Abstract
Background:
Surgical resection of colorectal liver metastases (CLM) is an established form of treatment. Limited data exists on the value of sequential hepatic and pulmonary metastasectomy. We analysed patients who underwent sequential liver and lung resections for CLM.
Methods:
A total of 910 patients who underwent liver resection for CLM between January 2000 and December 2007, were analysed to identify patients with resectable pulmonary metastases (n= 43; 4.7%). Patient demographics, overall survival and survival difference between synchronous and metachronous pulmonary metastasectomy groups were compared. In addition, outcomes in the ‘liver and lung resection’ group were compared with a matched group of ‘liver resection only’ patients (matched for age, primary disease stage, interval to liver resection and liver disease stage).
Results:
Forty-three patients (median age 62, range 43–83 years, 22 males) underwent sequential liver and lung resection. A total of 36 patients underwent major hepatic resections, 18 patients had bilobar disease and the median number of liver lesions resected was 3 (range 1–5 lesions). Ten patients had synchronous liver and lung metastases. The median interval between liver and lung metastasectomy was 25 months (range 2–88 months). A total of two patients underwent major lobectomies, three patients had bilateral disease and the median number of lung lesions resected was one (range 1–3). The 1-, 3- and 5-year overall survival rates after first metastasectomy were 100%, 87.1% and 53.9%, respectively, with a median survival of 42 months.
Patients:
Undergoing metachronous pulmonary metastasectomy had better 1-, 3- and 5-year survival rates than those with synchronous disease (100%, 88.9% and 60.9% vs. 100%, 75% and 0%, respectively; P= 0.02, log rank test). There was no significant survival difference between the ‘liver and lung resection’ and the ‘liver resection only’ groups.
Conclusion:
Sequential liver and lung resection for CLM is associated with good long-term survival in selected patients, except in those presenting with synchronous lung and liver metastases.
Keywords: Sequential resections, liver and lung metastasectomy
Introduction
The most common sites for haematogenous metastases from colorectal carcinoma are the liver and lung.1 Surgical resection of hepatic and pulmonary colorectal metastases is an established form of treatment for stage 4 colorectal cancer.2–7 Studies have reported 5-year survival of up to 58% after isolated hepatic metastasectomy.5,8–11 Similarly, 5-year survival rates of 36–45% were reported after resection of lung metastases from colorectal cancer.7,12–14 However, limited data are available on the value of sequential resections of liver and lung colorectal cancer metastases. A study on the outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases, by Miller et al. concluded that surgical resection was associated with prolonged survival in selected patients.15 Patients with a longer disease-free interval between metastases and those with single liver lesions had the best outcomes.
The purpose of this study was to evaluate the long-term outcome of sequential hepatic and pulmonary metastasectomy from colorectal cancer and to identify factors that predict survival.
Patients and methods
All patients who underwent liver resection for colorectal cancer metastases at the University Hospital of Birmingham between January 2000 and December 2007 were retrospectively analysed to identify patients who had either synchronous or metachronous pulmonary metastases. Synchronous pulmonary metastasis was defined as the development of a lung lesion within 6 months of liver resection. All patients included in the study had received adjuvant chemotherapy after resection of primary colorectal cancer.
The data on liver resections were obtained from the prospectively maintained Liver Unit database at the Queen Elizabeth Hospital, Birmingham. The lung resection data were retrieved from the Pathology database at the Birmingham Heartlands Hospital.
Selection criteria
The criteria for hepatic metastasectomy were as follows: technically resectable liver secondaries leaving behind an adequate hepatic residual volume, and the absence of extra-hepatic metastasis other than resectable pulmonary metastases. Similarly the criteria for pulmonary metastasectomy included technically resectable lung metastases, absence of extra-thoracic metastasis other that resectable liver metastases and adequate cardiopulmonary function enabling complete resection of all pulmonary tumours.
Surgical techniques
During liver resection, all patients were explored using a subcostal incision. A thorough intra-abdominal exploration was carried out to identify any extra-hepatic disease. Intra-operative ultrasound was used in all patients to assess the number and extent of liver tumours and their relation to vascular anatomy. Pulmonary resection was performed after a minimum of 6–8 weeks after liver resection, after assessing the patient as being fit for a thoracotomy.
Follow-up
After hepatic or pulmonary metastatic resection, patients were closely followed up clinically, radiologically and biochemically. Surveillance computed tomography (CT) scans of the thorax, abdomen and pelvis were performed at 12 and 24 months after resection or when clinically indicated or when the tumour markers were elevated. Serum carcinoembryonic antigen (CEA) levels were measured every 3 months.
Data analysis
The following parameters were analysed: patient demographics, Duke's stage of primary colorectal cancer, number of liver metastases, interval between primary colorectal surgery and liver resection and between liver and lung resections. The overall 1-, 3- and 5-year survival after first metastasectomy and the survival difference between synchronous and metachronous pulmonary metastasectomy were also analysed. Survival difference was also compared between the ‘liver and lung resection’ group and a matched ‘liver resection only’ group without pulmonary metastases. Both these groups were matched for age, gender, stage of primary colorectal cancer, number of liver metastases and interval between colorectal surgery and liver resection.
Statistics
Data analysis was done with SPSS for Windows® software (version 13; Chicago, IL, USA). The overall survival was evaluated using Kaplan–Meier analysis and survival curves were compared using the Log-rank test. Factors possibly predicting survival were evaluated by univariate analysis.
Results
Of the 910 patients who had liver resection (median age, range, gender) for colorectal metastases during the study period, 43 patients (4.7%) underwent sequential liver and lung resections. The median age at the time of presentation of liver metastasis was 62 years (range 43–83 years). There were 22 men and 21 women.
The primary colorectal tumour was staged as Duke's B in 9 patients, Duke's C in 28 patients and Duke's D in 6 patients, all of whom had synchronous liver metastases. None of these six patients had lung metastasis at the time of detection of colonic and liver disease. The interval between colonic surgery and liver resection ranged from 4 to 66 months with a median of 19 months. None of the patients had simultaneous colonic and liver metastasis resection.
All the patients received adjuvant chemotherapy after colonic resection. Twenty-six patients received 5-FU/Folinic acid, 13 patients received oxaliplatin-based chemotherapy and 4 patients had irinotecan-based chemotherapy. Patients with metachronous liver metastasis went on to have surgical resection of metastasis first followed by additional chemotherapy. Patients with synchronous lung metastasis had sequential resection of liver and lung lesions followed by additional chemotherapy, if fit. However, patients with metachronous lung metastasis had resection without further chemotherapy, as they would have had chemotherapy after liver resection.
Hepatic resection
A total of 44 liver resections were performed on 43 patients. The type of liver resections performed is shown in Fig. 1. One patient who had non-anatomical resection of segment 4 initially developed recurrence within 5 months. He subsequently underwent a right hemihepatectomy with non-anatomical resection. Major liver resection, defined as resection of 3 or more Couinaud's segments was performed in 36 patients (81.8%). Eighteen patients (40.9%) had bilobar disease.
Figure 1.
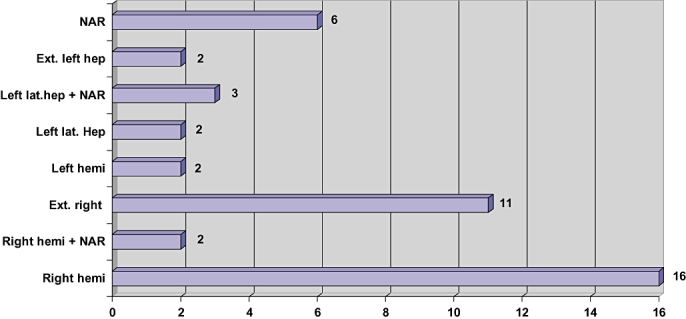
Type of liver resections performed
Final histopathological examination of resected liver specimens confirmed the number of liver lesions to vary from 1 to 5, with the median being 3. Nine patients had solitary liver metastases, 12 patients had 2 lesions, 17 patients had 3, 4 patients had 4 and 2 patients had 5 lesions each. All tumours were resected with histological clear margins (median 5 mm, range 3 mm to 5 cm)
Pulmonary resection
The median interval between liver and lung metastasectomy was 25 months (range: 2–88 months). Ten patients had synchronous liver and lung metastasis and the remaining 33 patients had metachronous metastasis.
A total of 45 pulmonary resections were performed on 43 patients. Two patients developed recurrence, one within 3 months and the other in 12 months, necessitating repeat resections. Wedge resection of pulmonary metastases was performed in all but two patients, both of whom had lobectomies. Three patients had bilateral disease. The most common site of pulmonary metastasis was the right lower lobe in 23 patients (51.1%) followed by the left lower lobe in 12 patients (26.7%). The median number of lung metastasis resected was 1 (range: 1–3).
Overall survival and prognostic factors
The overall survival was calculated from the time of first metastasectomy. The actuarial overall survival after sequential liver and lung metastasectomy was 100% at 1 year, 87.1% at 3 years and 53.9% at 5 years, with a median survival of 42 months (range:3–96 months) (Fig. 2). Analysis of the risk factors revealed that the presence of metachronous liver and lung metastases was significantly associated with better survival than synchronously detected lesions (P= 0.02) (Fig. 3), although age, gender (Fig. 4), Duke's stage of primary colorectal tumour (Fig. 5), number of liver and lung lesions (Fig. 6) and the interval between primary colorectal cancer resection and liver surgery were not (Table 1). The 1-, 3- and 5-year overall survival for the metachronous pulmonary metastasectomy group was 100%, 88.9% and 60.9%, respectively, with a median survival of 52 months, compared with 100%, 75% and 0%, respectively, for the synchronous pulmonary metastasectomy group with a median survival of 12 months.
Figure 2.
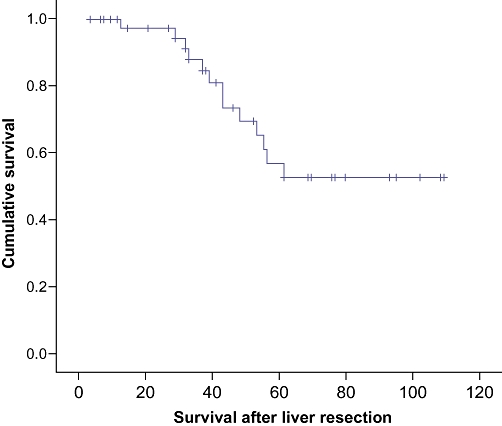
Cumulative survival curve, calculated from first metastasectomy for patients who underwent both hepatic and pulmonary metastatic resections
Figure 3.
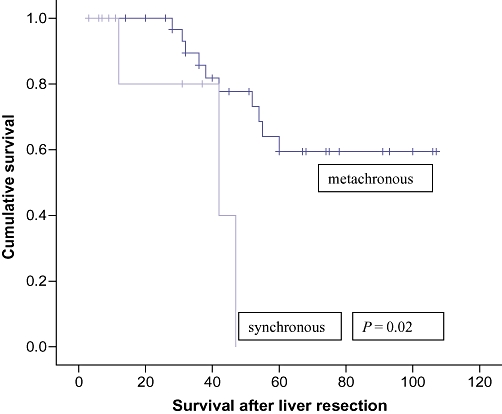
Cumulative survival curves comparing synchronous vs. metachronous pulmonary metastasectomy groups
Figure 4.
Cumulative survival curves comparing male and female subgroups
Figure 5.
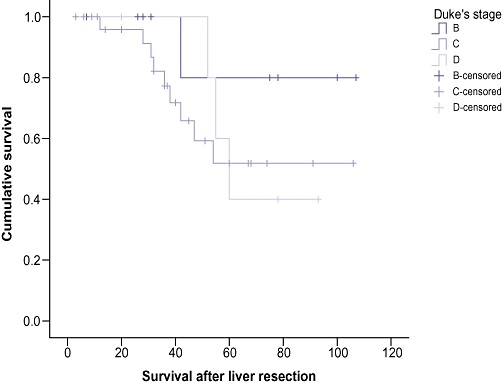
Cumulative survival curves comparing Dukes' stage of the primary colonic cancer
Figure 6.
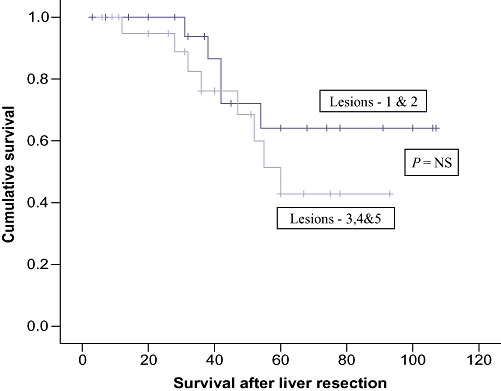
Cumulative survival curves comparing number of liver lesions resected
Table 1.
Statistical analysis of factors predicting survival after hepatic and pulmonary metastasectomy
| Factors predicting survival | P-value |
|---|---|
| Age | 0.711 |
| Gender | 0.600 |
| Duke's stage of primary colorectal cancer | 0.438 |
| Number of liver metastases | 0.120 |
| Number of lung metastases | 0.530 |
| Interval between primary and liver surgery | 0.740 |
| Synchronous vs. metachronous liver/lung metastases | 0.020a |
Statistically significant.
Patient's overall survival in the ‘liver and lung resection’ group was comparable with that of matched ‘liver resection only’ group, without pulmonary metastasis. The 1-, 3- and 5-year survival of the ‘liver and lung resection’ group was 100%, 87.1% and 53.9%, respectively, as compared with 96.9%, 84.5% and 51.5% in the ‘liver resection’ only group.
Discussion
The results of this study have demonstrated that good long-term survival could be achieved after combined liver and lung resections for metastases from colorectal cancer in selected group of patients.
The overall 5-year survival in the present study was 53.9%. This was comparable to the 5-year survival rates reported by Mineo et al. and Takahashi et al.16,17 The high 5-year survival rates could be attributed to the aggressive surgical approach, selection of patients with biologically less aggressive disease, strict post-operative follow-up, early identification of metastasis and newer generation of chemotherapeutic agents. Even although adjuvant oxaliplatin-based chemotherapy has been proved to improve survival, Takahashi et al. obtained similar 5-year survival rates with surgical treatment only.17 None of their patients received chemotherapy. They believed that this selected group of patients might have unique tumour characteristics that favour better outcome.
Controversy still exists over the prognostic factors which predict better survival after surgical resection. Our study demonstrated that overall survival is significantly better if pulmonary metastasis developed more than 6 months after liver resection (metachronous group) as compared with those who developed within 6 months of liver resection (synchronous group) (5-year survival of 60.9% vs. 0%). Several studies have confirmed similar results.18–20 Nagakura et al. reported that sequentially detected hepatic and pulmonary metastases was the strongest favourable prognostic factor.20 These authors concluded that simultaneous detection of these metastases does not warrant resection.
A number of other factors have been mentioned to significantly affect outcome after resection. These include age, number of metastasis, short disease free interval, high levels of CEA before metastasectomy, mediastinal nodal involvement and bilateral or multiple lung metastasis.15,16,18,19,21–24 The type of liver and lung resection has not been proven to influence outcome. However, several studies have shown better survival with adequate margin clearance.3–6 The present study analysed factors such as age, gender, stage of primary colorectal cancer, number of liver and lung lesions, interval between primary and liver surgery and none of them were found to influence outcome. It is possible that as the number of patients in this study was small, we were underpowered to identify a true prognostic factor. This could also reflect the inclusion of the selected group of patients with favourable tumour characteristics.
Comparing the survival difference between patients who underwent hepatic and pulmonary metastasectomy with those who had isolated hepatic metastasectomy (without pulmonary metastasis), we found a marginally better 5-year survival in the former group (53.9% vs. 51.5%), although the difference was not statistically significant. Miller et al. reported that the 5-year survival after liver and lung resection was 49% as compared with only 37% after hepatectomy for colorectal metastasis.15 Similar findings were shown by Shah and associates.25 This supports the notion that multisite metastases from colorectal cancer should not preclude potential curative resection. The likely reason for this finding could be the fact that this group of patients is representative of a subset of patients with favourable tumour biology. Particularly patients developing second resectable metastasis after first metastasectomy represent a different subgroup compared with the cohort of patients undergoing hepatic metastasectomy, most of whom develop extensive extra-hepatic disease.
The limitations of this study are the small size of the study group and retrospective nature of data analysis, even although these patients were identified on a prospective database. This highly selected patient population is likely to have favourable tumour characteristics, and is probably different than the population of patients with colorectal metastasis alone. Despite the limitations, the study does suggest a favourable outcome in patients with a longer interval between hepatic and pulmonary resection, which needs to be verified in large multicentre cohorts/studies.
In conclusion, sequential liver and lung resection for metastases from colorectal cancer is associated with satisfactory long-term survival in selected patients, with the exception of those presenting with synchronous liver and lung metastases.
Conflicts of interest
None declared.
References
- 1.Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50:1362–1366. [PubMed] [Google Scholar]
- 2.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver: prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 4.Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–471. doi: 10.1016/s0002-9610(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okumura S, Kondo H, Tsuboi M, Nakayama H, Asamura H, Tsuchiya R, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg. 1996;112:867–874. doi: 10.1016/S0022-5223(96)70085-5. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcome following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley AL, Chapman WC, Wright JK, Marsh JW, Geevarghese S, Blair KT, et al. Surgical experience with hepatic colorectal meatstasis. Am Surg. 1999;65:560–566. [PubMed] [Google Scholar]
- 10.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. doi: 10.1002/bjs.1800780711. [DOI] [PubMed] [Google Scholar]
- 11.Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998;22:268–276. doi: 10.1007/s002689900381. [DOI] [PubMed] [Google Scholar]
- 12.Friedel G, Pastorino U, Buyse M, Ginsberg RJ, Girard P, Goldstraw P, et al. Resection of lung metastases: long-term results and prognostic analysis based on 5206 cases- the International Registry of Lung Metastases. Zentralbl Chir. 1999;124:96–103. [PubMed] [Google Scholar]
- 13.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 14.McCormack PM, Butt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases: 10 year results. Arch Surg. 1992;127:1403–1406. doi: 10.1001/archsurg.1992.01420120037006. [DOI] [PubMed] [Google Scholar]
- 15.Miller G, Biernacki P, Kemeny NE, Gonen M, Downey R, Jarnagin WR, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205:231–238. doi: 10.1016/j.jamcollsurg.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Mineo TC, Ambrogi V, Tonini G, Bollero P, Roselli M, Mineo D, et al. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg. 2003;197:386–391. doi: 10.1016/S1072-7515(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi S, Nagai K, Saito N, Konishi M, Nakagohri T, Gotohda N, et al. Multiple resections for hepatic and pulmonary metastases of colorectal carcinoma. Jpn J Clin Oncol. 2007;37:186–192. doi: 10.1093/jjco/hym006. [DOI] [PubMed] [Google Scholar]
- 18.Robinson BJ, Rice TW, Strong SA, Rybicki LA, Blackstone EH. Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer. J Thorac Cardiovasc Surg. 1999;117:66–76. doi: 10.1016/s0022-5223(99)70470-8. [DOI] [PubMed] [Google Scholar]
- 19.Murata S, Moriya Y, Akasu T, Fujita S, Sugihara K. Resection of both hepatic and pulmonary metastases in patients with colorectal cancer. Cancer. 1998;83:1086–1093. doi: 10.1002/(sici)1097-0142(19980915)83:6<1086::aid-cncr6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Nagakura S, Shirai Y, Yamato Y, Yokoyama N, Suda T, Hatakeyama K. Simultaneous detection of colorectal carcinoma liver and lung metastases does not warrant resection. J Am Coll Surg. 2001;193:153–160. doi: 10.1016/s1072-7515(01)00970-x. [DOI] [PubMed] [Google Scholar]
- 21.Reddy RH, Kumar B, Shah R, Mirsadraee S, Papagiannopoulos K, Lodge P, et al. Staged pulmonary and hepatic metastasectomy in colorectal cancer- is it worth it? Eur J Cardiothorac Surg. 2004;25:151–154. doi: 10.1016/j.ejcts.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Gough DB, Donohue JH, Trastek VA, Nagorney DM. Resection of hepatic and pulmonary metastases in patients with colorectal cancer. Br J Surg. 1994;81:94–96. doi: 10.1002/bjs.1800810134. [DOI] [PubMed] [Google Scholar]
- 23.Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, et al. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001;71:975–979. doi: 10.1016/s0003-4975(00)02522-4. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Kawamura M, Ishihara T. Surgical treatment for both pulmonary and hepatic metastases from colorectal cancer. J Thorac Cardiovasc Surg. 1999;118:1090–1096. doi: 10.1016/S0022-5223(99)70106-6. [DOI] [PubMed] [Google Scholar]
- 25.Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]


