Abstract
Pain control is one of the most challenging aspects in the management of chronic pancreatitis. Total pancreatectomy can successfully relieve the intractable abdominal pain in these patients but will inevitably result in insulin-dependent diabetes. Islet autotransplantation aims to preserve, as far as possible, the insulin secretory function of the islet cell mass thereby reducing (or even removing) the requirement for exogenous insulin administration after a total pancreactomy. Despite the relatively small number of centres able to perform these procedures, there are important technical variations in the details of their approaches. The aim of this review is to provide details of the current surgical practice for total pancreatectomy combined with islet autotransplantation, and outline the potential advantages and disadvantages of the variations adopted in each centre.
Keywords: total pancreatectomy, islet, autotransplantation
Introduction
Chronic pancreatitis is a condition associated with irreversible morphological and functional abnormalities secondary to a prolonged pancreatic inflammation and fibrosis.1 Steatorrhoea, malabsorption, diabetes and abdominal pain of varying degree are the key clinical features. While malabsorption as a result of steatorrhoea can be managed with oral pancreatic enzyme supplements and diabetes with insulin, pain management is at best challenging and not infrequently intractable. The severity of the pain experienced does not always correlate with the apparent extent of the morphological changes demonstrated by radiological studies and this frequently complicates the clinical management. Fortunately the majority of patients can be managed medically within a multidisciplinary setting but a proportion of patients may experience disabling pain that severely affects their quality of life and renders them chronically dependent on opiates.2
Treatment options are limited but include extensive surgery in an attempt to remove or dramatically reduce symptoms, less invasive endoscopic procedures which aim to alleviate chronic pain and medical management which encompasses analgesia and regional blocks (with almost inevitable symptom recurrence). Total pancreatectomy, although a radical approach, is occasionally the only treatment option that can provide complete relief in these patients.3 The indications for the operation include patients with small duct disease without focal abnormalities or those in whom an endoscopic drainage procedure or previous resectional surgery have failed to relieve pain.4,5 Total pancreatectomy is technically challenging and has traditionally been associated with high morbidity and mortality rates6 but the development of specialist centres has improved the operative and peri-operative management resulting in outcomes comparable to those of a pylorus-preserving pancreactico-duodenectomy.7–9 The benefit of pain relief must nevertheless be considered in the context of the inevitable consequences of the procedure. These include exocrine insufficiency and insulin-dependent diabetes, although it is important to remember that the vast majority of patients will already require pancreatic supplements and that without treatment 50–60% of patients with chronic pancreatitis will eventually require insulin. Autologous islet transplantation when employed after total pancreatectomy may prevent the onset of diabetes and even when some exogenous insulin is required, because the infused islets almost always results in the long-term production of C-peptide and insulin, diabetic control is simplified.10,11
Patient selection and initial assessment
Patients with chronic pancreatitis are generally referred for total pancreatectomy after other treatments have failed to adequately control their symptoms. The severity of the pain that patients experience frequently does not correlate with the gross morphological changes in the gland and the diagnosis of chronic pancreatitis is often reached by considering the combination of clinical symptoms, metabolic features and radiological and/or endoscopic results. Most of the patients with chronic pancreatitis that are referred for surgical assessment have already had a number of investigations over long periods. These generally include metabolic studies for pancreatic endocrine and exocrine functions, computed tomography, magnetic resonance imaging, laparoscopy, endoscopic retrograde cholangiopancreatogrphy and, in some centres, endoscopic ultrasound.
The Leicester, Minnesota, Cincinnati and Alabama groups have all adopted a multidisciplinary approach in the patient selection process for total pancreatectomy with/without islet autotransplantation.3,5,12,13Table 1 shows the basic demographics of the patients who underwent pancreatectomy and islet autotransplantation in these centres. The aetiology of chronic pancreatitis in these patients was quite similar amongst the centres except for the Cincinnati series which had a much lower proportion of chronic pancreatitis secondary to alcohol.
Table 1.
Comparison of demographics and aetiology of patients in different centres
| Demographics | Leicester9 | Cincinnati12 | Minneapolis10,17,19 | Alabama3 |
|---|---|---|---|---|
| Age (mean, years) | 43 | 38 | 35 | 44 |
| Gender (M : F ratio) | 1 : 1.1 | 1 : 2 | 1 : 2.4 | 1 : 0.9 |
| Body mass index (mean, kg/m2) | 22 | 26 | N/A | 22 |
| Median duration of symptoms (months) | 65 (25–287) | N/A | 72 (12–240) | N/A |
| Aetiology (% patients) | ||||
| Alcohol | 36% | 4% | 20% | 35% |
| Idiopathic | 48% | 87% | 50% | 31% |
| Biliary | 10% | Not specified | 12% | 4% |
| Others | 6% | 9% | 18% | 30% |
N/A, Data not available.
The islet autotransplantation programme in Leicester was established in 1994 and after referral all patients are formally assessed by a multidisciplinary team. Patients undergo laboratory testing of their exocrine and endocrine functions by means of a glucose tolerance test, serum HbA1c level, oral butterfat test and faecal elastase 1 estimations. The multi-disciplinary team consists of a pancreatic surgeon, a diabetologist, a gastroenterologist, a pain specialist, an anaesthetist and a medical psychologist. Any member of the multi-dsiciplinary team can veto the decision to proceed with surgery if it is felt inappropriate. A clinical nurse specialist provides these patients with information and counselling regarding the procedure as well as introducing the potential candidates to two patients who have had a total pancreatectomy and islet autotransplantation (one who underwent the procedure within 12 months and the other more than 12 months ago) to facilitate their understanding of immediate and long-term post-operative recovery and gain realistic expectations. A further meeting is then scheduled in the clinic at least a month after the assessment to discuss consent with the patients (and their family if appropriate).
In Leicester, islet isolations are routinely attempted in patients with normal glucose tolerance tests who undergo total pancreatectomy for intractable pain, regardless of the severity of morphological changes to the pancreas. Patients with abnormal glucose tolerance tests may be considered for an islet autotransplant after discussion in the outpatients (with the aim of producing some background insulin production). Performing the procedure in these patients is based on the understanding that any beta cell mass, and the consequent insulin production, will result in improved diabetic control and reduce the long-term complication rate and a similar approach is adopted at the University of Minnesota.5 Patients who already require insulin are not considered for islet autotransplantation but total pancreatectomy will still be considered for pain relief. For patients where splenic preservation is not considered to be an option and in centres where splenectomy is performed routinely with total pancreatectomy, pre-operative vaccinations for Haemophilus influenza, Pneumococcus and Meningococcus are given.
The surgical approach to total pancreatectomy
Total pancreatectomy aims to completely remove the diseased pancreas, abolishing rather than reducing the chronic pain. The advantage over partial pancreatic resection or a surgical drainage procedure is that there is no potential for pancreatic duct leakage and the diseased gland is completely excised. However, it is a lengthy and complex procedure particularly if patients have had previous pancreatic surgery or severe inflammation, particularly pseudocyst formation.
There are similarities and differences in the surgical approach to total pancreatectomy among the three largest islet autotransplantation series worldwide9,10,12,14–16 and these are shown in Table 2.
Table 2.
Comparison of the surgical approaches adopted by the three largest published series of total pancreatectomy and islet autotransplantation
| Leicester series (55 to date) | Minnesota series (>200 to date) | Cincinnati series (>130 to date) | |
|---|---|---|---|
| Pancreatic resection and duodenectomy | Total with partial duodenectomy Pylorus preserving and preserving 4th part of duodenum Pancreas resected as a whole | Earlier series were near total with preservation of entire duodenum Past 15 years, partial duodenectomy, preserving pylorus and 4th part of duodenum Pancreas resected as a whole | For near-total pancreatectomy, a small rim (<5%) of pancreas left along with the C-loop of the duodenum, with the common bile duct and pancreaticoduodenal artery and entire duodenum left intact For total pancreatectomy, partial duodenum with or without preserving the pylorus Pancreas divided at the level of superior mesenteric artery with the distal portion sent for islet isolation while dissection around the head of pancreas continues |
| Spleen | Spleen preserving, supplied by the short gastric vessels | Splenectomy performed unless it retains an absolutely normal appearance after hilar ligation | Routine splenectomy |
| Reconstruction | End-to-end duodeno-duodenostomy or end-to-side duodenojejunostomy Choledochoduodenostomy | End-to-end duodeno-duodenostomy or end-to-side duodenojejunostomy Choledochoduodenostomy | Not required in near-total pancreatectomy. Side-to-side gastrojejunostomy or end-to-side duodenojejunostomy End-to-side hepaticojejunostomy |
Some aspects of the operation are common among the centres. Generally, the blood supply of the pancreas is preserved for as long as possible to minimize the warm ischaemic time. This can be achieved by preserving the venous drainage as well as the arterial supply of the pancreas via the splenic vessels until the point of pancreas removal. Meticulous dissection and attention to haemostasis is crucial as the risk of bleeding is increased because full heparinization is carried out prior to islet infusion and the islet infusion induces transient portal hypertension.
Preservation of the whole duodenum was originally advocated in pancreatic resections for benign diseases to retain the physiological bile secretion but this approach is associated with an increased complication rate as the vascular supply to the retained duodenum is compromised secondary to interruption of the pancreaticoduodenal arcade.10,17 Nevertheless total pancreatectomy with partial duodenectomy is still preferred by some centres.18,19 It is theoretically also preferable to keep the pancreatic capsule intact and avoid transection of the pancreas intra-operatively (Fig. 1) to facilitate the collagenase digestion and to minimize the period of warm ischaemia. However in some centres this is felt to prolong the procedure (and the warm ischaemia time) unduly and thus negate the potential advantage that this offers and consequently this practice has not been routinely adopted in all centres.15
Figure 1.
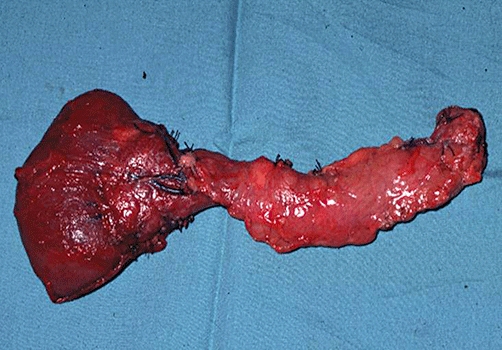
Pancreas resection with capsule intact and along with part of the duodenum
During the early development of the procedure it was believed that splenectomy could not be avoided but with the recognition of the important role that the spleen plays in maintaining immunological functions, in the mid 1990s a number of groups attempted splenic preservation and demonstrated that not only was it possible but it could be achieved in the majority of patients. It has subsequently been shown that in the absence of previous pseudocyst formation that has involved the splenic vessels then they can be preserved. If both vessels cannot be preserved, the splenic vein may be retained but the artery must not be left in isolation. Even in patients where this is not possible the spleen is usually viable when the short gastric vessels are not involved in the fibrotic process. Our experience in Leicester is that even with the temporary rise in portal pressure from the subsequent islet infusion this can safely be achieved in almost all patients.9,14 Other centres still have a lower threshold for splenectomy as a result of concerns over the risks of variceal formation in the short gastric veins with the potential for late intestinal bleeding and painful splenomegaly, although this has not been our experience.5
Islet isolation and preparation
In the United Kingdom, islet isolation must be performed in a the Human Tissue Authority accredited laboratory and a Food and Drug Administration approved laboratory in the United States.
After excision of the pancreas, the pancreatic duct is cannulated and the organ distended with collagenase and digested according to the modified automated method widely described.13,20–22
Islet purification is not a compulsory step of islet processing and transplantation. The purpose of islet purification is to reduce the volume of infusion in order to avoid an excessive rise in portal pressure, however, this must be balanced against the risk of wastage of islet cells.23 Islet purity is determined subjectively by a visual assessment using two 100-µl sampling strips. A comparison is made comparing the relative quantity of dithizone-stained islets (red) to unstained acinar tissue (yellow).24 Islet yields are expressed as international islet equivalents (IEQ).25,26
In Leicester, we prefer to transplant whole pancreas digest if possible as our earlier experience clearly demonstrated that purification compromised the number of islets recovered.20 At least 40% of islet cell mass will be lost during purification and this is generally less than the amount that would need to be discarded if unpurified digest was employed. At the University of Minnesota, islet purification is preferred except in patients where the tissue volume is so low that there would be no advantage.5
Before transplantation, islet preparations are suspended in a 50 : 50 solution of 20% human serum albumin (Zenalb 20, BioProducts, Laboratories, Elstree, UK) and M199 transplant media (Cambrex, Woking, UK). We infuse the islets into the left lobe of the liver via the portal vein (accessed by way of the middle colic or recannalated umbilical vein) and 5000 units of intravenous heparin is administered routinely (immediately prior to islet infusion) to prevent intra-portal thromboses.
Route of islet transplantation/portal vein access
The most common site for islet autotransplantation is the liver via intraportal infusion. Other sites had been explored both in animal models and in humans with the hope of avoiding the associated risks of portal hypertension, venous thrombosis and infarction. Intrasplenic islet transplantation had successfully led to insulin independence in some animal models but was associated with a high morbidity in humans as a result of splenic infarctions and venous thromboses.21,27–30 The portal system can be accessed via a number of different routes and we routinely infused the islet into the left lobe of the liver alone to allow for a salvage procedure in the event of serious complications.
Umbilical vein
This method of portal venous access has been used for many years for total parenteral nutrition in neonates (where the umbilical vein is patent) and occasionally in adults with intractable vascular access problems. The obliterated umbilical vein (the ligamentum of teres hepatis) can be easily identified and recanalized with a Bakes dilator after transection of the falciform ligament, giving access to the left portal vein for the infusion of islet isolates (Fig. 2). To date, we have used this technique in 16 patients with ultrasound evidence that the vast majority of islets are successfully transplanted into the left lobe of the liver.31 The vein can also be exteriorized by pulling the ligamentum teres through a 10-mm laparoscopic port in the epigastrium and then after being sutured to the skin and covered with a sterile dressing can be accessed for up to 5 days post-operatively for the measurement of portal pressure or theoretically for the infusion of pharmacologically active agents. We have used this technique to monitor portal pressures and we have experienced no intra-operative or post-operative complications related to this.32 In addition, the umbilical vein can also be approached above the umbilicus under local anaesthetic and with the demonstrated safety of this access route would appear to be an ideal route for allotransplantation and has the additional advantage that the exteriorised vein would facilitate repeated transplants if they were required.
Figure 2.
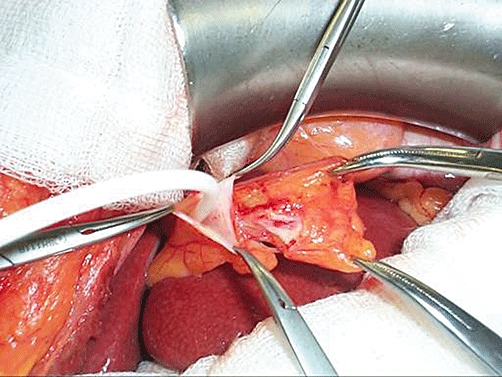
Infusion of islet via a catheter inserted into the recanalized umbilical vein
Middle colic vein or mesenteric vein
Prior to developing a technique for use of the umbilical vein we used to infuse islets into the left lobe of the liver via the middle colic vein. The middle colic vein was identified intra-operatively and cannulated within the transverse mesocolon in a manner similar to other mesenteric vein approach described in the literature.33 Although this can be accessed easily through a minilaparotomy with a Rocky Davies incision, it requires the patient to remain anaesthetized during the islet administration and repeated infusions are not feasible as the vein cannot be exteriorized.
Omental vein
The Minnesota group described the omental vein approach in which an omental tongue around a venous tributary can be fashioned into a pedicle that can then be exteriorized through a transverse lateral abdominal wall incision.34 This approach confers similar advantages to the umbilical vein approach as it allows portal pressure measurements. In addition should the primary total pancreatectomy and gastrointestinal reconstruction be completed significantly before the islet isolation process is completed, the patient can be woken up and the islet infusion performed in the recovery area, thereby reducing the overall duration of anaesthesia.
Transhepatic
Transhepatic islet infusion can be performed under local anaesthesia (generally with sedation) under imaging guidance.35 This is an advantage in the setting of islet allotransplantation and the approach has been adopted by the Edmonton group and is included in their protocol.36 Unfortunately this method of intraportal islet infusion has been associated with frequent minor and occasionally major complications (similar to those experienced with liver biopsy) and these include bleeding, damage to neighbouring structures and thromboses.37,38
Results of islet autotransplantation
The largest series of islet autotransplantation to date are from Minneapolis, Leicester, Cincinnati and Alabama. The outcomes traditionally used to measure the success of total pancreatectomy with islet autotransplantation include metabolic function (insulin requirement), pain control (narcotics requirement), quality of life and the morbidity and mortality associated with the surgery.
Metabolic function
Table 3 compares the results of metabolic function of the four largest series. Islet equivalents serve as an indirect measurement of the β-cell mass infused. The number of islet equivalents isolated is influenced by a number of factors including the extent of fibrosis of the pancreas, previous pancreatic resection and previous surgical drainage procedures. The wide range and standard deviations of islet equivalents reported in the literature reflects the variability in islet isolation experienced by all centres.
Table 3.
Islet yield and metabolic outcome
| Minneapolis | Leicester | Cincinnati | Alabama | |
|---|---|---|---|---|
| Published series where data obtained | Sutherland et al. 200841 | Garcea et al. 20099Webb et al. 200820a | Ahmad et al. 200512 | Argo et al. 20083 |
| Sample size (period of study) | 193 (1977–2007); metabolic follow-up available for 173 patients | 50 (1996–2006) | 45 (2000–2004) | 27 (2005–2007) |
| Time between surgery and islet infusion | Cold ischaemia time = 58+/−24 min Islet preparation time 3.4+/−1.1 h | 2 to 4 h (unpublished data) | ∼4 h | Not reported |
| Islet yield | 1977–1990 (n = 23) IEQ/kg body weight = 2582 +/− 3194 Median 1375 (49–12470) 1991–1994 (n = 15) IEQ/kg body weight = 5296 +/− 4395 Median 4588 (111–17035) 1995–2000 (n = 27) IEQ/kg body weight = 3181 +/− 2523 Median 3121 (225–10000) 2001–2007 (n = 107) IEQ/kg body weight = 3150 +/− 1955 Median 3054 (23–8558) | Median IEQ/g pancreas = 1876 (249–12271)aMedian IEQ transplanted = 130029 (24332–958078)aMedian IEQ/kg body weight = 2245 (405–20385)a | Insulin independet subgroup IEQ/g pancreas = 7304 +/− 1722 IEQ yield = 413542 +/− 70985 IEQ/kg body weight = 6635 +/− 229 Insulin dependent subgroup IEQ/g pancreas = 5025 +/− 968 IEQ yield = 297889 +/− 49480 IEQ/kg body weight = 3799 +/− 629 | Mean IEQ/g pancreas = 2739 +/− 477 Mean IEQ transplanted = 82904 +/− 18223 Mean IEQ/kg body weight = 1331 +/− 304 |
| Glucose control (insulin requirement and C-peptide level) | 32% had full and 33% had partial metabolic function at some post-transplant point | 12 patients (24%) had significant periods (median 16.5 months) of insulin independencea0.17 IU/kg body weight vs. 0.22 IU/kg body weight insulin requirement for purified vs. non-purified groups (P = 0.32)a100% C-peptide positive.a | 40% insulin independence 24% requires 1–20 units/day 20% requires 20–40 units/day 16% requires >40 units/day | None reported insulin independence Mean insulin 17.4 units/day, C-peptide 1 ng/ml, HbA1c 6.7% at 3-months follow up Mean insulin 23 units/day, C-peptide 1.7 ng/ml, HbA1c 7.5% at 6-months follow up |
| Factors influencing metabolic outcome | Islet function correlated with IEQ/kg body weight (P < 0.05). Islet function demonstrated at 1 year for: 32% of recipients with <2500 IEQ/kg had islet function at 1 year 79% of recipients with 2501–5000 IEQ/kg 86% of recipients with >5000 IEQ/kg. | Insulin requirements were similar in recipients of purified and non-purified islets. (P = 0.32)aTrend of increase insulin requirement over time was observed but not statistically significant.a | Higher IEQ/kg body weight in the insulin independent subgroup (P = 0.04) | – |
sample size = 46
The length of time between excision of the pancreas and islet infusion is consistent (between 3 and 4 h) across the series. This is largely as a result of the fact that total pancreatectomy, islet isolation and islet transplantation were carried out in the same site. The pancreata are subjected to a minimal period of cold ischaemia in autotransplantation as opposed to those in allotransplantation where the cold ischaemia time can be highly variable and may have a significant effect on the quantity and quality of the islets isolated.39,40 Islet purification does not improve insulin independence in islet autotransplantation.20
Insulin independence is achieved in up to 40% of patients after total pancreatectomy with islet autotransplantation41 and C-peptide production can be demonstrated in all patients. C-peptide levels also increase after an oral glucose tolerance test and demonstrate little deterioration with time after transplantation.20 Evidence from islet allotransplantation has demonstrated that stimulated C-peptide levels are an early marker of islet allograft function and recipients who are C-peptide positive (even though they may be insulin dependent) are metabolically more stable.42,43 The same is true for islet autotransplantation and C-peptide production is likely to confer long-term protection in a similar fashion to insulin-treated diabetes where preservation of some insulin production improves long-term glycaemic control.
Higher islet equivalents per kilogram of body weight have been shown to correlate with insulin independence15,41 although the correlation is not as straightforward as long-term insulin independence had been demonstrated in patients who received as little as 882 IEQs/kg body weight.44 In addition, a significant proportion of patients who are not insulin independent after islet autotransplantation only require very small amounts of exogenous insulin to achieve excellent control of their blood sugar.3,12,20,41
Islet autotransplantation and allotransplantation contrast in many aspects. Islet allografts can be obtained from single or multiple donor(s) and be given as repeated infusions. Immunosuppression is essential to prevent allograft rejection despite the undesirable diabetogenic side-effect of even the latest steroid free regimens. Finally donor characteristics and duration of cold ischaemia are extremely variable in allotransplantation (principally due to the longer cold ischaemia times with islet allografts). These differences give rise to the discrepancy in the results between islet autotransplantation and islet allotransplantation. Despite the greater number of islet equivalents (per kilogram of body weight) that are transplantated during islet allografting (from single or multiple donors), insulin independence after islet allotransplantation is poorly sustained when compared with islet autotransplantation. Only 71% of recipients of islet allografts who achieved insulin independence retained this status at 1 year and this figure reduces to 52% at 2 year whereas 74% of islet autotransplantation patients who were insulin dependent remained so at 2 years post transplant.41,45
Pain control and quality of life
One of the goals of total pancreatectomy is to improve quality of life by alleviating the intractable abdominal pain which renders these patients dependent on opioid analgesics. All leading centres for total pancreatectomy with islet autotransplantation have demonstrated a significant improvement in pain score after surgery and a significant reduction in the use of opiate analgesia (Table 4). The Cincinnati series also reported a significant improvement in quality of life using a validated standard assessment questionnaire (SF-36).15 In Leicester, a questionnaire was sent out to patients to obtain their assessment on the success of total pancreatectomy in terms of pain relief and none of the patients felt that they had not benefited from the surgery.9
Table 4.
Pain control following total pancreatectomy and islet autotransplantation
| Centres | Minneapolis | Leicester | Cincinnati | Alabama |
|---|---|---|---|---|
| Published series (where data was extracted from) | Gruessner et al. 200419aWahoff et al. 199510b | Garcea et al. 20099 | Ahmad et al. 200512Rodriguez Rilo et al. 200315c | Argo et al. 20083 |
| Opiate requirements | 40% resolved, 32% improved, 12% no change, 16% lost to follow-upa81% of narcotic dependent patients pre-op became independent post-opb | 91% patients requiring opiates pre-op → 40% post-op Only 16% requiring opiate after 60 months | Morphine equivalents: 206 mg (0–1120 mg) pre-op → 90 mg (0–520 mg) post-op (P = 0.005) | 14.3% no narcotic use; 50% decreased; 35.7% no change at 3-months follow up 60% reported no narcotic use; 20% decreased; 20% no change at 6-months follow up |
| Pain score | Pain score 9.6 +/− 0.9 → 3.8 +/− 1.5 (P < 0.05)b | Severity of pain: 10 → 3 (P < 0.05) Frequency of pain: 10 → 2 (P < 0.05) | Pain rating index 37 → 11c (P < 0.01) | Not reported |
Sample size = 112;
Sample size = 48;
Sample size = 22
Morbidity and mortality surrounding surgery
Total pancreatectomy with islet autotransplantation is only carried out in highly specialized centres with both surgical expertise and accredited scientific laboratory facilities. As a consequence, the surgical results after total pancreatectomy in these units are excellent with peri-operative mortality rates of around 2%. Table 5 summarizes the surgical outcomes of total pancreatectomy with islet autotransplantation in the leading centres.
Table 5.
Operative details and surgical morbidity and mortality
| Centres | Minneapolis | Leicester | Cincinnati | Alabama |
|---|---|---|---|---|
| Published series (where data was extracted from) | Sutherland et al. 200841Manciu et al. 199951aWahoff et al. 199510b | Garcea et al. 20099Clayton et al. 200352c | Ahmad et al. 200512Rodriguez Rilo et al. 200315d | Argo et al. 20083 |
| Operative duration | Mean 9 +/− 1.5 ha | Median 8 h (4–11 h) | Mean 533 min (325–725 min) | Mean 6.9 +/− 0.18 h |
| Blood loss | Median 2000 ml (350–12000 ml)a | Median 400 ml (0–2000 ml)c | Mean 563 ml (190–1900 ml) | Mean 632.6 +/− 100.5 ml |
| Length of hospital stay | Median 25 days (9–82 days)a | Median 20 days (8–144 days) | Mean 15.2 days (5–40 days)d | Mean 10.1 +/− 0.78 days |
| Complications | Overall complication reported: 25%b | Overall major complication: 15% | Major: 18%d; minor: 64%d | Overall complication reported: 56% |
| Operative mortality | 2%b | 2% | 6.7% | 0% |
Sample size = 41;
Sample size = 48;
Sample size = 40;
Sample size = 22
Discussion
Total pancreatectomy, although a radical procedure and a significant surgical challenge, may be the only treatment option for a subgroup of patients with chronic pancreatitis. These patients suffer from relentless abdominal pain which frequently produces an appalling quality of life despite maximal medical management. The operation can successfully remove the pain that these patients experience and this is evident by the significant reduction in analgesia requirement reported. However, a small proportion of patients remain opioid dependent. It is important to note that patients with chronic pancreatitis often have other co-morbidities which continue to cause pain symptoms necessitating opioid analgesia. In addition, prolonged opioid dependence in some of the patients may have led to addiction accounting for their ongoing requirement post pancreatectomy.
Without preservation of the islet cell mass exogenous insulin therapy is required post-operatively to prevent the complications of diabetes but may require intensive regimens and subject these patients to the risk of serious hypoglycaemia.5 In addition, retention (or restoration) of some insulin production considerably simplifies the management of diabetes and reduces the long-term complication rate.38
Autotransplantation of islets recovered from the resected pancreas will preserve a proportion of the beta cell mass and result in some endogenous insulin production. Even although insulin independence cannot not be achieved in a significant proportion of patients after islet autotransplantation, the control of their diabetes is superior to those patients who have a total pancreatectomy where islet transplantion is not possible.9
The first total pancreatectomy with islet autotransplantation was carried out in 1977 at the University of Minnesota, Minneapolis.13 Since then, there have been over 300 total or near-total pancreatectomies with islet autotransplantation performed worldwide, with promising results in terms of insulin independence and long-term islet graft function.5,15 In addition, the sustained C-peptide secretion may have a protective effect against some undesirable diabetic complications.20 The three largest series of islet autotransplantation are from Minneapolis and Cincinnati (United States of America) and Leicester (United Kingdom). All series describe similar populations with comparable demographics and the aetiology of their chronic pancreatitis. The success of islet autotransplantation compares favourably with that of islet allotransplantation for patients with insulin-dependent diabetes despite the advances after the widespread application of the Edmonton's protocol, and the long-term results are considerably better.46–49 The differences are almost certainly explained by allograft rejection and the diabetogenic effects of even the steroid-free immunosuppressive regimens required in patients receiving allotransplants.
There is considerable evidence for a direct correlation between islet yield and insulin independence,50 and in patients who have exhausted all non-surgical options early referral may facilitate assessment and a decision about suitability for treatment. It is likely that treatment at an earlier stage would improve the outcome of an islet autotransplantation, particularly if it could be performed prior to the development of significant endocrine deficiency associated with the disease progression. Arguably, in patients with severe abdominal pain secondary to small duct disease and in those where endoscopic or percutaneous procedures have been unsuccessful, total pancreatectomy and islet autotransplantation should be considered the first and definitive surgical treatment. This could significantly reduce the operative risks as a result of the dense adhesions and fibrosis that are often encountered in patients who had previous surgical procedure(s). In addition, by maximizing the islet yield it increases the potential for insulin independence and improves the patient's quality of life at an earlier stage. Efforts directed towards the education of patients, general practitioners and gastroenterologist are essential to facilitate this early referral of patients with chronic pancreatitis for assessment in units that can offer islet autotransplantation.
Conflicts of interest
None declared.
References
- 1.Anderson D. Mechanisms and emerging treatments of the metabolic comnplications of chronic pancreatitis. Pancreas. 2007;35:1–15. doi: 10.1097/mpa.0b013e31805d01b0. [DOI] [PubMed] [Google Scholar]
- 2.Bornman PC, Marks IN, Girdwood AW, Berberat PO, Gulbinas A, Buchler MW. Pathogenesis of pain in chronic pancreatitis: ongoing enigma. World J Surg. 2003;27:1175–1182. doi: 10.1007/s00268-003-7235-x. [DOI] [PubMed] [Google Scholar]
- 3.Argo JL, Contreras JL, Wesley MM, Christein JD. Pancreatic resection withislet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74:530–536. [PubMed] [Google Scholar]
- 4.Helling T. Surgical management of chronic pancreatitis and the role of islet cell transplantation. Curr Surg. 2003;60:463–469. doi: 10.1016/S0149-7944(02)00789-4. [DOI] [PubMed] [Google Scholar]
- 5.Blondett J, Carlson A, Kobayashi T, Jie T, Belling M, Hering B, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007;87:1477–1501. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt C, Gall FP, Muhe E, Lauterwald A. Is total pancreatectomy still responsible treatment for chronic pancreatitis? Langenbecks Arch Chir. 1979;350:129–137. doi: 10.1007/BF01234295. [DOI] [PubMed] [Google Scholar]
- 7.Nathan H, Wolfgang CL, Edil BH, Choti MA, Herman JM, Schulick RD, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 8.Muller M, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- 9.Garcea G, Weaver J, Phillips J, Pollard CA, Illouz SC, Webb MA, et al. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients. Pancreas. 2009;38:1–7. doi: 10.1097/MPA.0b013e3181825c00. [DOI] [PubMed] [Google Scholar]
- 10.Wahoff D, Papalois B, Najarian J, Kendall D, Farney A, Leone J, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222:562–575. doi: 10.1097/00000658-199522240-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton H, Davies J, Pollard C, White S, Musto P, Dennison A. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the Leicester General Hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad S, Lowy A, Wray C, D'Alessio D, Choe K, James L, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in pateints with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland DE, Matas AJ, Najarin JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58:365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- 14.White S, Sutton C, Weymss-Holden S, Berry D, Pollard C, Rees Y, et al. The feasibility of spleen-preserving pancreatectomy for end-stage chronic pancreatitis. Am Surg. 2000;179:294–297. doi: 10.1016/s0002-9610(00)00333-0. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez Rilo H, Ahmad S, D'Alessio D, Iwanaga Y, Kim J, Choe K, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978–989. doi: 10.1016/j.gassur.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Manciu N, Beebe D, Tran P, Gruessner R, Sutherland D, Belani K. Total pancreatectomy with islet cell autotransplantation: anesthetic implications. J Clin Anesth. 1999;11:576–582. doi: 10.1016/s0952-8180(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 17.Farney A, Najarian J, Nakhleh R, Lloveras G, Field M, Gores P, et al. Autotransplantation of dispersed pancreatic islet tissue combined with total or near-total pancreatectomy for treatment of chronic pancreatitis. Surgery. 1991;110:427–437. [PubMed] [Google Scholar]
- 18.Robertson G, Dennison A, Johnson P, London N. A review of pancreatic islet autotransplantation. Hepatogastroenterology. 1998;45:226–235. [PubMed] [Google Scholar]
- 19.Gruessner R, Sutherland D, Dunn D, Najarian J, Jie T, Hering B, et al. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2004;198:559–567. doi: 10.1016/j.jamcollsurg.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 20.Webb M, Ilouz S, Pollard C, Gregory R, Mayberry J, Tordoff S, et al. Islet auto transplantation following total pancreatectomy. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 21.White S, London N, Johnson P, Davies J, Pollard C, Contractor H, et al. The risks of total pancreatectomy and splenic islet autotransplantation. Cell Transplant. 2000;9:19–24. doi: 10.1177/096368970000900103. [DOI] [PubMed] [Google Scholar]
- 22.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl. 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 23.Casey JJ, Lakey JR, Ryan EA, Paty BW, Owen R, O'Kelly K, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74:913–915. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- 24.London NJ, Chadwick DR, Johnson PRV, White S, Robertson GSM, et al. Approaches to islet purification. In: Lanza P, Chick W, editors. Pancreatic Islet Transplantation. Austin, TX: RG Landes; 1995. pp. 23–58. [Google Scholar]
- 25.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 26.White SA, Contractor HH, Hughes DP, Johnson PR, Clayton HA, Bell PR, et al. Influence of different collagenase solvents and timing of their delivery on porcine islet isolation. Br J Surg. 1996;83:1350–1355. doi: 10.1002/bjs.1800831008. [DOI] [PubMed] [Google Scholar]
- 27.White S, Robertson G, Davies J, Rees Y, London N, Dennison A. Splenic infarction after total pancreatectomy and autologous islet transplantation into the spleen. Pancreas. 1999;18:419–421. doi: 10.1097/00006676-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 28.White S, Birch J, Forshaw M, Power D, Dennison A. Splenic human islet autotransplantation: anatomical variations of splenic venous drainage. Transplant Proc. 1998;30:314. doi: 10.1016/s0041-1345(97)01284-0. [DOI] [PubMed] [Google Scholar]
- 29.Kneteman N, Warnock G, Evans M, Nason R, Rajotte R. Prolonged function of canine pancreatic fragments autotransplanted to the spleen by venous reflux. Transplantation. 1990;49:679–681. doi: 10.1097/00007890-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Gray D, Warnock G, Sutton R, Peters M, McShane P, Morris P. Successful autotransplantation of isolated islets of Langerhans in the cynomolgus monkey. Br J Surg. 1986;73:850–853. doi: 10.1002/bjs.1800731029. [DOI] [PubMed] [Google Scholar]
- 31.Ong SL, Pollard CA, Rees Y, Garcea G, Webb MA, Illouz SC, et al. Ultrasound changes within the liver after total pancreatectomy and intrahepatic islet cell autotransplantation. Transplantation. 2008;85:1773–1777. doi: 10.1097/TP.0b013e31817348d6. [DOI] [PubMed] [Google Scholar]
- 32.Kelkar A, White SA, White SD, Davies JE, Clayton H, Pollard C, Dennison A. A simple method for islet infusion during total pancreatetomy and islet autotransplantation: allows portal pressure measurement and possible therapeuric access. Acta Diabetol. 2003;40:197. [Google Scholar]
- 33.Osama Gaber A, Chamsuddin A, Fraga D, Fisher J, Lo A. Insulin independence achieved using the transmesenteric approach to the portal vein for islet transplantation. Transplantation. 2004;77:309–311. doi: 10.1097/01.TP.0000101509.35249.A0. [DOI] [PubMed] [Google Scholar]
- 34.Nath DS, Kellogg TA, Sutherland DE. Total pancreatectomy with intraportal auto-islet transplantation using a temporarily exteriorised omental vein. Am Col Surg. 2004;199:994–995. doi: 10.1016/j.jamcollsurg.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Owen RJ, Ryan EA, O'Kelly K, Lakey JR, McCarthy MC, Paty BW, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229:165–170. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 37.Ryan E, Paty B, Senior P, Shapiro A. Risks and side effects of islet transplantation. Curr Diab Rep. 2004;4:304–309. doi: 10.1007/s11892-004-0083-8. [DOI] [PubMed] [Google Scholar]
- 38.Ryan E, Lakey J, Rajotte R, Korbutt G, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 39.Kim SC, Han DJ, Kang CH, We YM, Back JH, Kim YH, et al. Analysis on donor and isolation-related factors of successful isolation of human islet of Langerhans from human cadaveric donors. Transplant Proc. 2005;37:3402–3403. doi: 10.1016/j.transproceed.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 40.Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland DE, Gruessner AC, Carlson AM, Blondet JJ, Balamurugan AN, Reigstad KF, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paty BW, Senior PA, Lakey JR, Shapiro AM, Ryan EA. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther. 2006;8:165–173. doi: 10.1089/dia.2006.8.165. [DOI] [PubMed] [Google Scholar]
- 43.Baidal DA, Faradji RN, Messinger S, Froud T, Monroy K, Ricordi C, et al. Early metabolic markers of islet allograft dysfunction. Transplantation. 2009;87:689–697. doi: 10.1097/TP.0b013e318195c249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb MA, Illouz SC, Pollard CA, Musto PP, Berry D, Dennison AR. Long-term maintenance of graft function after islet autotransplantation of less than 1000 IEQ/kg. Pancreas. 2006;33:433–434. doi: 10.1097/01.mpa.0000236732.54488.9e. [DOI] [PubMed] [Google Scholar]
- 45.2008 Collaborative Islet Transplant Registry (CITR) Annual Report. Available from URL: http://www.citregistry.com (accessed 13 September 2009.
- 46.Shapiro A, Ricordi C, Hering B, Auchincloss H, Lindblad R, Robertson R, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 47.Ryan E, Paty B, Senior P, Bigam D, Alfadhili E, Kneteman N, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 48.Robertson R, Lanz K, Sutherland D, Kendall D. Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes. 2001;50:47–50. doi: 10.2337/diabetes.50.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland D, Gruessner A, Carlson A, Blondett J, Balamurugan A, Reigstad K, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pyzdrowski K, Kendall D, Halter J, Nakhleh R, Sutherland D, Robertson R. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med. 1992;327:220–226. doi: 10.1056/NEJM199207233270402. [DOI] [PubMed] [Google Scholar]
- 51.Manciu N, Beebe DS, Tran P, Gruessner R, Sutherland DE, Belani KG. Total pancreatectomy with islet cell autotransplantation: anesthetic implications. J Clin Anesth. 1999;11:576–582. doi: 10.1016/s0952-8180(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 52.Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the leicester general hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]


