, a few vials may have super-cooled slightly more than the majority of the batch. Further, visual observation indicated that the ice fog was less dense under full-load condition, as compared to a load of only one shelf. Hence, with a full load, the ice fog was introduced several times (at least four times) in rapid succession to insure that all vials were seeded. Nevertheless, all vials on all the shelves were nucleated within 5 min. One obvious solution to this problem would be to use sparging tubes inside the chamber, which should result in a denser and more homogenous ice fog. In a manufacturing environment, the best practice would be to use the CIP/SIP lines in the dryer since their placement is designed to cover the entire dryer interior. Even with low pressure in the chamber, the ice fog distribution under full load was not sufficiently uniform to result in ice nucleation as quickly as typically found with the single shelf experiments. Thus, there is a possibility of some Ostwald ripening under full-load condition.1 Nevertheless, with the reduced pressure ice fog technique as used here, the average product resistance was within experimental error for both the 100% and 33.3% of full load runs with 4 mL of 5% sucrose (Fig. 2, Table III). Thus, using the reduced pressure ice fog technique, there did not appear to be a significant effect of Ostwald ripening even under the full-load conditions. Also under full-load conditions, due to higher thermal load, the shelf surface temperature is expected to run slightly colder than under partial load condition. This could result in lower product temperature and slightly longer drying time for the full-load conditions. In fact, the drying time increases about 17% (with a decrease in product temperature by ∼1°C) in going from 33.3% to 100% of full load, consistent with a recent report (10).
Fig. 2.
Product resistance as a function of dry layer thickness for 5% sucrose solution for a fixed fill volume of 4 mL and different load conditions. Triangles = three shelves and circles 1 shelf. The difference between datasets is roughly within the normal experimental error of 10–15%
Table III.
Product Temperature and Average Dry Layer Resistance (Over 0.5 cm Dry Layer Thickness) During Primary Drying for Different Load Condition
| Experimental Conditions | MTM (°C) | TC Center (°C) | <Rp> |
|---|---|---|---|
| 10% Sucrose, 1 shelf | −35.4 | −34.3 ± 0.1 | 4.2 |
| 5% Sucrose, 1 shelf | −35.8 | −35.3 ± 0.1 | 3.1 |
| 5% Sucrose, 3 shelf | −36.9 | −36.9 ± 0.6 | 3.7 |
Reproducibility of product resistance data is within ±10–15%. Fill volume = 4 mL. Product temperature reported is the average during the steady state primary drying. MTM product temperature is at the bottom center of the vial (i.e., MTM product temperature at the interface is corrected for the small gradient across the ice).
<R p > = mean dry layer resistance over 0–0.5 cm of dry layer, in cm2 Torr h/g
Specific Surface Area
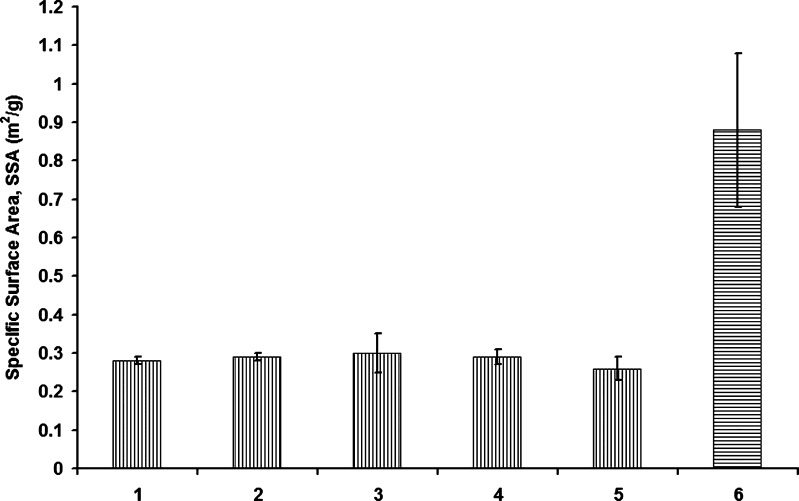
During primary drying, sublimation of ice creates pores in the solute matrix, and the dimensions of the pores are a direct reflection of the size and geometry of the ice crystals formed during freezing. Thus, the nucleation temperature determines the ice crystal structure and, hence, the specific surface area. Further, it has been shown that the specific surface area correlates with degree of super-cooling and product resistance (5). Specific surface area was measured for the center vials for all the freeze-drying runs listed in Table I. Within experimental error, there is no difference in specific surface area for different solute concentrations, different fill volumes, or different load conditions (Fig. 3), suggesting that with the use of the reduced pressure ice fog technique, the solutions nucleated at very nearly the same temperature (−10°C).2 Further, the low standard deviation in specific surface area suggests good inter-vial homogeneity in ice nucleation temperature when using the reduced pressure ice fog technique. It is important to note that the specific surface area for the samples freeze-dried normally, without applying the ice fog technique, was 0.88 with a standard deviation of 0.2 (n = 3) (5), nearly an order of magnitude larger standard deviation than found with the reduced pressure ice fog technique.
Fig. 3.
Specific surface area with standard deviation bars (n = 3) for 5% and 10% sucrose solution for different fill volume and load condition. KEY: 1: 10% sucrose, one shelf, 4 mL; 2: 10% sucrose, one shelf, 2 mL; 3: 5% sucrose, one shelf, 4 mL; 4: 5% sucrose, three shelves, 4 mL; 5: 5% sucrose, one shelf, 2 mL; 6: 5% sucrose, one shelf, 5-mL fill without ice fog
CONCLUSION
Using a partial vacuum in the chamber during introduction of the cold nitrogen, the ice fog technique has been improved to allow more rapid and more uniform nucleation. The reduced pressure technique should also allow easier scale-up of the ice fog nucleation technique to production scale dryers. The reduced pressure ice fog technique resulted in rapid ice nucleation at the desired temperature under widely different process and formulation conditions. Thus, the reduced pressure ice fog technique seems to be a promising method for temperature controlled ice nucleation, thereby improving the uniformity of drying as well as eliminating what is perhaps the most important scale-up problem, the difference in spontaneous ice nucleation between the laboratory and the clean environment of manufacturing. Future work should focus on applying this technique to pilot and commercial scale freeze-dryers.3
Footnotes
However, the previous report (5) used the ice fog technique with only one shelf and, hence, would have required rather long time (30 min or even longer) to result in ice nucleation in every vial on all the shelves under full load condition. This would result in variable Ostwald ripening and, hence, variable drying rate with full load.
We note that the small difference in product resistance between 5% and 10% sucrose (Tables II and III) are not reflected in a measurable difference in specific surface area, perhaps due to a combination of experimental errors dominating the expected small difference in specific surface area.
We note that the ice-fog technique carries no risk of sterility compromise as long as the cold nitrogen introduced is sterile filtered and the interior of the dryer is sterilized, presumably by steam. The technique basically consists of pumping out most of the air and introducing sterile filtered cold nitrogen gas back into the chamber. Thus, sterile ice crystals are formed from the sterile humid gas in the chamber, with some of the ice crystals being introduced into the vials.
References
- 1.Kochs M, Koerber C, Heschel I, Nunner B. The influence of the freezing process on vapor transport during sublimation in vacuum-freeze-drying of macroscopic samples. Int J of Heat and Mass Transfer. 1993;36(7):1727–1738. doi: 10.1016/S0017-9310(05)80159-0. [DOI] [Google Scholar]
- 2.Roy ML, Pikal MJ. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. J Parenter Sci Technol. 1989;43(2):60–66. [PubMed] [Google Scholar]
- 3.Searles JA, Carpenter JF, Randolph TW. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J Pharm Sci. 2001;90(7):860–871. doi: 10.1002/jps.1039. [DOI] [PubMed] [Google Scholar]
- 4.Rowe TW. A technique for the nucleation of ice. International Symposium on Biological Product Freeze-Drying and Formulation. Switzerland: Geneva; 1990. [Google Scholar]
- 5.Rambhatla S, Ramot R, Bhugra C, Pikal MJ. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of super-cooling. AAPS PharmSciTech. 2004;5(4):e58. doi: 10.1208/pt050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nail SL, Johnson W. Methodology for in-process determination of residual water in freeze-dreid products. Dev biol Stand. 1991;74:137–151. [PubMed] [Google Scholar]
- 7.Milton N, Pikal MJ, Roy ML, Nail SL. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J Pharm Sci Technol. 1997;51(1):7–16. [PubMed] [Google Scholar]
- 8.Tang X, Nail SL, Pikal MJ. Evaluation of manometric temperature measurement, a process analytical technology tool for freeze-drying: part II measurement of dry-layer resistance. AAPS PharmSciTech. 2006;7(4):93. doi: 10.1208/pt070493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pikal MJ, Shah S, Senior D, Lang JE. Physical chemistry of freeze-drying: measurement of sublimation rates for frozen aqueous solutions by a microbalance technique. J Pharm Sci. 1983;72(6):635–650. doi: 10.1002/jps.2600720614. [DOI] [PubMed] [Google Scholar]
- 10.Patel SM, Thummala A, Jameel F, Pikal MJ. The effect of dryer load on freeze-drying process design: comparison of load condition on laboratory and pilot scale freeze-dryer. AAPS National Biotech Conference; 2008; Toronto, Canada.