Abstract
Background:
Despite increasing numbers of reports, biliary tract intraductal papillary mucinous neoplasm (BT-IPMN) is not yet recognized as a unique neoplasm. The aim of the present study was to define the presence of BT-IPMN in a large series of resected biliary neoplasms.
Methods:
From May 1994 to December 2006, BT-IPMN cases were identified by reviewing pathology specimens of all resected cholangiocarcinomas and other biliary neoplasms when cystic, papillary or mucinous features were cited in pathology reports.
Results:
BT-IPMN was identified in 23 out of 253 (9%) specimens using the strict histopathological criteria of IPMN. The most common presenting symptom was abdominal discomfort which was present in 15 patients (65%). Only one of the original operative pathology reports used the term IPMN; 16 (70%) used the terms cystic, mucinous and/or papillary. BT-IPMN was isolated to non-hilar extra-hepatic ducts in 12 (52%), intra-hepatic ducts in 6 (26%) and hilar extra-hepatic ducts in 5 patients (22%). Carcinoma was found in association with BT-IPMN in 19 patients (83%); 5-year survival was 38% after resection.
Conclusion:
BT-IPMN occurs throughout the intra- and extra-hepatic biliary system and can be identified readily as a unique neoplasm. Broader acceptance of BT-IPMN as a unique neoplasm may lead to a better understanding of the pathogenesis of biliary malignancies.
Keywords: intraductal papillary mucinous neoplasm, biliary, papillary, mucinous
Introduction
Since the 1960s, a variety of mucin-secreting, papillary and/or cystic lesions of the intra- and extra-hepatic biliary tract have been recognized and reported with increasing frequency.1–4 We have previously reported our experience with such lesions at our institution.5,6 The pathological similarities between these lesions and intraductal papillary mucinous neoplasms (IPMN) of the pancreas have been suggested.7–9 The recognition of these similarities coincided with widespread acceptance of the nomenclature of IPMN for pancreatic lesions in the late 1990s.10 Despite these reports, biliary tract IPMN (BT-IPMN) is currently neither broadly nor officially recognized as a unique biliary neoplasm outside of the pancreas.
We hypothesized that BT-IPMN is a distinct clinicopathological entity with features analogous to IPMN of the pancreas but with its own separate identity. The aim of the present study was to define the presence of BT-IPMN in a large series of resected biliary neoplasms.
Methods
Between May 1994 and December 2006, all intra- and extra-hepatic cholangiocarcinomas resected were selected for further review in order to identify cases of BT-IPMN (n= 223). Slides from resected intra-hepatic, extra-hepatic and perihilar biliary neoplasms other than cholangiocarcinoma were also selected for further review if the original pathology reports cited findings of cystic, papillary or mucinous features (n= 22). This methodology is similar to a study conducted at our institution that identified patients with pancreatic IPMN prior to use of the IPMN nomenclature.11
Review of pathology slides was done by two experienced hepato-pancreato-biliary pathologists at our institution (DB and TS); discordant pathological reviews were discussed by the two pathologists, and consensus was reached in the final pathological diagnosis. BT-IPMN was defined histologically as a mucinous and papillary neoplasm showing clear origin from biliary epithelium with solitary or diffuse intraductal growth. We excluded all lesions originating in the periampullary region of the duodenum or in the pancreatic ducts (i.e. pancreatic IPMN), lesions in which clear origin from biliary ducts could not be determined and lesions with ovarian or mesenchymal stroma. This working definition is identical to the histological definition for pancreatic IPMN but mandates that the origin of the lesion be within the biliary tract. Each case that met the histological criteria for BT-IPMN was assessed for the presence of a macroscopic intra-luminal lesion and intra-luminal mucin per international guidelines.12
Each case of biliary tract IPMN was classified as IPMN with adenoma, moderate dysplasia, carcinoma in situ or invasive carcinoma according to the guidelines of the World Health Organization.13 Patients with invasive carcinoma associated with BT-IPMN were staged according to guidelines in the American Joint Committee on Cancer –Cancer Staging Manual, 6th edition.14 Additionally, pre-resection biopsies of patients with BT-IPMN were reviewed and assessed for the presence of BT-IPMN.
The medical records of patients identified to have BT-IPMN were reviewed. Demographics, clinical characteristics, pathology and outcomes were analysed. The Accurint system was used to augment information regarding death.15 The Accurint system is a commercially available database containing more than 20 billion records from 400 sources including dates of death. Data were summarized using descriptive statistics: median and range for continuous variables, and count (per cent) for categorical variables. Survival was analysed using the Kaplan–Meier method. Statistical analyses were performed using the SAS software package, v9.1 (SAS Institute Inc, Cary, NC, USA).
Results
Clinical characteristics (Table 1)
Table 1.
Clinical characteristics of patients with BT-IPMN
| Demographics | n | UICC stage (n= 17) | n |
| Age (median years; range) | 68 (31–81) | Intra-hepatic (n= 3) | |
| Male gender (%) | 10 (48) | I | 1 |
| II | 1 | ||
| Presenting symptoms | n (%) | IIIA | 0 |
| Abdominal discomfort | 15 (65) | IIIB | 0 |
| Jaundice | 9 (39) | IIIC | 1 |
| Weight loss | 8 (35) | IV | 0 |
| Extra-hepatic (n= 14) | |||
| Lesion location | n (%) | IA | 8 |
| Extra-hepatic | 17 (52) | IB | 2 |
| Hilar | 5 (22) | IIA | 1 |
| Non-hilar | 12 (52) | IIB | 3 |
| Intra-hepatic | 6 (26) | III | 0 |
| IV | 0 | ||
| Procedure types | n (%) | ||
| Pancreatoduodenectomy | 6 | Pre-operative labs (serum) | Median (range) |
| Hepatic & BD resection | 6 | Total bilirubin (mg/dL) | 3.2 (0.4–15.6) |
| BD resection only | 5 | Alk. Phos. (U/l) | 501 (162–1409) |
| Hepatic resection | 5 | ||
| Lobectomy | 4 | ||
| Segmentectomy | 1 | ||
| Cholecystectomy | 1 |
BT-IPMN was identified in 23 out of 245 specimens reviewed (48% men; median age = 68 years; range 31–81 years). BT-IPMN was identified in 15 out of 223 patients with resected cholangiocarcinoma reviewed and in 8 out of 22 patients with resected biliary tract lesions with cystic, papillary or mucinous features cited in their original pathology reports. Prior to diagnosis, symptoms were present for a median of 30 days (range 3 days to 7 years). The most common presenting symptom was abdominal discomfort occurring in 15 out of 23 patients (65%). Pre-operative serum total bilirubin concentration and alkaline phosphatase activity were available in 15 patients. Serum total bilirubin was >2.0 mg/dl in 7 patients and serum alkaline phosphatase activity was increased in all 15 patients.
Pre-operative evaluation included endoscopic retrograde cholangiography in 14 patients, computed tomography in 12, ultrasonography in 7, magnetic resonance imaging in 3 and percutaneous transhepatic cholangiography in 2. Characteristic findings on computed tomography (Fig. 1) included the presence of an intra-luminal lesion originating from the biliary tract with proximal bile duct dilation.
Figure 1.
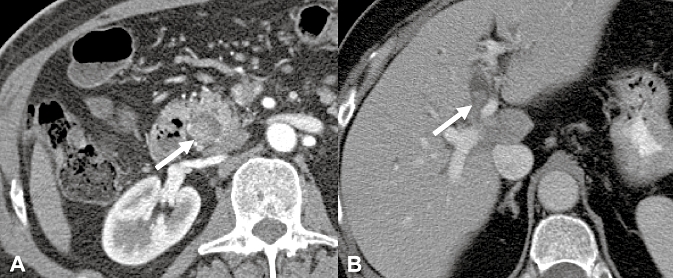
Computed tomography depicting an intra-luminal polypoid lesion originating from the biliary tract in extra-hepatic (a) and hilar (b) locations
BT-IPMN was isolated to the extra-hepatic bile ducts in 17 (74%) and the intra-hepatic bile ducts in 6 (26%) patients. Pancreatoduodenectomy was performed in six, combined major hepatic resection and extra-hepatic bile duct resection in six, bile duct resection in five, hepatic resection in five and cholecystectomy in one patient. Cholecystectomy was performed on one patient for presumed symptomatic cholelithiasis; an IPMN isolated to the cystic duct was found incidentally. The presence of large amounts of intra-ductal mucin was noted during the time of the operation in five patients.
There were two deaths within 60 days of operation. One patient underwent resection of the common bile duct with Roux-en-Y hepaticojejunostomy and died 3 days after discharge; this patient's post-operative course was complicated by an acute myocardial infarction and bile leak requiring a 17-day hospitalization. A second patient underwent a pancreatoduodenectomy for a distal common bile duct neoplasm and died 2 weeks after discharge; this patient's post-operative course was complicated by acute intra-abdominal haemorrhage post-operatively requiring reoperation, renal failure and anastomotic leak from the pancreaticojejunostomy requiring a 36-day hospitalization.
Complications occurred in eight patients (35%). Bile leaks occurred in three and bleeding in the immediate post-operative period occurred in two patients. One patient each had a leak at the pancreaticojejunostomy, intra-abdominal abscess, acute renal failure, pneumonia, pneumothorax, fascial dehiscence, wound infection and bacteraemia.
Pathology (Tables 2 and 3)
Table 2.
Original pathology report diagnoses, the presence of cystic, mucinous and/or papillary features as noted in dictated operative reports and pathology reports
| Patient no. | Original pathology report diagnosis | Reported operative features |
Original pathology report features |
Lesion size (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| Mucin | Cystic | Papillary | Mucin | Cystic | Papillary | |||
| 1 | Adenocarcinoma | ✓ | 1.0 | |||||
| 2 | Adenocarcinoma | 1.7 | ||||||
| 3 | Adenocarcinoma | 2.6 | ||||||
| 4 | Adenocarcinoma | ✓ | ✓ | 3.0 | ||||
| 5 | Adenocarcinoma | 4.0 | ||||||
| 6 | Adenocarcinoma | 4.8 | ||||||
| 7 | Adenocarcinoma | ✓ | ✓ | 5.0 | ||||
| 8 | Adenocarcinoma | ✓ | 7.5 | |||||
| 9 | Adenocarcinoma | 11.0 | ||||||
| 10 | Adenocarcinoma | ✓ | ✓ | Not available | ||||
| 11 | Adenocarcinoma | ✓ | 4.0 | |||||
| 12 | Adenocarcinoma | ✓ | ✓ | 2.3 | ||||
| 13 | Biliary papillomatosis | ✓ | ✓ | ✓ | 4.0 | |||
| 14 | Cholangiocarcinoma | ✓ | 2.0 | |||||
| 15 | Cholangiocarcinoma | ✓ | 2.0 | |||||
| 16 | Cholangiocarcinoma | ✓ | ✓ | 2.8 | ||||
| 17 | Cholangiocarcinoma | ✓ | ✓ | 3.5 | ||||
| 18 | Cystadenoma | ✓ | ✓ | ✓ | ✓ | 1.6 | ||
| 19 | Cystadenoma | ✓ | ✓ | 1.8 | ||||
| 20 | Cystic neoplasm | ✓ | ✓ | ✓ | 15.0 | |||
| 21 | Cystic neoplasm | ✓ | ✓ | ✓ | ✓ | ✓ | 7.5 | |
| 22 | IPMN with carcinoma | ✓ | ✓ | 2.0 | ||||
| 23 | IPMN with carcinoma | ✓ | 2.3 | |||||
Table 3.
Results of features noted on review of original imaging studies, gross pathology reports and pathology slides
| Patient no. | Macro intraluminal lesion | Intraluminal mucin | Lesion mocation | IPMN type |
|---|---|---|---|---|
| 1 | ✓ | Distal extra-heptic | Invasive carcinoma | |
| 2 | ✓ | ✓ | Distal extra-heptic | Invasive carcinoma |
| 3 | ✓ | ✓ | Distal extra-heptic | Invasive carcinoma |
| 4 | Distal extra-heptic | Invasive carcinoma | ||
| 5 | ✓ | Intra-hepatic | Invasive carcinoma | |
| 6 | Distal extra-heptic | Invasive carcinoma | ||
| 7 | ✓ | Distal extra-heptic | Invasive carcinoma | |
| 8 | ✓ | ✓ | Hilar extra-hepatic | Invasive carcinoma |
| 9 | Intra-hepatic | Invasive carcinoma | ||
| 10 | ✓ | Hilar extra-hepatic | Invasive carcinoma | |
| 11 | ✓ | Distal extra-heptic | Invasive carcinoma | |
| 12 | ✓ | Distal extra-heptic | Invasive carcinoma | |
| 13 | ✓ | ✓ | Hilar extra-hepatic | Invasive carcinoma |
| 14 | Hilar extra-hepatic | Invasive carcinoma | ||
| 15 | ✓ | Distal extra-heptic | Invasive carcinoma | |
| 16 | ✓ | Hilar extra-hepatic | Invasive carcinoma | |
| 17 | ✓ | ✓ | Intra-hepatic | Invasive carcinoma |
| 18 | ✓ | Distal extra-heptic | Adenoma | |
| 19 | ✓ | ✓ | Intra-hepatic | Invasive carcinoma |
| 20 | ✓ | Intra-hepatic | Adenoma | |
| 21 | ✓ | ✓ | Intra-hepatic | Adenoma |
| 22 | ✓ | ✓ | Distal extra-heptic | Invasive carcinoma |
| 23 | ✓ | Distal extra-heptic | Dysplasia |
IPMN, intraductal papillary mucinous neoplasm.
Pre-operative biopsies were performed in 14 patients. Six patients underwent direct endoscopic biopsy at our institution, and had material available for repeat analysis. None of the original preoperative pathology reports used the term IPMN; 4 (29%) used the terms ‘cystic’, ‘mucinous’, and/or ‘papillary’. Repeat pathological review confirmed the presence of BT-IPMN in four out of six patients with pre-operative biopsies.
Only two of the original pathology reports of the operative resection specimens used the term IPMN; 16 (70%) used the terms ‘cystic’, ‘mucinous’ and/or ‘papillary’. The diagnosis of BT-IPMN was based on the presence of an intraluminal neoplasm with papillary cyto-architecture; these criteria are in keeping with the accepted histological definition of pancreatic IPMN10 (Fig. 2b). The neoplastic epithelium was sub-classified as intestinal in 10, pancreatobiliary in 12 and oncocytic in one (Fig. 2c–e, respectively).
Figure 2.
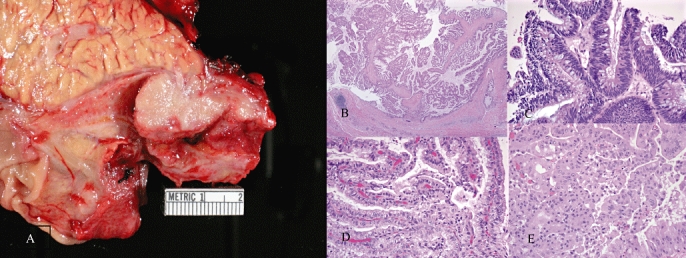
Photograph A shows a distal common bile duct intraductal papillary mucinous neoplasm (IPMN) with characteristic gross polypoid features. Photographs B–E show haematoxylin and eosin stains of different biliary tract (BT)-IPMNs with papillary cytoarchitecture within the benign portion (b), intestinal epithelium (c), pancreatobiliary epithelium (d) and oncocytic epithelium (e)
Pre-operative imaging studies and gross descriptions of the resected tumours indicated that macroscopic intraluminal lesions were present in 19 patients (83%). Intraluminal mucin was identifiable upon review of haematoxylin and eosin stains in eight patients (35%). Using this review for intraluminal mucin on pathology slides, as well as the original operative reports and gross pathology descriptions, at total of 17 (74%) unique patients had evidence of intraluminal mucin.
Reassessment using the WHO classification for pancreatic IPMN revealed four patients with non-invasive lesions (three with adenoma and one with moderate dysplasia) and 19 patients with invasive adenocarcinoma. All invasive adenocarcinomas were accompanied by carcinoma in situ in the non-invasive component. None of the 23 patients had only carcinoma in situ without tissue invasion. Staging information was available for 17 out of 19 patients with invasive malignancy; 3 patients were staged according to AJCC Guidelines for intrahepatic bile duct malignancies and 14 according to extrahepatic bile duct malignancies (Table 1).
Recurrence and survival
Out of the 19 patients who had invasive carcinoma in association with BT-IPMN, 2 patients underwent R1 resections, one of whom is alive without evidence of recurrence 32 months after resection. The other patient recurred 8 months post-operatively. Out of the four patients who did not have invasive carcinoma in association with BT-IPMN, one had IPMN without dysplasia at the resection margin and one patient had dysplasia at the resection margin. There has been no evidence of recurrence or disease-related death in these patients or in any of the patients without malignancy. At last follow-up (median = 5.5 years), 9 out of 19 patients with invasive BT-IPMN had evidence of recurrent carcinoma. The median survival of all patients with BT-IPMN with invasive carcinomas was 2.8 years, and overall 5-year survival was 38% (95% CI 20–72%, Fig. 3).
Figure 3.
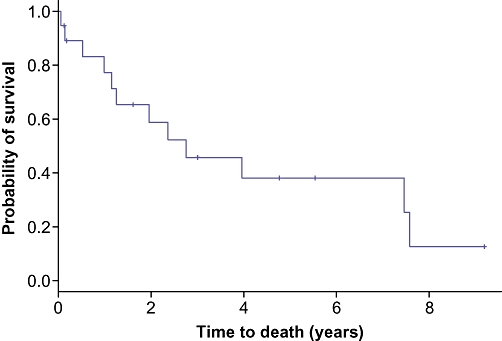
Kaplan–Meier curve depicting the survival for 19 patients who had carcinoma in association with BT-IPMN. Survival was 77% at 1 year (n= 13; 95% CI 60–100%), 46% at 3 years (n= 7; 95% CI 27–78%) and 38% at 5 years (n= 4; 95% CI 20–72%)
Discussion
Our findings suggest that BT-IPMN is a lesion that has a meaningful occurrence, is found throughout the intra- and extra-hepatic biliary tract (including the cystic duct) and can be identified readily on histopathological review using the criteria developed by the WHO. In our institution, BT-IPMN represented 7% of all biliary neoplasms resected between 1994 and 2006. During the same period as this study, 10% of pancreatic resection specimens at our institution harboured IPMN.
Defining BT-IPMN as a distinct entity has important ramifications. First, various biliary neoplasms that have been described previously as adenomas, papillomatosis, adenocarcinoma, cholangiocarcinoma, cystic lesions and mucin-secreting lesions could be defined more clearly and consistently as BT-IPMN. Also, this delineation may allow study of malignant change in the biliary epithelium such that, as with pancreatic carcinoma associated with IPMN, biliary carcinoma arising in BT-IPMN may be recognized to behave differently than the typical cholangiocarcinoma.
The term ‘papillary carcinoma’ enjoys widespread usage in neoplasms of the biliary tract. We and others16,17 suggest that papillary neoplasms in this location, including papillomatosis, are best considered the biliary counterpart to pancreatic IPMN and represent a non-invasive neoplasm that may give rise to invasive carcinoma. Similar to what occurs within the pancreas, as others have previously recognized,18,19 invasive carcinoma in the setting of BT-IPMN resembles typical adenocarcinoma often with mucinous (colloid) morphology but is rarely papillary. Although invasive papillary structures can be seen histologically,18 this very rarely occurs within the biliary tract in our experience and was not found in any patient in our study. Along those lines, invasive papillary structures can occur theoretically without the presence of IPMN. Thus, our definition of BT-IPMN is not a wholesale reclassification of papillary carcinoma. It is likely, however, that a significant number of lesions that have been previously described as papillary carcinoma are typical sclerotic adenocarcinoma with adjoining IPMN; in this setting, ‘papillary’ refers to what we feel would be better described as IPMN. Whether the lack of papillary changes in the invasive component is secondary to the malignant phenotype of cholangiocytes or is in response to the extracellular matrix is unknown.
During the course of this study, not every cystic lesion of the bile duct reviewed was reclassified as IPMN. Similar to cystic lesions of the pancreas, the differentiation between BT-IPMN and cystadenoma or cystadenocarcinoma of the biliary tract was dependent on the presence of mesenchymal stroma in the latter. The original diagnosis of cystadenoma or cystic biliary neoplasm in three patients in this study highlights the potential need for clarification of certain unusual cystic biliary tract neoplasms similar to that which has evolved for cystic pancreatic lesions, leading to the adoption of the WHO classification for IPMN. Thus, all ‘biliary’ cystic, mucinous or papillary neoplasms do not necessarily present as BT-IPMN, although 36% of such neoplasms in our series proved to be BT-IPMN.
Similarities between biliary and pancreatic IPMN can be explained given the shared embryological development of the bile duct and the main pancreatic duct from the hepatic diverticulum in the foregut mesoderm,20 but important differences may exist. As in our study, when reviewing the historical presence of pancreatic IPMN at our institution, Tollefson et al. found that abdominal discomfort was the most common presenting symptom.11 In contrast, Tollefson et al. reported jaundice in 62% of pancreatic IPMN patients compared with 39% in our study. This difference is likely as a result of the various sites of origin of BT-IPMN, from within the liver itself to the distal common bile duct, thus producing a constellation of symptoms more similar to cholangiocarcinoma.21 Additionally, the frequency of invasive carcinoma in patients with resected BT-IPMN appears to be greater than in patients with resected pancreatic IPMN. In our study, 83% of patients with resected BT-IPMN had invasive carcinoma compared with only 30% of patients with resected pancreatic IPMN at our institution.19
Other potential differences between BT-IPMN and its pancreatic counterpart are worth noting. Diffuse involvement of the pancreas is often seen with pancreatic IPMN. Interestingly, we found evidence of BT-IPMN occurring in a diffuse manner in only one patient who was originally diagnosed with papillomatosis. Additionally, pancreatic IPMN is known to recur after resection even in benign cases.19,22 We found no evidence of recurrent BT-IPMN-adenoma after resection in our cohort. Also, gross cystic dilatation was noted in the original operative reports in only six patients in our study: three had intra-hepatic lesions, two had extra-hepatic distal lesions and one had an extra-hepatic hilar lesion. Although gross cystic dilatation is often associated with pancreatic IPMN, this feature is highly variable and is not a prerequisite for diagnosis of pancreatic IPMN according to the WHO criteria.13 Whether the apparent differences between BT-IPMN and its pancreatic counterpart are because of different tumour biology or are a product of the difference between the physical structures of the biliary and pancreatic ductal systems is unclear. Zen et al. demonstrated that pancreatic IPMN overall has lower CK-20 expression and a higher frequency of gastric cell-type tumours than BT-IPMN.17 This difference is less apparent, however, when excluding branch-duct IPMNs of the pancreas which express rarely CK-20 and are commonly gastric cell-type tumours. It is therefore plausible that BT-IPMNs are more closely related to main duct IPMNs of the pancreas. In any case, these features highlight the heterogeneous nature of all IPMNs irrespective of the biliary counterpart.
International consensus guidelines define IPMN as a ‘grossly visible, non-invasive, mucin-producing, predominantly papillary or, rarely flat, epithelial neoplasm’.12 These guidelines were created in order to clearly delineate IPMN from pancreatic intraepithelial neoplasm (PanIN) which can be impossible to differentiate by microscopic evaluation of routine haematoxylin and eosin stains only. In fact, the presence of a macroscopic intraluminal lesion and visible mucin on the surface of the tumour are the main characteristics differentiating IPMN and PanIN. In our study, macroscopic intra-luminal lesions were present in 83% of patients with BT-IPMN. Although the epithelium of the lesions in each patient found to have BT-IPMN in this study was mucinous, the presence of intraluminal mucin was variable which is similar to findings of other studies.17,23 We found documentation or evidence on review of pathology slides of visible intraluminal mucin in 74% of patients with BT-IPMN in our study. It is possible that intraluminal mucin was present in more patients but the fixation and staining process makes the detection of mucin unreliable and is a significant limitation of this study. In any case, as with cystic dilatation, the presence of mucin is considered to be variable even in pancreatic IPMN.12,13 As Adsay suggests, mucin production and even cystic dilatation may be dependent on ‘the location of the primary process and subsequent mechanical changes in the ducts’.24
In our study, all the patients with BT-IPMN clearly met the histological criteria for IPMN. Although it is quite feasible that a minority of these patients truly did not have the macroscopic criteria for IPMN, we are hampered by the retrospective nature of this study as such features are not uniformly documented and re-evaluation of gross specimens is impossible. Evaluation of gross specimens is the best opportunity for assessing macroscopic features. At most, the minority of patients in this study who truly do not meet the macroscopic criteria for IPMN had lesions which might be better described as biliary intra-epithelial neoplasms (BilIN). This idea only further supports the concept that these biliary lesions are the counterpart of pancreatic ductal lesions and may share similar pathogenesis. In fact, as a result of guidelines developed in Asia,25 international consensus guidelines that include the experiences of western clinicians were developed for the diagnosis of BilIN.23 One of the contentions of this inter-observer group is that BilIN and BT-IPMN, or ‘biliary IPN’ as they refer to it, are major precursor lesions associated with the development of cholangiocarcinoma. Inter-observer guidelines for BT-IPMN have been proposed but are not yet completed.
Our findings suggest that patients with BT-IPMN may have a survival similar to its pancreatic counterpart. A recent study at our institution19 indicates that survival after resection of invasive pancreatic carcinoma in the setting of IPMN is similar to survival after curative resection of pancreatic ductal adenocarcinoma without IPMN when compared stage for stage (31% vs. 24% survival at 5 years, respectively). Interestingly, the survival for invasive pancreatic carcinoma associated with IPMN is similar to the survival at 5 years (38%) for patients with invasive carcinoma associated with BT-IPMN after resection in our study. Although the limited number of patients (n= 23) available for analysis in our study prevents an absolute conclusion, these trends in our data warrant further investigation.
Comparing the survival of patients with invasive carcinoma in association with BT-IPMN to patients with cholangiocarcinoma alone may be more revealing and useful than comparing BT-IPMN with IPMN of the pancreas. Survival at 5 years for patients with resected cholangiocarcinoma varies from 8–30% depending both on location of the lesion and the particular study reviewed.26–28 Studies at our institution found that 5-year survival was 26% for patients with resected hilar cholangiocarcinoma28 and 20% for patients with resected distal common bile duct cholangiocarcinoma.29 The small size of our study prevents broader conclusions, but the overall 5-year survival of 38% in our study suggests better survival for patients with carcinoma associated with BT-IPMN.
As previously shown,30–33 depth of invasion is an important predictor of survival after resection of cholangiocarcinoma. This may play a role in the greater survival seen in our study for patients with BT-IPMN and may not reflect an intrinsic difference in tumour biology. Interestingly, Albores-Saavedra et al. suggest that, in contrast to typical cholangiocarcinoma, invasive papillary cholangiocarcinoma (which we would consider to be invasive BT-IPMN) grows towards the bile duct lumen prior to invading the bile duct wall.30 We did not assess depth of invasion for the patients with BT-IPMN in this study or patients with typical cholangiocarcinoma and cannot comment on how the depth of invasion may play a role in our cohort.
Two original pathology reports of patients who underwent resection of a BT-IPMN used the term ‘IPMN’. These patients who underwent resection after nomenclature for IPMN of the pancreas had been adopted at our institution; nine other patients underwent resection after this time point as well but were not diagnosed originally with IPMN. This observation highlights both the need for an awareness of these histological findings as a distinct entity and consideration for BT-IPMN as the appropriate nomenclature for these lesions within the biliary tract.
Our study has several limitations not discussed above. First, although our study suggests that BT-IPMN is a distinct pathological entity and is clinically meaningful, we only reviewed the pathology of resected lesions. These findings require further studies to confirm the presence of this entity outside of resected specimens and to establish accurately the actual incidence and prevalence of BT-IPMN. We maintain that BT-IPMN exists as a unique neoplasm and is often unrecognized, similar to the story with IPMN of the pancreas. Second, our data on recurrence are not as robust as survival data; a large proportion of our patient population is referred from distant locations and thus seeks long-term follow-up elsewhere. More complete follow-up imaging of BT-IPMN will not only provide better data on patterns of failure, but also on progression of adenoma to invasion and development of other extra-biliary neoplasms as occurs in pancreatic IPMN.34 In conclusion, we maintain that BT-IPMN is an identifiable neoplasm that occurs with meaningful frequency in resected biliary tract neoplasms. We provide strong histopathological data to establish this neoplasm as a unique clinicopathologic entity. Similar to pancreatic IPMN, universal acceptance of BT-IPMN as a unique neoplastic spectrum may lead to a better understanding of the pathogenesis of biliary malignancies. This concept may modify our approach to selected biliary tract malignancies which could result in improved survival.
Conflicts of interest
None declared
References
- 1.Helpap B. Malignant papillomatosis of the intrahepatic bile ducts. Acta Hepatogastroenterol (Stuttg) 1977;24:419–425. [PubMed] [Google Scholar]
- 2.Neumann RD, LiVolsi VA, Rosenthal NS, Burrell M, Ball TJ. Adenocarcinoma in biliary papillomatosis. Gastroenterology. 1976;70(5):779–782. Pt. 1. [PubMed] [Google Scholar]
- 3.Kokubo T, Itai Y, Ohtomo K, Itoh K, Kawauchi N, Minami M. Mucin-hypersecreting intrahepatic biliary neoplasms. Radiology. 1988;168:609–614. doi: 10.1148/radiology.168.3.2841715. [DOI] [PubMed] [Google Scholar]
- 4.Cattell RB, Braasch JW, Kahn F. Polypoid epithelial tumors of the bile ducts. N Engl J Med. 1962;266:57–61. doi: 10.1056/NEJM196201112660201. [DOI] [PubMed] [Google Scholar]
- 5.Davies W, Chow M, Nagorney D. Extrahepatic biliary cystadenomas and cystadenocarcinoma. Report of seven cases and review of the literature. Ann Surg. 1995;222:619–625. doi: 10.1097/00000658-199511000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capizzi PJ, Rosen CB, Nagorney DM. Intermittent jaundice by tumor emboli from intrahepatic cholangiocarcinoma. Gastroenterology. 1992;103:1669–1673. doi: 10.1016/0016-5085(92)91194-9. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, et al. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389–393. doi: 10.1055/s-2000-8996. [DOI] [PubMed] [Google Scholar]
- 8.Oshikiri T, Kashimura N, Katanuma A, Maguchi H, Shinohara T, Shimizu M, et al. Mucin-secreting bile duct adenoma – clinicopathological resemblance to intraductal papillary mucinous tumor of the pancreas. Dig Surg. 2002;19:324–327. doi: 10.1159/000064570. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita Y, Fukuzawa K, Taketomi A, Aishima S, Yoshizumi T, Uchiyama H, et al. Mucin-hypersecreting bile duct neoplasm characterized by clinicopathological resemblance to intraductal papillary mucinous neoplasm (IPMN) of the pancreas. World J Surg Oncol. 2007;5:98. doi: 10.1186/1477-7819-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longnecker DS, Adler G, Hruban R, Klöppel G. Intraductal papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press; 2000. pp. 237–241. [Google Scholar]
- 11.Tollefson MK, Libsch KD, Sarr MG, Chari ST, DiMagno EP, Urrutia R, Smyrk TC. Intraductal papillary mucinous neoplasm: did it exist prior to 1980? Pancreas. 2003;26:e55–e58. doi: 10.1097/00006676-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 13.Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH, World Health Organization . International Classification of Tumors. 2nd edn. Berlin: Springer; 1996. Histologic typing of tumors of the exocrine pancreas; pp. 219–251. [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th edn. New York: Springer; pp. 131–138.pp. 145–150. [Google Scholar]
- 15.Accurint®, a LexisNexis® commercial location and research online service. http://www.accurint.com.
- 16.Longnecker DS, Terhune PG. The case for parallel classification of biliary tract and pancreatic neoplasms. Mod Pathol. 1996;9:828–837. [PubMed] [Google Scholar]
- 17.Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 18.Hoang MP, Murakata LA, Katabi N, Henson DE, Albores-Saavedra J. Invasive papillary carcinomas of the extrahepatic bile ducts: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 2002;15:1251–1258. doi: 10.1097/01.MP.0000036450.61830.8E. [DOI] [PubMed] [Google Scholar]
- 19.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. discussion 646. [DOI] [PubMed] [Google Scholar]
- 20.Keith L, Moore TVNP. The Developing Human: Clinically Oriented Embryology. 8th edn. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 21.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701–709. doi: 10.1038/modpathol.3800788. [DOI] [PubMed] [Google Scholar]
- 24.Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007;20(Suppl. 1):S71–S93. doi: 10.1038/modpathol.3800706. [DOI] [PubMed] [Google Scholar]
- 25.Zen Y, Aishima S, Ajioka Y, Haratake J, Kage M, Kondo F, et al. Proposal of histological criteria for intraepithelial atypical/proliferative biliary epithelial lesions of the bile duct in hepatolithiasis with respect to cholangiocarcinoma: preliminary report based on interobserver agreement. Pathol Int. 2005;55:180–188. doi: 10.1111/j.1440-1827.2005.01816.x. [DOI] [PubMed] [Google Scholar]
- 26.Jonas S, Thelen A, Benckert C, Biskup W, Neumann U, Rudolph B, et al. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg. 2009;249:303–309. doi: 10.1097/SLA.0b013e318195e164. [DOI] [PubMed] [Google Scholar]
- 27.Nanashima A, Sumida Y, Abo T, Nagasaki T, Takeshita H, Fukuoka H, et al. Patient outcome and prognostic factors in intrahepatic cholangiocarcinoma after hepatectomy. Hepatogastroenterology. 2007;54:2337–2342. [PubMed] [Google Scholar]
- 28.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. discussion 523–515. [DOI] [PubMed] [Google Scholar]
- 29.Bortolasi L, Burgart LJ, Tsiotos GG, Luque-De Leon E, Sarr MG. Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg. 2000;17:36–41. doi: 10.1159/000018798. [DOI] [PubMed] [Google Scholar]
- 30.Albores-Saavedra J, Murakata L, Krueger JE, Henson DE. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer. 2000;89:508–515. doi: 10.1002/1097-0142(20000801)89:3<508::aid-cncr5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: univariate and multivariate analysis. Hepatogastroenterology. 2002;49:311–316. [PubMed] [Google Scholar]
- 32.Strasberg SM. Resection of hilar cholangiocarcinoma. HPB Surg. 1998;10:415–418. doi: 10.1155/1998/24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riall TS, Stager VM, Nealon WH, Townsend CM, Jr, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803–813. doi: 10.1016/j.jamcollsurg.2007.01.015. discussion 813–804. [DOI] [PubMed] [Google Scholar]


