Abstract
Objective
The aim of this study was to evaluate the differences in normal brain MRI findings between under 3.0 Tesla (T) and 1.5T MRI conditions with the use of the fluid attenuated inversion recovery (FLAIR) sequences.
Materials and Methods
Eleven normal adults underwent imaging with the use of the FLAIR sequences on both 1.5T and 3.0T scanners. Two neuroradiologists compared the signal intensity (SI) of the centrum semiovale (CS), pulvinar thalami (PT) and normal iron deposit structures (IDSs) on the 3.0T and 1.5T FLAIR images, and they evaluated three MRI findings qualitatively: high SI of CS; low SI of PT; low SI of IDS. We also evaluated signal-to-noise ratios (SNRs) for the CS, PT, red nucleus and cerebellar dentate nucleus on the FLAIR images.
Results
Based on qualitative analyses, the 3.0T FLAIR images showed all three MRI findings for all cases. Low SI for the PT in seven cases (64%), high SI of the CS in one case (9%) and low SI of the cerebellar dentate nucleus in one case (9%) were visualized only on 3.0T FLAIR images. The mean SNRs of the PT, red nucleus and dentate nucleus in patients where 3.0T FLAIR imaging was performed were significantly lower as compared with the SNRs on 1.5T FLAIR images. The SNR of the CS was not significantly different between under the two magnetic field strengths (p > 0.05).
Conclusion
We have demonstrated that normal, high and low SIs of the CS, PT and IDS on 3.0T FLAIR images were depicted more frequently and more prominently as compared with those on 1.5T FLAIR images in normal adult brains.
Keywords: Magnetic resonance (MR), Fluid attenuated inversion recovery (FLAIR), Normal Brain, 3.0 Tesla
Fluid attenuated inversion recovery (FLAIR) imaging is a useful routine sequence of brain imaging to assess lesions in the patients with brain parenchymal and meningeal lesions. In normal healthy individuals, FLAIR hyperintensity, such as periventricular white matter hyperintensity, has been demonstrated with the use of 1.5 Tesla (T) MRI. There have been reports on the normal MRI findings of the FLAIR images using 1.0T and 1.5T MR scanners (1, 2). With the use of 1.0T and 1.5T, one interesting MRI finding of the normal brain is the high signal intensity (SI) of the corticospinal tract. One recent study reported diffuse parenchymal white matter FLAIR hyperintensity that was more common and prominent at a field strength of 3.0T as compared to that of 1.5T in healthily volunteers (3). In addition, we have demonstrated that the 3.0T FLAIR images showed several different MRI findings of the normal brain as compared to those of the 1.5T FLAIR images. These differences include different SI of the centrum semiovale (CS), pulvinar thalamus (PT) and the normal iron deposit structures (IDSs) on the 3.0T images. The IDSs include the substantia nigra, red nucleus, globus pallidus, putamen and cerebellar dentate nucleus.
Although the signal-to-noise ratio (SNR) for 3.0T systems is not double the SNR of 1.5T systems, the SNR increases approximately linearly with the constant magnetic induction field (4-7). A better SNR is associated with increased diagnostic sensitivity and specificity for a variety of neurological conditions. With the profound opportunities that are available with the transition to higher field MR scanners, it is critical to establish normative data to maximize the diagnostic accuracy and specificity.
The aim of the present study was to evaluate the differences in normal brain MRI findings between under 3.0T and 1.5T MRI conditions with the use of the FLAIR sequences.
MATERIALS AND METHODS
Subjects and Protocol
Eleven healthy adults (six men and five women; age range, 26-71 years; mean age, 55 years) without any known disease were enrolled in this study after obtaining written informed consent. Each subject was extensively interviewed before undergoing MR examinations. None of the subjects had any history of neurological symptoms and none of the subjects had any disease that could have affected the central nervous system. No formal bedside physical or neurological examination or neuropsychiatric testing was performed. None of the subjects was taking any medication.
The subjects underwent fast FLAIR MR imaging of the brain at field strengths of both 1.5T and 3.0T (Signa, GE Medical Systems, Waukesha, WI) with a maximum period of five hours between the two studies. The FLAIR images covering the whole brain were scanned with the same scan parameters (TI = 2250 ms, TR = 11002 ms, eTE = 135 ms, 19 axial sections, echo train length of 16, 512 × 192 matrix, acquisition time of 6 minutes 36 seconds) for both scanners.
Qualitative Image Analysis
The image data was randomized and keep anonymous for the analysis. Two neuroradiologists carefully compared the signal intensities of the CS, PT and IDSs on the 3.0T FLAIR images with the signal intensities on the 1.5T FLAIR images. The evaluation process was carried out by consensus agreement of both investigators. The following criteria were used for evaluating the MRI findings for the selected anatomic locations. High SI of the CS was seen when the SI of the CS was higher than that of the subcortical white matter SI. Low SI of the PT and IDSs were seen, which was indicative of the lower SI of the two regions as compared to that of the surrounding structures. First, we evaluated the presence of these three MRI findings. The following grading system was used to evaluate the differences in cases that showed the three MRI findings with the use of both scanners. The scores included -2, only the 1.5T FLAIR images had the MRI findings; -1, the 1.5T FLAIR images had more increased conspicuity of the MRI findings than did the 3.0T FLAIR images; 0, non-visualization of the MRI findings on the FLAIR images; 1, the 3.0T FLAIR images had more increased conspicuity of the MRI findings than did the 1.5T FLAIR images; 2, only the 3.0T FLAIR images had more increased conspicuity of the MRI findings; 3, the same pattern of MRI findings was seen on both FLAIR images.
Quantitative Image Analysis
The two sets of FLIAR images were transferred to a workstation for further quantitative analysis of the SNR, as described below.
Region of interest placement: With the close supervision of an experienced neuroradiologist, oval regions of interest (ROIs) were placed in both CSs (22 ROIs), in the PT (22 ROIs), in the red nuclei (22 ROIs), in the dentate nuclei (22 ROIs) and in ghosting-free parts of the image background (11 ROIs). The size of the ROIs varied, depending on the location; the mean area of the ROIs in the CS was 180 mm2 and the mean area of the ROIs in the PT was 32 mm2. The mean area of the ROIs in the red nuclei was 13 mm2 and the mean area of the ROIs in the dentate nuclei was 16 mm2. The mean area of the ROIs in the background noise was 700 mm2. By use of the ROI data, the SNRs were calculated by one investigator.
Calculation of SNRs: SNRs were calculated in all the studies according to the following equation: SNR = SItissue/SD SInoise, where SItissue is the mean SI of the ROIs placed in the anatomical regions and SD SInoise is the standard deviation of the SI of the background noise.
Statistical Analysis
The Wilcoxon signed rank test was used for statistical analysis to compare the SNRs of the FLAIR images between the two field strengths. A p value of less than 0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed with SPSS statistical software (SPSS for Windows, version 12.0; SPSS, Chicago, IL).
RESULTS
Qualitative Image Analysis
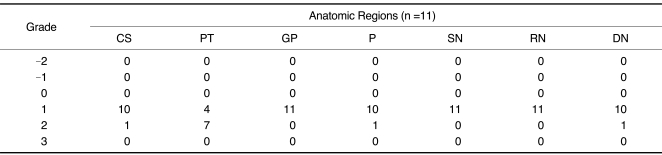
All the 3.0T FLAIR images had all three MRI findings. Especially, the low SI of the PT in seven cases (64%), the high SI of the CS in one case (9%), the low SI of the cerebellar dentate nucleus in one case (9%) and the low SI of the putamen in one case (9%) were only visualized on the 3.0T FLAIR images (Fig. 1). For all the positive cases, the 3.0T FLAIR images showed a score of 2 for these anatomical structures (Fig. 2). The low SI of the globus pallidus, red nucleus and substantia nucleus were visualized for all cases on the 1.5T and 3.0T FLAIR images. The low SI of the PT was visualized on the 1.5T FLAIR images for only four cases (36%), and this is in contrast to 100% visualization on the 3.0T FLAIR images. The FLAIR MRI findings in the anatomic regions are summarized in Table 1.
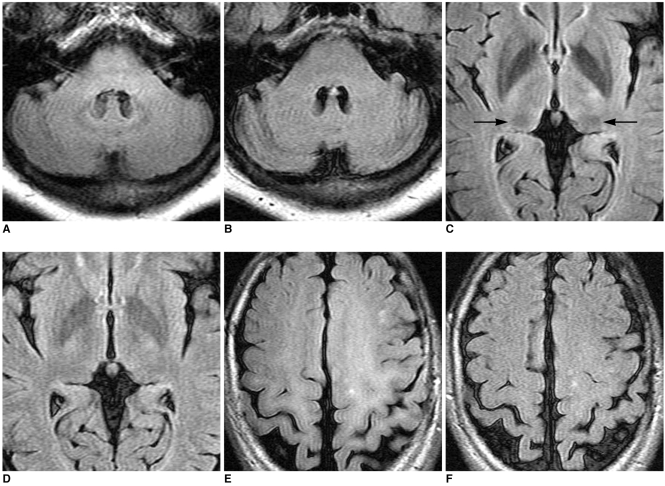
Fig. 1.
FLAIR images of 3.0T (A, C, E) show low signal intensities at dentate nucleus of cerebellum (A) and pulvinar thalami (arrows in C) and high signal intensities at both centrum semiovales (E), but 1.5T FLAIR images (B, D, F) do not show these findings in several cases.
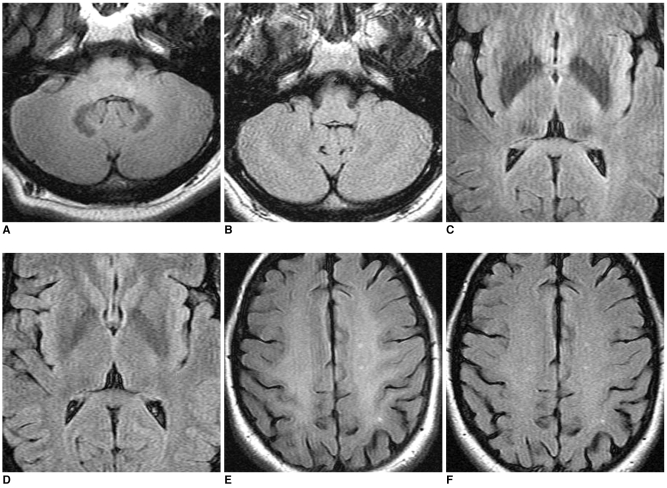
Fig. 2.
In cases with three FLAIR MRI findings with both MR scanners, FLAIR images of 3.0T (A, C, E) show increased conspicuity of low signal intensities at dentate nucleus of cerebellum (A) and both pulvinar thalami (B) and high signal intensities at both centrum semiovales (E), as compared with 1.5T (B, D, F).
Table 1.
Qualitative Analysis of FLAIR MRI Findings in Different Anatomic Regions
Note.-FLAIR = fluid attenuated inversion recovery, CS = centrum semiovales, PT = pulvinar thalami, GP = globus pallidus, P = putamen, SN = substantia nigra, RN = red nuclei, DN = dentate nuclei
Grade: -2 = only 1.5T FLAIR images had three MRI findings (high signal intensity of CS; low signal intensity of PT; low signal intensity of iron deposit structures), -1 = 1.5T FLAIR images had more increased conspicuity of three MRI findings than did 3.0T, 0 = non-visualization of three MRI findings in FLAIR images, 1 = 3.0T FLAIR images had more increased conspicuity of three MRI findings than did 1.5T, 2 = only 3.0T FLAIR images had three MRI findings, 3 = same pattern of three MRI findings on both FLAIR images
Quantitative Image Analysis
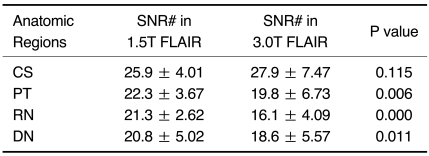
Signal-to-noise ratios were measured in the 11 cases. The mean SNRs of the PT, red nucleus and dentate nucleus in the cases where 3.0T FLAIR imaging was performed were significantly lower as compared with the SNRs in the patients where 1.5T FLAIR imaging was performed. The SNR of the CS was not significantly different between the two field strengths (p > 0.05) (Table 2).
Table 2.
SNR of FLAIR Images: 1.5T versus 3.0T
Note.-SNR = signal-to-noise ratio, FLAIR = fluid attenuated inversion recovery, CS = centrum semiovales, PT = pulvinar thalami, RN = red nuclei, DN = dentate nuclei
# Mean SNR + standard deviation
DISCUSSION
FLAIR imaging produces heavily fast spin echo (FSE) T2-weighted cerebrospinal fluid-nulled images by coupling an inversion pulse followed by a long inversion time. The heavy T2-weighting sequences demonstrate that normal white matter is not uniform (1). In normal individuals, the 1.5T FLAIR images show high SI due to unmyelinated fibers in the posterior CS bilaterally, and the high SI is faint and ill-defined (2). In this study, qualitative analysis demonstrated that the 3.0T FLAIR images increased the conspicuity of the high SI for the CS, even though quantitative analysis did not show a significant difference between the two field strengths. This normal MRI finding is often misdiagnosed as white matter disease. The causes that induce signal change have not yet been determined. One possible explanation is a 'dielectric resonance effect'. This effect is manifested as central hyperintensity of the deeper structures and relative hypointensity of the more superficial structures. This effect is accentuated at higher fields and it is clearly evident with the use of quadrature coils on the T1-weighted images (8). In this study, we used a quadrature type head coil. Another possible explanation is the difference of shortening of the T2 relaxation time of the unmyelinated and myelinated fibers of the white matter on the 3.0T imaging. Neema et al. (3) reported that only the 3.0T FLAIR images showed diffuse posterior parenchymal white matter hyperintensity in all the study cases. In addition, those investigators explained that this finding might represent normal hypomyelination relative to the anterior white matter, leading to higher water content (9, 10). In our study, visual analysis showed that the CS had more prominent high SI on the 3.0T FLAIR images in all the normal volunteers, although quantitative analysis did not demonstrate a difference of the SNR in the CS between the two field strengths.
High field strength (≥3.0T) increases magnetic susceptibility as compared to 1.5T and this phenomenon decreases the SI of IDSs on the T2-weighted or T2*-weighted images. Physiological IDSs have an abundant amount of ferritin with aging or for various neurodegenerative disorders. Ferritin in the nonheme iron form has shown more prominent image contrast on the spin echo T2-weighted images as compared to the FSE T2-weighted and T2*-weighted gradient-echo (GRE) images, and the putamen showed no significant difference between the image contrast on the T2*-weighted GRE and FSE at a field strength of 1.5T (8). Allkemper et al. (11) demonstrated that the sensitivity of FSE to susceptibility effects increased with increasing field strength, resulting in a significantly improved depiction of IDSs at a field strength of 3.0T.
The pulvinar nucleus of the thalamus is not a well-known normal iron deposition area. In 1995, Schenck (12) reported that the PT had a relatively short T2 relaxation time (63 msec) as compared to that of the putamen (64 msec) at a field strength of 1.5T. For a higher field strength (4.0T), the PT T2 relaxation time (38 msec) was decreased more than that of the putamen (43 msec). This finding is in good agreement with the PT low SI of our cases. However, we do not know the exact mechanism of this finding. We know that the iron concentration of the iron-rich regions is a major determinant factor of the T2 relaxation time. However, there is no absolute correspondence between the iron concentration and the T2 relaxation time. The T2 relaxation time can be determined not only by the iron concentration, but also by additional factors such as the state of aggregation and the chemical composition of the iron-containing compounds. We cannot explain the exact mechanism of the PT low SI, but the iron concentration may play a role for the low SI. Decreased SI of the thalamus has been reported for abnormal conditions such as multiple sclerosis (13, 14). A further study is required to evaluate the normal iron deposition in the pulvinar nucleus with aging. To the best of our knowledge, our FLAIR imaging findings of low SI of the PT are the first to be reported in the medical literature.
There are several limitations to our study. Our findings should be considered preliminary because of the small sample size. We used the same imaging parameters for both field strengths. In real practice, the echo time (TE) is optimized for image quality depending on the field strength. The change of TE could change the SI of the anatomic regions on the T2-weighted FLAIR images.
In conclusion, we have demonstrated the normal high SI of the CS and the low SI of the PT and IDSs on the 3.0T FLAIR images. These normal findings should be considered when interpreting the 3.0T FLAIR images of the brain.
Footnotes
This work was supported by a brain research promoting grant from Keimyung University Dongsan Medical Center in 2001.
References
- 1.Hajnal JV, De Coene B, Lewis PD, Baudouin CJ, Cowan FM, Pennock JM, et al. High signal regions in normal white matter shown by heavily T2-weighted CSF nulled IR sequences. J Comput Assist Tomogr. 1992;16:506–513. doi: 10.1097/00004728-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gawne-Cain ML, Silver NC, Moseley IF, Miller DH. Fast FLAIR of the brain: the range of appearances in normal subjects and its application to quantification of white-matter disease. Neuroradiology. 1997;39:243–249. doi: 10.1007/s002340050402. [DOI] [PubMed] [Google Scholar]
- 3.Neema M, Guss ZD, Stankiewicz JM, Arora A, Healy BC, Bakshi R. Normal findings on brain fluid-attenuated inversion recovery MR images at 3T. AJNR Am J Neuroradiol. 2009;30:911–916. doi: 10.3174/ajnr.A1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein MA, Huston J, 3rd, Lin C, Gibbs GF, Felmlee JP. High-resolution intracranial and cervical MRA at 3.0T: technical considerations and initial experience. Magn Reson Med. 2001;46:955–962. doi: 10.1002/mrm.1282. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein WA, Glover GH, Hardy CJ, Redington RW. The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med. 1986;3:604–618. doi: 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Linera J. 3T MRI: advances in brain imaging. Eur J Radiol. 2008;67:415–426. doi: 10.1016/j.ejrad.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Chang KH, Na DG, Song IC, Kim SJ, Kwon BJ, et al. Comparison of 1.5T and 3T 1H MR spectroscopy for human brain tumors. Korean J Radiol. 2006;7:156–161. doi: 10.3348/kjr.2006.7.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque TL, Miki Y, Kanagaki M, Takahashi T, Yamamoto A, Konishi J, et al. MR contrast of ferritin and hemosiderin in the brain: comparison among gradient-echo, conventional spin-echo and fast spin-echo sequences. Eur J Radiol. 2003;48:230–236. doi: 10.1016/s0720-048x(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 9.Murakami JW, Weinberger E, Shaw DW. Normal myelination of the pediatric brain imaged with fluid-attenuated inversion-recovery (FLAIR) MR imaging. AJNR Am J Neuroradiol. 1999;20:1406–1411. [PMC free article] [PubMed] [Google Scholar]
- 10.Ashikaga R, Araki Y, Ono Y, Nishimura Y, Ishida O. Appearance of normal brain maturation on fluid-attenuated inversion-recovery (FLAIR) MR images. AJNR Am J Neuroradiol. 1999;20:427–431. [PMC free article] [PubMed] [Google Scholar]
- 11.Allkemper T, Schwindt W, Maintz D, Heindel W, Tombach B. Sensitivity of T2-weighted FSE sequences towards physiological iron depositions in normal brains at 1.5 and 3.0T. Eur Radiol. 2004;14:1000–1004. doi: 10.1007/s00330-004-2241-4. [DOI] [PubMed] [Google Scholar]
- 12.Schenck JF. Imaging of brain iron by magnetic resonance: T2 relaxation at different field strengths. J Neurol Sci. 1995;134:10–18. doi: 10.1016/0022-510x(95)00203-e. [DOI] [PubMed] [Google Scholar]
- 13.Drayer B, Burger P, Hurwitz B, Dawson D, Cain J. Reduced signal intensity on MR images of thalamus and putamen in multiple sclerosis: increased iron content? AJR Am J Roentgenol. 1987;149:357–363. doi: 10.2214/ajr.149.2.357. [DOI] [PubMed] [Google Scholar]
- 14.Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC. Comparison of multiple sclerosis lesions at 1.5 and 3.0 tesla. Invest Radiol. 2003;38:423–427. doi: 10.1097/01.RLI.0000065426.07178.f1. [DOI] [PubMed] [Google Scholar]