Abstract
Background:
The aim of the present study was to determine whether raised pre-operative serum creatinine increased the risk of renal failure after liver resection.
Method:
Data were studied from 1535 consecutive liver resections. Outcomes in patients with pre-operative creatinine ≤124 µmol/l (Group 1) were compared with those with pre-operative creatinine ≥125 µmol/l (Group 2).
Results:
The median age of the 1446 (94.3%) patients resected in Group 1 was 62 years compared with 67 years in the 88 (5.7%) patients in Group 2 (P < 0.0001). Similarly this latter group had double the number of patients who were American Society of Anesthesiologists (ASA) III or IV (34.1% vs. 15.2%, P= 0.00004). Overall, the incidence of post-operative renal failure requiring haemofiltration was low (0.9%) but significantly more in Group 2 patients (5.7% vs. 0.6, P= 0.0007). In addition, patients in Group 2 were more likely to suffer acute kidney injury post-operatively (18.2% vs. 4.3%, P < 0.0001). Patients with acute kidney injury had significantly higher blood loss. Although there was no difference in mortality, patients in Group 2 had higher post-operative morbidity (37.5%) than Group 1 (21.7%, P= 0.0006), with the incidence of cardiorespiratory complications being higher in Group 2 (25.9% vs. 8.9%, P= 0.0025).
Conclusions:
After liver resection, renal failure is rare but patients with an elevated creatinine pre-operatively are at an increased risk of both renal and non-renal complications.
Keywords: liver resection, complications, renal failure, acute kidney injury
Introduction
Twenty years ago, blood loss and subsequent blood transfusion was recognized as important in the short- and longer-term outcomes for patients undergoing liver resection and this remains the case today.1,2 Consequently, surgical and anaesthetic techniques have evolved in the quest for ‘bloodless surgery’ and now peri-operative blood transfusion is the exception rather than the rule.3
Two key manoeuvres are used to reduce blood loss during hepatic transection: portal triad clamping and low central venous pressure (CVP) anaesthesia. Portal triad clamping, first described by Pringle in 1908, reduces hepatic arterial and portal venous bleeding.4,5 Although a recent European survey demonstrated that the use of inflow occlusion is not universal, it did confirm that most hepatic surgeons resort to it in difficult cases and that experienced surgeons are more likely to use it routinely.6 During the transaction, a low CVP reduces back bleeding from hepatic veins.7–9 These techniques test the patients' cardiovascular reserve. Obstructing the portal blood flow causes venous congestion of the bowel and in combination with warm ischaemic liver injury, results in a flush of anaerobic metabolites and cytokines back into the circulation on release of the clamp.10 Low CVP anaesthesia relies on patients being maintained in a hypovolaemic state until liver resection has been completed.7,8 This is in contrast to most other major surgical procedures, where patients have large volumes of crystalloid and colloid peri-operatively. Moreover, vasodilators are often used to further reduce CVP, leading to distributive changes in blood flow.7 A potential consequence of such circulatory changes is acute tubular necrosis (ATN), with subsequent renal impairment or failure.11
Studies have shown that 3–7% of patients require renal replacement therapy after liver resection.8,11 The aim of this study was to determine the outcome of liver resection performed under low CVP anaesthesia with intermittent inflow occlusion, in patients with a pre-operative serum creatinine ≥125 µmol/l (compared with patients with a serum creatinine ≤124 µmol/l). The primary endpoint was the requirement for renal replacement therapy as a consequence of acute kidney injury, with overall peri-operative morbidity and mortality as secondary endpoints.
Methods
Study design
This was a study of consecutive patients undergoing elective hepatic resection at a single tertiary referral centre between February 1991 and August 2008. Patient data were collected prospectively on a database (Access®, Microsoft Corporation, Redmond, WA, USA) comprising 268 data fields. This was compiled contemporaneously using standardized proformas and encompassed patient symptoms, pre-operative assessment, surgical treatment, post-operative course, histopathology and long-term outcomes. Patients operated on prior to February 1991 were excluded, as this was prior to the introduction of low CVP anaesthesia in our unit. A single patient who was on long-term renal replacement therapy was also excluded from the study.
All patients had their urea and electrolytes measured pre-operatively. The upper limit of the normal reference range for creatinine in our unit was 124 µmol/l, representing two standard deviations above the mean. Patients were divided into two groups depending on their pre-operative serum creatinine: Group 1 – those with a serum creatinine ≤124 µmol/l and Group 2 – those with a serum creatinine ≥125 µmol/l.
The surgical and anaesthetic techniques used for liver resection have been previously described by this unit and are used as a matter of routine.7 During the transection of the hepatic parenchyma, low CVP anaesthesia and intermittent portal inflow occlusion were used. The CVP was controlled by balancing judicious fluid and intravenous nitroglycerine administration against blood loss, with the aim of mainataing a CVP 0–4 mmHg during transection. These parameters were usually acheived, without compromising systemic hypotension. In this event, however, vasopressors (e.g. dopamine, phenylephrine) and fluid boluses were used to maintain what was considered safe cardiovascular status by the anaesthetist. Inflow occlusion was typically performed for 20 min, followed by 5 min reperfusion time, and then repeated. Inflow occlusion was kept to a minimum, ideally less than 60 min in total.
The nomenclature and extent of hepatic resection were recorded according to the Brisbane 2000 Terminology of Liver Anatomy and Resections.12 Major liver resection was defined as the resection of three or more hepatic segments. A minor resection was defined as the resection of fewer than three segments, including wedge resections.
Post-operative care was standardized. All patients had level 2 care for the first 24 h. Patients requiring ventilatory or circulatory support were admitted to the intensive care unit for level 3 care. Peri-operative pain relief was provided with either thoracic epidural analgesia or more recently, a regional intermuscular bupivicaine infusion combined with patient controlled-analgesia and paracetemol.13 Fluid management comprised a background infusion of Gelofusin® (B. Braun, Melsungen, Germany) and 10% dextrose, with additional boluses of Gelofusin® dictated by urine output. Patients were allowed to eat and drink from day one as tolerated. Non-steroidal anti-inflammatory drugs (NSAIDs) were used routinely for post-operative analgesia in patients once they were eating, unless they had specific contraindications (allergy, asthma, peptic ulceration and renal impairment).
Peri-operative mortality was defined as a death during the same hospital admission or within 90 days of the date of the operation. Post-operative complications were classified as slight, relevant (delayed patient discharge) or life threatening (complications requiring urgent or level 3 care with medical or surgical intervention).
Glomerular filtration rate (GFR) is now routinely estimated in some laboratories. The data collected on the patients studied in this paper largely pre-dates this. Furthermore, patients' weight was not recorded, preventing retrospective estimation of GFR. The patients are therefore considered in two groups, based on their serum creatinine as stated above. Serum creatinine gives an approximate guide to glomerular filtration rate and when raised, is suggestive of renal impairment. Serum creatinine can be affected by muscle mass and hydration status.14 However, the large size of the study controls for this type of inherent variation amongst patients. Post-operative change in serum creatinine was used to determine the presence or absence of an acute kidney injury, as defined in a consensus statement from the Acute Kidney Injury Network.15 Patients were regarded as having acute kidney injury if the serum creatinine increased by 50% or more, or by 26.4 µmol/l or more above the pre-operative level within 48 h of surgery. The severity of the injury was staged from I to III, as detailed in Table 3.
Table 3.
The frequency of acute kidney injury in patients undergoing hepatic resection with curative intent. Patients are grouped according to whether they have normal (≤124 µmol/l) (Group 1) or elevated (≥125 µmol/l) (Group 2) pre-operative serum creatinine
| N |
Group 1 |
Group 2 |
P-value | |
|---|---|---|---|---|
| 1447 | 88 | |||
| Acute kidney injury | Stage I | 50 (3.5) | 11 (12.5) | 0.0001a |
| Stage II | 9 (0.6) | 3 (3.4) | 0.0275b | |
| Stage III | 3 (0.2) | 2 (2.3) | 0.0290b | |
| Any stage (Total) | 62 (4.3) | 16 (18.2) | 0.0001a | |
| Patients requiring haemofiltration | 9 (0.6) | 5 (5.7) | 0.0007b | |
Quantification of acute kidney injury, based on serum creatinine measurements within the first 48 h of surgery.15
Stage I: Cr ≥ 1.5–2.0 fold baseline or a rise of 26.4 µmol/l.
Stage II: Cr = 2.0–3.0 fold baseline.
Stage III: Cr > 3.0 fold baseline or ≥354 µmol/l.
χ2-test.
Fisher's exact test.
Numbers in parentheses represent percentages.
Study outcome measures
The primary outcome measure of this study was post-operative renal failure necessitating haemofiltration. Secondary outcomes measured included the development of an acute kidney injury (as defined above). Intra-operative parameters such as operation time (taken from induction of anaesthesia to the patient leaving the operating room at the end of surgery), blood loss, post-operative morbidity and mortality were also compared.
Statistical analysis
Data were entered on a Statistical Package for the Social Sciences (version 9) software package (SPSS Inc., Chicago, IL, USA). Continuous data was expressed as the median, with the range in parenthesis. Analysis of categorical data was performed using the Pearson χ2 test or Fisher's exact test if events were ≤5. Continuous variables were analysed using the Mann–Whitney U-test.
Results
During the study period, 1535 patients underwent liver resection in our unit. Of these, 88 (5.7%) had a pre-operative serum creatinine ≥125 µmol/l (Group 2). The patient demographics of both groups are shown in Table 1. Patients in Group 2 had a higher median age compared with those in Group 1 (67 years vs. 62 years, P < 0.0001) and were more likely to be male (89.8% vs. 56.4%, P < 0.0001). The indication for surgery and the prevalence of background liver disease were similar in both groups. However, patients in Group 2 had a significantly higher ASA,16 with a greater proportion of them being ASA grades 3 and 4 (P= 0.00004).
Table 1.
Patient demographic characteristics and operative details of patients undergoing hepatic resections with a curative intent. Patients are grouped according to whether they have normal (≤124 µmol/l) (Group 1) or elevated (≥125 µmol/l) (Group 2) pre-operative serum creatinine
| Group 1 n = 1447 | Group 2 n = 88 | P-value | |
|---|---|---|---|
| Median age (range) | 62 (21–86) | 67 (21–81) | <0.0001a |
| Gender | |||
| Male | 816 (56.4) | 79 (89.8) | <0.0001b |
| Indication for liver resection | |||
| Colorectal metastases | 1194 (82.5) | 75 (85.2) | 0.51b |
| Other neoplasms | 207 (14.3) | 11 (12.5) | 0.63b |
| Benign disease | 46 (3.2) | 2 (2.3) | 1.0c |
| Background liver histology | |||
| Normal | 1138 (78.6) | 68 (77.3) | 0.76b |
| Steatotic | 252 (17.4) | 18 (20.4) | 0.56b |
| Cirrhotic | 12 (0.8) | 0 (0) | 0.47c |
| Unknown | 45 (3.2) | 2 (2.3) | 1.0c |
| ASA Score | |||
| I and II | 1018 (70.4) | 48 (54.5) | 0.0018b |
| III and IV | 222 (15.3) | 30 (34.1) | 0.00004b |
| Unknown | 207 (14.2) | 10 (11.4) | 0.4419b |
| Major liver resection | 880 (60.8) | 46 (52.3) | 0.112b |
Mann-Whitney U-test.
χ2-test.
Fisher's exact Test.
Numbers in parentheses represent percentages unless otherwise stated.
ASA, American Society of Anaesthesia pre-operative assessment score.16
The magnitude of the liver resection, duration of the surgery and blood loss was similar in both groups (Table 2). The median (range) duration of inflow occlusion was 39 (5–104) min in Group 1, comparable to that of 38 (8–110) min in Group 2 (P= 0.7107). The median length of stay was longer in patients in Group 2 [9 (4–33) days], compared with the patients in Group 1 [8 (2–97) days], but this failed to reach statistical significance (P= 0.052).
Table 2.
Intra- and post-operative details of patients undergoing hepatic resections with a curative intent. Patients are grouped according to whether they have normal (≤124 µmol/l) (Group 1) or elevated (≥125 µmol/l) (Group 2) pre-operative serum creatinine (median and range)
| Risk factors | Group 1 n= 1447 | Group 2 n= 88 | P-value |
|---|---|---|---|
| Duration of surgery (min) | 240 (60–730) | 260 (90–675) | 0.065a |
| Duration of inflow occlusion (min) | 39 (5–104) | 38 (8–110) | 0.7107a |
| Blood loss (ml) | 350 (10–9998) | 372 (10–9252) | 0.125a |
| Length of hospital stay | 8 (2–97) | 9 (4–33) | 0.052a |
Mann–Whitney U-test.
After liver resection, serum creatinine was measured daily and patients were stratified according to their pre-operative creatinine levels (Fig. 1). Patients in Group 2 were arbitrarily subdivided into those with a pre-operative creatinine between 125 µmol/l and 154 µmol/l (n= 72) and those whose serum creatinine was 155 µmol/l or more (n= 16). Figure 1 shows that there was little change in serum creatinine in the immediate post-operative period, even for patients with a pre-operative serum creatinine between 125 µmol/l and 154 µmol/l. However, in patients with a pre-operative serum creatinine ≥155 µmol/l, there was a much less predictable effect of liver surgery on post-operative serum creatinine levels, as shown by the wide inter-quartile range in this group (Fig. 1).
Figure 1.
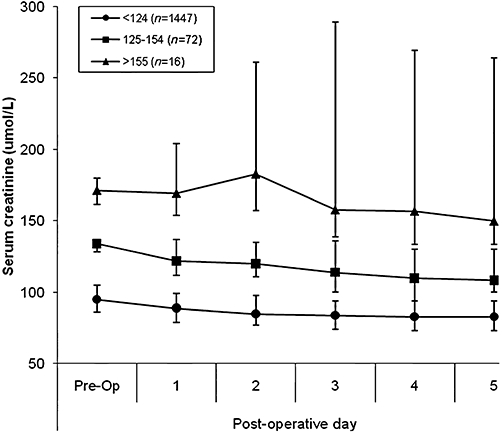
Chart showing the effect of hepatic resection on post-operative changes in serum creatinine in three patient groups according to pre-operative serum creatinine (<124, 125-154 and >155 µmol/l) The data are shown as the median and inter-quartile range of each group.
The primary outcome measure of this study was post-operative renal failure necessitating haemofiltration. Overall, the incidence of this post-operative complication was extremely low [14/1535 patients (0.9%)] (Table 3). However, a significantly higher percentage of patients in Group 2 [5/88 (5.7%)] required haemofiltration, compared with those in Group1 [9/1447 (0.6%), P= 0.0007]. Factors considered to have pre-disposed or contributed to patients developing acute renal failure following surgery are shown in Table 4. The majority of patients (5 of 9) in Group 1 who required haemofiltration had a significant peri-operative event. Three suffered significant blood loss leading to transient hypotension, one had an air embolus and another a myocardial infarction that contributed to or precipitated acute renal failure. In contrast, three out of the five patients in Group 2 that required haemofiltration underwent an uneventful surgical procedure. One patient in group 2 was given NSAIDs, contrary to the usual protocol.
Table 4.
Factors considered to have predisposed or directly contributed to acute renal failure requiring haemofiltration. Patients are grouped according to whether they have normal (≤124 µmol/l) (Group 1) or elevated (≥125 µmol/l) (Group 2) pre-operative serum creatinine
| Patient | Diabetes | IHD/HT | Peri-operative event | NSAIDs | Late sepsis/multi-organ failure | |
|---|---|---|---|---|---|---|
| Group 1 | 1 | ✓ | ||||
| 2 | Hypotension | |||||
| 3 | Hypotension | |||||
| 4 | ✓ | |||||
| 5 | Hypotension | |||||
| 6 | ||||||
| 7 | ✓ | ✓ | Air Embolus | |||
| 8 | ✓ | ✓ | ||||
| 9 | ✓ | ✓ | MI | |||
| Group 2 | 1 | ✓ | ✓ | |||
| 2 | ✓ | |||||
| 3 | ✓ | |||||
| 4 | ✓ | |||||
| 5 | ✓ | ✓ |
HT, hypertension; IHD, ischaemic heart disease; MI, myocardial infarction; ✓, predisposing factor present.
Table 3 also shows the number of patients in Groups 1 and 2 who developed an acute kidney injury (Stage I–III) in the immediate post-operative period. It shows that significantly more patients in Group 2 (18.2%) suffered an acute kidney injury compared with those patients in Group 1 (4.3%, P= 0.0001). The median blood loss of the patients who developed an acute kidney injury was significantly higher in both groups [Group 1: 629.5 vs. 330 ml (P < 0.0001), Group 2: 662.5 vs. 372 (P= 0.003)]. Furthermore, the operation time was significantly longer [Group 1: 300 vs. 240 min (P < 0.0001), Group 2: 292.5 vs. 260 min (P= 0.043)]. There was no difference in inflow occlusion times. Acute kidney injury increased the length of hospital stay in Group 1: 11.5 vs. 8 days (P < 0.0001) but not significantly in Group 2: 10 vs. 9 days (P= 0.073). None of the patients who required post-operative haemofiltration required long-term dialysis. Amongst the patients who required haemofiltration, two out of nine died in Group 1 and one out of five died in Group 2.
Differences in morbidity and mortality were studied as secondary outcome measures in the two groups of patients. While there was no significant difference in mortality between the groups (1.7 vs. 3.4%, P= 0.21), patients in Group 2 had significantly higher post-operative morbidity (37.5%) compared with those in Group 1 (21.7%, P= 0.0006). Furthermore, they were significantly more likely to be life threatening (Table 5). There were 397 complications in 314 patients in Group 1 and 55 complications in 33 patients in Group 2. Thus not only was post-operative morbidity more common in patients in Group 2, but also these patients were more likely to experience multiple complications. Figure 2 shows that there was no difference in the frequency of surgical complications (e.g. biliary fistula, wound infection), or hepatic insufficiency between the two groups. However, patients in Group 2 had a significantly higher incidence of cardiac, respiratory and renal/urinary tract complications post-operatively.
Table 5.
Post-operative morbidity and mortality of patients undergoing hepatic resections with a curative intent. Patients are grouped according to whether they have normal (≤124 µmol/l) (Group 1) or elevated (≥125 µmol/l) (Group 2) pre-operative serum creatinine
| Post-operative adverse events | Group 1 n = 1447 | Group 2 n = 88 | P-value |
|---|---|---|---|
| All complications | 314 (21.7) | 33 (37.5) | 0.0006a |
| Slight | 108 (34.4) | 11 (33.3) | 0.0863a |
| Relevant | 141 (44.9) | 13 (39.4) | 0.1275a |
| Life threatening | 65 (20.7) | 9 (27.3) | 0.0147a |
| Mortality | 25 (1.7) | 3 (3.4) | 0.2137b |
χ2-test.
Fisher's exact test.
Numbers in parentheses represent percentages.
Figure 2.
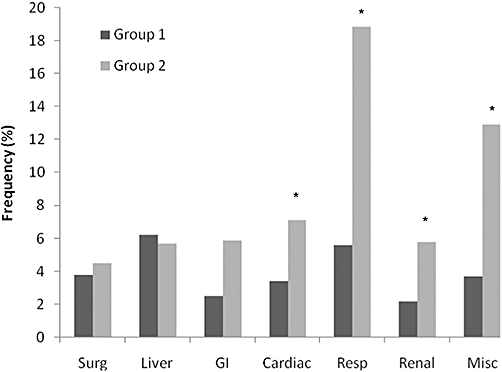
Chart showing the relative frequency of individual complications between Groups 1 and 2. There were 397 complications in 314 patients in Group 1 (creatinine ≤ 124 µmol/l) and 55 complications in 33 patients in Group 2 (creatinine ≥ 125 µmol/l) *P < 0.05 (χ2-test).
Discussion
This study shows that the incidence of acute renal failure requiring renal replacement therapy after liver resection is low (<1%). This is a testament to modern surgery, anaesthesia and critical care. It also confirms that the routine use of low CVP anaesthesia and intermittent inflow occlusion is safe. However, patients with a pre-operative serum creatinine ≥125 µmol/l have a 4.3-fold risk of acute kidney injury and a 9.5-fold risk of requiring haemofiltration compared with those with a pre-operative serum creatinine ≤124 µmol/l. The group of patients who suffered acute kidney injury had significantly higher blood loss and operation time. Furthermore, they have significantly more non-renal complications, particularly respiratory. Although these findings may be expected, this study provides clear evidence that performing liver resections in patients with serum creatinine ≥125 µmol/l is associated with greater morbidity, where little other evidence exists. Although patients in group 2 appeared to have a more complex course, their median hospital stay was not significantly increased. The data analysed in this study covers a period of 17 years and the median length of hospital stay following uncomplicated liver resection has undoubtedly reduced during this period, in keeping with modern surgical practice. This factor has probably reduced the observed impact of complications on the length of hospital stay.
After liver resection, the low rate of renal failure in this series is the same (0.9%) as another reported series of 5853 patients undergoing major abdominal surgery and lower than other reported rates after liver resection (3–7%).8,11,17 The series of patients studied by Melendez routinely had low CVP anaesthesia and portal triad clamping. The anaesthetic technique was similar to that used in our patients, with respect to fluid restriction but differed in that their patients were positioned 15° head down. Moreover, in their study nitrates were used when required, rather than routinely. Saner does not detail their anaesthetic technique.11
There are three main factors which may contribute to the development of renal failure after liver resection. Elderly patients and those with conditions such ashypertension, atherosclerosis or chronic kidney disease are at risk.18 These patients have a reduced capacity for neurohumeral autoregulation of glomerular blood flow during surgery and thus an increased risk of ATN.18 Certainly, the patients in Group 2 were significantly older, with more comorbidity as indicated by their higher ASA scores, compared with those in Group 1. Specific co-morbidity was not routinely recorded in our database; however, it would be of interest to determine the role of diabetes, hypertension and ischaemic heart disease in the future. Peri-operative use of NSAIDs may also impair normal autoregulation of glomerular perfusion through inhibition arteriolar dilatory prostaglandins.18 In this study, oral NSAIDs were used routinely for post-operative analgesia once patients were tolerating diet, but avoided in patients with renal impairment.
The second factor relates to the ‘hit’ of surgery. Two key players in the pathogenesis of ATN are hypovolaemia and renal damage by inflammatory mediators.11 Both these events are predicable in every hepatic resection that employs low CVP anaesthesia and portal inflow occlusion. A raised pre-operative serum creatinine appears to clearly identify the group of patients that are most susceptible to this surgical ‘hit’.
The third factor which contributes to the aetiology of renal failure after liver resection is a low perfusion state either secondary to cardiac dysfunction or distributive circulatory changes, such as sepsis or hepatorenal failure.11,18 Patients often have more than one factor contributing to post-operative renal dysfunction. The potential consequences of acute kidney injury include increased risk of mortality and may contribute to the development of chronic kidney disease.15
Despite the differences in age and comorbidity, this study showed no difference in the rate of surgical complications between the two groups. Systemic complications accounted for the higher rates of post-operative morbidity in patients with raised serum creatinine. However, the relative risk of complications in this series of patients (1.72) is similar to that previously observed in patients with a raised serum creatinine in another large series reported by Jarnagin (1.78).19 In this study of 1803 patients, 59.2% with an elevated serum creatinine suffered post-operative morbidity, compared with 45.0% with normal serum creatinine (P= 0.118). When these patients were grouped according to those with complications vs. those with no complications, the group with complications had higher mean serum creatinine levels (0.92 vs. 0.99 mg/dl). Elevated serum creatinine was also associated with higher mortality (6.3% vs. 2.9%, P= 0.037). Other authors have also found that raised pre-operative serum creatinine predicts in hospital mortality.20 However, in this Asian series, a much greater proportion of patients (59.2%) had a background of chronic liver disease.20
In conclusion, while renal failure following liver resection is rare, patients with an elevated pre-operative creatinine are at increased risk of both renal and non-renal complications. Identification of this high-risk group dictates that level 2 critical care is mandatory for these patients in the early post-operative period, in order to optimize fluid balance and cardiac output, while maintaining a low threshold for haemofiltration. Furthermore, we have identified a group of patients who need different advice in the process of obtaining informed consent. This study also reinforces the need to minimize blood loss in liver surgery. However, the majority of patients with renal impairment can be offered a major liver resection with a successful post-operative outcome.
Acknowledgments
The authors thank Christine Murray and Verity Bonwitt for their input in to data recording and management.
Conflicts of interest
None declared.
References
- 1.Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–687. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw IM, Rees M, Welsh FK, Bygrave S, John TG. Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable long-term survival. Br J Surg. 2006;93:457–464. doi: 10.1002/bjs.5323. [DOI] [PubMed] [Google Scholar]
- 4.Pringle JH. Notes on the arrest of hepatic haemorrhage due to trauma. Ann Surg. 1908;48:541. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Bilt JD, Livestro DP, Borren A, van Hillegersberg R, Borel Rinkes IH. European survey on the application of vascular clamping in liver surgery. Dig Surg. 2007;24:423–435. doi: 10.1159/000108325. [DOI] [PubMed] [Google Scholar]
- 7.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–1529. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 8.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 9.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204–211. doi: 10.1093/bja/aeh195. [DOI] [PubMed] [Google Scholar]
- 11.Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40:1221–1224. doi: 10.1016/j.transproceed.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 12.Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden OJ, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 13.Basu S, Tamijmarane A, Bulters D, Wells J, John T, Rees M. An alternative method of wound pain control following hepatic resection: a preliminary study. HPB (Oxford) 2004;6:186–189. doi: 10.1080/13651820410030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough PA. Beyond serum creatinine: defining the patient with renal insufficiency and why? Rev Cardiovasc Med. 2003;4(Suppl. 1):S2–S6. [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Society of Anestheiologists. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 17.Longo WE, Virgo KS, Johnson FE, Oprian CA, Vernava AM, Wade TP, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 18.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 19.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2000;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]


