Abstract
CT is increasingly being used for evaluating the cardiovascular structures and airways in the patients with congenital heart disease. Multi-slice CT has traditionally been used for the evaluation of the extracardiac vascular and airway abnormalities because of its inherent high spatial resolution and excellent air-tissue contrast. Recent developments in CT technology primarily by reducing the cardiac motion and the radiation dose usage in congenital heart disease evaluation have helped expand the indications for CT usage. Tracheobronchomalacia associated with congenital heart disease can be evaluated with cine CT. Intravenous contrast injection should be tailored to unequivocally demonstrate cardiovascular abnormalities. Knowledge of the state-of-the-art CT imaging techniques that are used for evaluating congenital heart disease is helpful not only for planning and performing CT examinations, but also for interpreting and presenting the CT image findings that consequently guide the proper medical and surgical management.
Keywords: Computed tomography (CT) techniques, Multi-slice CT, Congenital heart disease
The recent developments in CT techniques are characterized by faster speed, longer anatomic coverage, a more flexible ECG-synchronized scan and a lower radiation dose, and these advances have noticeably increased the cardiac applications of CT. This increasing role of CT also includes the evaluation of congenital heart disease (1-3). Minimization of the radiation exposure delivered by CT is an important issue particularly for children (4, 5). Various dose reduction techniques are currently available for cardiac CT as a result of the efforts to reduce the CT dose (6, 7). Thus, cardiac radiologists should be familiar with the CT techniques that are associated with a cardiac protocol and dose reduction. The CT imaging techniques for congenital heart disease are not the same as those for acquired heart disease: they are different according to the imaged anatomic structures, the purposes of the study and the study population evaluated with CT (e.g. children and adults with congenital heart disease). The state-of-the-art CT imaging techniques for acquired heart disease have been extensively appraised and frequently updated, while those for congenital heart disease have not been thoroughly reviewed in the literature. In this article, I review the current CT imaging techniques for congenital heart disease. These include the CT scan techniques, the dose reduction techniques and the methods for intravenous injection of contrast agent. The current clinical applications of CT and the future developments in CT technology are also discussed.
ECG-SYNCHRONIZATION
Among the various CT parameters, temporal resolution is the most important one for evaluating congenital heart disease because the study population for this malady commonly includes children with high heart rates and limited ability to cooperate. Therefore, the fastest gantry rotation speed is preferred, and 270-280 ms is currently the fastest gantry rotation time. In contrast to the conventional interpolation algorithms for non-ECG-synchronized spiral scans, a dedicated image reconstruction algorithm using a half scan is employed for the ECG-synchronized scan. Thus, the temporal resolution of the dedicated algorithm corresponds to half of the gantry rotation time, for example, 135 ms for a gantry rotation time of 270 ms. The improved temporal resolution by a half scan itself significantly reduces cardiac pulsation artifacts on the CT images (8). Although non-ECG-synchronized spiral CT has been mainly used for assessing the extracardiac vascular abnormalities of congenital heart disease (1, 2, 9), the introduction of the ECG-synchronized scan with either retrospective gating or, more recently, prospective triggering is truly a step forward for the CT imaging of congenital heart disease (2, 3, 10, 11). ECG-synchronization enables us to accurately evaluate the coronary arteries (10, 11), the conotruncal and other intracardiac structures (3) and the ventricle function and volumetry (12). The highest temporal resolution, 75-83 ms, of the ECG-synchronized scan is currently achieved with dual-source CT (13, 14).
Non-ECG-Synchronized Spiral CT Scan
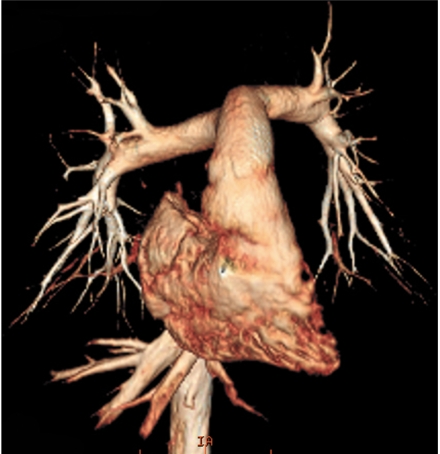
Non-ECG-synchronized spiral scanning is a simple, easy technique to perform CT in patients with congenital heart disease. Cardiac CT with this scan technique has been widely used since the introduction of multi-slice CT. The capability of multi-slice CT to produce submillimeter isotropic volume data greatly improves the image quality of the multiplanar reformatted and three dimensional CT images, which are vital for evaluating congenital heart disease (Fig. 1). A thinner collimation/slice thickness and a reconstruction interval corresponding to approximately half of the slice thickness (e.g. 50% overlapping) are recommended to achieve isotropic voxels. A pitch of 1 usually provides a good balance between image quality and the radiation dose. Pitches lower than 1 add a little in terms of image quality at the expense of an increased radiation dose. Conversely, high pitches (> 1) tend to degrade the image quality. It should be noted that high pitches actually result in little dose saving in a CT system that employs an effective mA instead of an mA. Based on CT physics, a thin slice thickness increases the image noise at the same radiation dose. Of note, a slight increase in slice thickness (for example, 0.75 mm rather than 0.6 mm at a collimation of 0.6 mm) offers a substantial benefit for image quality at the expense of an unnoticeable decrease of the longitudinal spatial resolution (Fig. 2). The z-flying focal spot technology increases the longitudinal spatial resolution, by a factor of approximately 1.4, by double sampling in the z-direction (15). The technique also reduces windmill artifacts that are caused by the cone beam geometry of multi-slice CT (15).
Fig. 1.
Volume-rendered CT image with non-ECG-synchronized spiral scan shows excellent anatomic details of pulmonary arteries in 12-year-old girl with repaired coarctation of aorta.
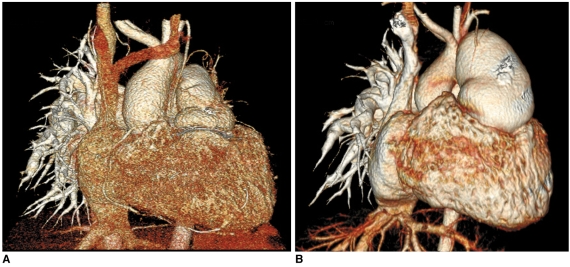
Fig. 2.
Effect of slice thickness on image quality of non-ECG-synchronized spiral CT in 12-year-old boy with pulmonary atresia and ventricular septal defect after Rastelli operation.
A. Volume-rendered CT image reconstructed from thin, overlapped axial images with 0.6-mm slice thickness at collimation of 0.6 mm appears quite grainy. That is because slice thickness is too thin at employed CT dose and this thin slice consequently increases image noise enough to degrade image quality. There are two ways to improve image quality in this situation: one is to slightly increase slice thickness and the other is to increase radiation dose a lot.
B. Slight increase in slice thickness to 0.75 mm substantially improves image quality of volume-rendered CT image. This strategy is highly recommended because its dose saving effect is great.
The scan technique may be acquired during either breath-holding or free-breathing. Thanks to the short scan time, the respiratory motion artifacts on the multi-slice CT images of free-breathing young children are surprisingly less remarkable than those on the single-slice CT images. On top of that, it is interesting to know that the origins and proximal segments of the coronary arteries are frequently observed, in 82% of the patients with congenital heart disease, on the non-ECG-synchronized spiral CT images (16). Thicker collimation is useful to shorten the CT scan time, which enables young children around 3-6 years of age to hold their breath during CT scan. In order to meet the need for a shorter breath-holding time for a child, higher pitches in addition to thicker collimation may be helpful to further reduce the scan time. These strategies allow reducing the frequency of sedation for a CT examination. On the other hand, thicker collimation is better than a thinner one for older children or adults because thicker collimation reduces the CT radiation dose and it improves the image quality of the CT images. Regarding the CT dose, we should know that unnecessary radiation exposure outside of a scan range, the so-called 'z-overranging' or 'z-overscanning', is inevitable with this scan technique and conventional collimation technology. The contribution of z-overranging to the total CT dose is not negligible, but it is actually enormous (up to 70%) particularly for pediatric CT with a short scan range, as is used for heart CT (17). Fortunately, adaptive collimation technology that can protect from z-overranging is currently being made available by some of CT manufacturers (18). When appropriate CT parameters are used, the radiation dose of the scan technique is usually less than 1.0 mSv in young children (9).
Retrospective ECG-Gated Spiral CT Scan
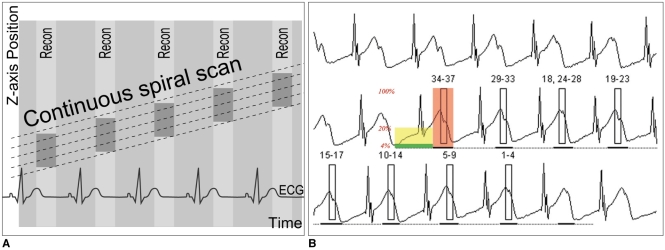
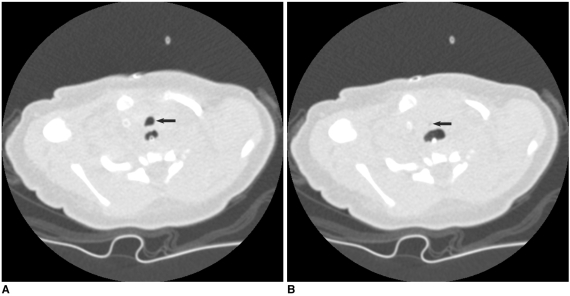
The relatively high radiation dose of retrospective ECG-gated spiral scanning is primarily attributed to the low pitch that is necessary to obtain gapless ECG-gated spiral data (Fig. 3). When a mode of a heart rate-dependent pitch is available, the radiation dose is reduced with a higher pitch that is automatically adapted to higher heart rates (19). However, it should be noted that the mode may degrade the image quality when the heart rate is unstable during the actual CT scan. If this is the case during a breath-holding test, then the use of manual selection of the target heart rates is better. A multi-segment reconstruction algorithm may be used to increase the temporal resolution. However, a single-segment reconstruction algorithm is usually preferred to a multi-segment one because the latter prolongs the scan time and thus it increases the variability of the heart rate. In addition, the temporal resolution of the multi-segment reconstruction algorithm is variable depending on the heart rates. Consequently, there is a higher chance to obtain better image quality with using a single-segment reconstruction algorithm. An optimal cardiac phase, which is important in applying ECG-controlled tube current modulation, depends on the heart rate. The mid-diastolic phase, the so-called 'diastasis', is chosen at lower heart rates, while the end-systolic phase is selected at higher heart rates (> 75-80 bpm). Then, a width with the full tube current should be determined around the selected cardiac phase. If the width is too broad, then there will be a dose penalty. In contrast, there will be an increased chance to lose the right cardiac phase if the width is too narrow. When the reason for performing cardiac CT is only for the morphologic evaluation, a 10% width of the cardiac cycle may be a good balance between the two factors (for example, 35-45% for the end-systolic phase and 65-75% for the mid-diastolic phase). The width should be broader than 10% when the heart rate is irregular or functional evaluation is indicated. The best cardiac phase with minimal motion may be selected either manually by previewing the multi-phase images at one or more slices or automatically by using a motion map (20). The scan time is relatively short, usually less than 10 s. Breath-holding is usually recommended because this scan mode is relatively vulnerable to respiratory motion artifacts (Fig. 4). Nevertheless, some investigators have attempted performing a retrospective ECG-gated spiral CT scan in free-breathing neonates (11). The effective dose estimates of this scan mode with ECG-controlled tube current modulation are 5-7 mSv for the patients with acquired heart disease (21) and 2-6 mSv for the patients with congenital heart disease (10) for dual-source CT.
Fig. 3.
Diagrams showing retrospective ECG-gated spiral CT scan.
A. As demonstrated on schema, spiral scan is acquired with low enough pitch to avoid gap in CT data. This spiral CT data is retrospectively reconstructed at specific cardiac phase by synchronizing with simultaneously acquired ECG data.
B. For ECG-controlled tube current modulation, period with 100% tube current should be appropriately determined depending on heart rates. End-systolic phase (red rectangle) is used at high heart rates. Tube current can be reduced to 20% (yellow rectangle) or 4% (light green rectangle) during rest of cardiac cycle.
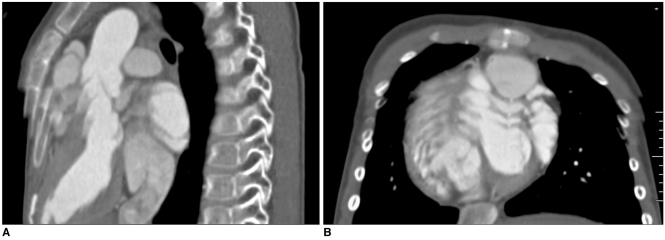
Fig. 4.
6-year-old boy with repaired tetralogy of Fallot. Sagittal (A) and short-axis (B) retrospective ECG-gated spiral CT images, which are affected by severe respiratory motion artifacts, are shown at midportion of scan range.
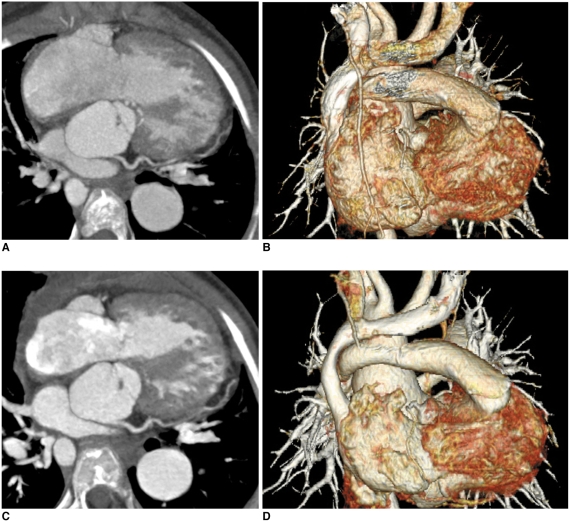
Prospective ECG-Triggered Sequential CT Scan
Prospective ECG-triggered sequential scanning is a low dose ECG-synchronization technique that can reduce the CT dose to 1-3 mSv in adults (22) and to 0.2-0.7 mSv in newborns and infants (10). In contrast to spiral scanning, it does not employ continuous table movement or a zoverranging effect (Fig. 5A). The degraded ECG-synchronization, seen as stair-step artifacts, may occur at irregular heart rates with this technique. The artifacts are less pronounced in the images acquired at the end-systolic phase because the phase is relatively constant irrespective of the heart rates (Fig. 5B). For the same reason, absolute trigger delay (ms) is preferred to relative trigger delay (percentage) for targeting the end-systolic phase. As compared with retrospective ECG-gated spiral scanning, the lack of capability for multi-phase functional evaluation has been regarded as a limitation of this technique. However, this limitation is partly overcome with a recently available option of longer data acquisition (Fig. 6). This longer data acquisition allows us to perform fine adjustment for the best cardiac phase at lower heart rates and even to evaluate cardiac motion at high heart rates if the acquisition time is long enough to cover the second half of the cardiac cycle (Fig. 6; Electronic supplementary material, animation 1).
Fig. 5.
Diagrams showing prospective ECG-triggered sequential CT scan.
A. Sequential scan is acquired without table movement at predefined cardiac phase, which is end-systolic phase in this diagram. Then, time period is necessary to move CT table to next scan. Thus, scan mode is commonly called 'step and shoot' mode.
B. ECG shows variable heart rates ranging from 54 bpm to 111 bpm. This variability in heart rates is commonly considered to be disadvantageous for performing prospective ECG-triggered sequential scan for mid-diastolic phase. However, this is not case when end-systolic phase is target. As demonstrated on this diagram, end-systolic phases can be consistently acquired at irregular heart rate with prospective ECG-triggered sequential scan. It should be noted that absolute delay, for instance, 240 ms in this case, must be used to have this benefit.
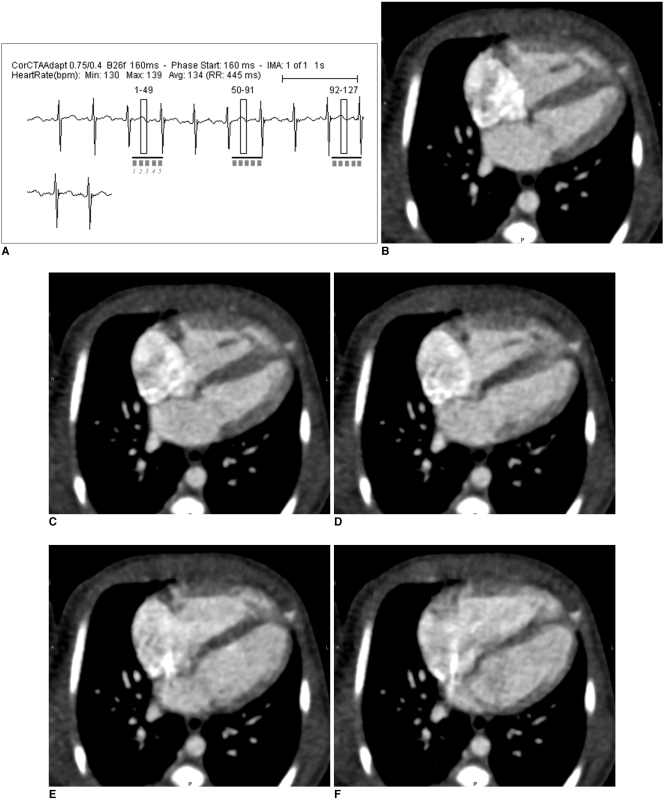
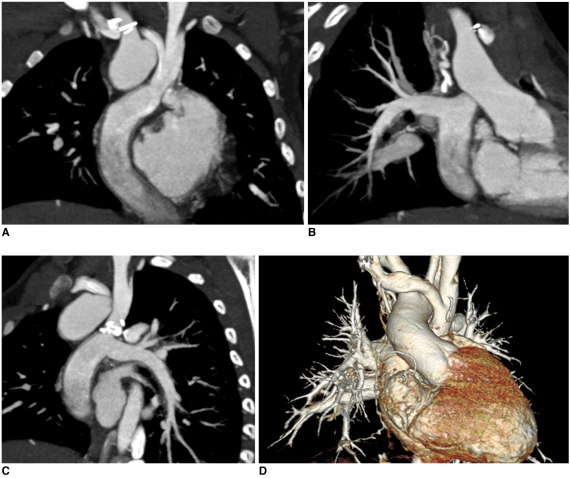
Fig. 6.
Multi-phase prospective ECG-triggered sequential CT scan.
A. Extended scan mode (0.38 s), in which period longer than necessary for single phase (0.2 s) is obtained, offers multi-phase study. Cardiac function can be evaluated with this scan mode at high heart rates (134 bpm in this case) because acquisition window covers both end-systole and end-diastole.
B-F. After scan, five cardiac phases are retrospectively reconstructed at approximately 40-ms intervals (B, 160 ms; C, 200 ms; D, 240 ms; E, 280 ms; F 340 ms).
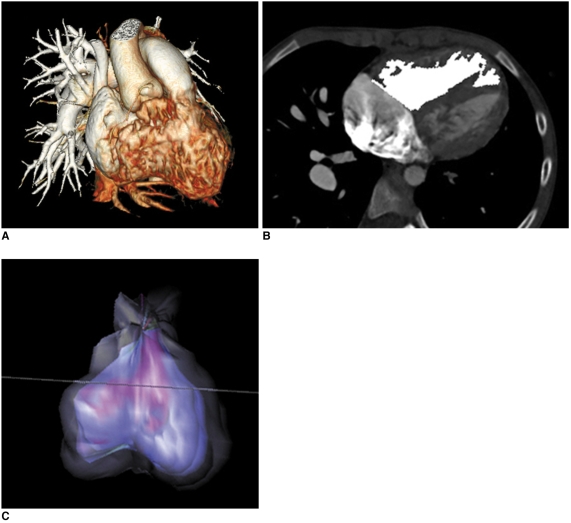
In contrast to retrospective ECG-gated spiral scanning, the respiratory motion artifacts tend to be less pronounced in a beam collimation or one slab scan. Therefore, this scan technique frequently provides motion-free CT images even in free-breathing young children (Fig. 7). However, various degrees of stair-step artifacts may occur between the adjacent slabs (Fig. 8A). Overlap (minimum value, 2.2-5.0 mm) between adjacent slabs, which is achieved by a table feed smaller than a beam collimation, makes some of the stair-step artifacts less conspicuous. A difference in cardiovascular enhancement is frequently noted between adjacent slabs because more than two heart beats are necessary to move the CT table for the next ECG-triggered scan, the socalled 'step and shoot' scan mode (Fig. 8B). Thus, a special intravenous injection method that prolongs the peak vascular enhancement may ameliorate this difference of cardiovascular enhancement. The total examination time is prolonged for the same reason, but it becomes shorter with the increased longitudinal coverage of a recently introduced multi-slice CT system, for instance, single gantry rotation time for 320-slice CT (23). As in spiral scanning, the use of a variable slice thickness other than a multiple of a detector collimation has recently become available for prospective ECG-triggered sequential scanning.
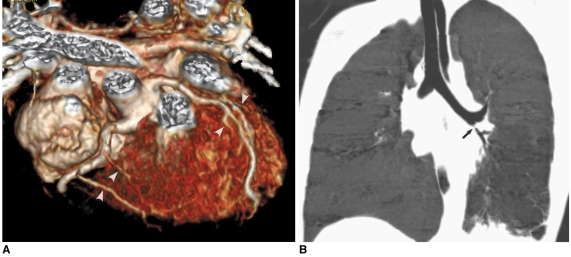
Fig. 7.
Prospective ECG-triggered sequential CT scan in free-breathing young children is relatively less susceptible to respiratory motion artifacts than is retrospectively ECG-gated spiral CT scan. With this scan mode, even side-branches (arrowheads) of coronary arteries (A) and stenosis (arrow) involving left bronchi (B) are clearly delineated without cardiac and respiratory motion artifacts in young children. Therefore, more invasive or sophisticated preparation procedures such as general anesthesia or controlled ventilation are not currently needed for only diagnostic purposes.
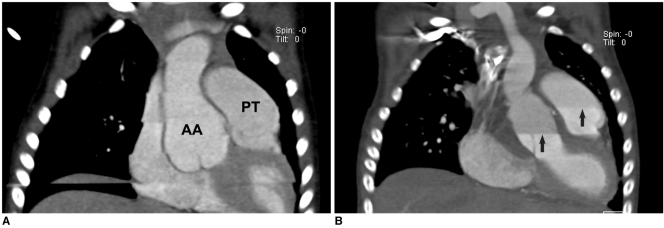
Fig. 8.
Artifacts on prospective ECG-triggered sequential CT scan.
A. Various degrees of stair-step artifacts derived from respiration motion are seen on coronal CT image. Artifacts are typically much more pronounced around diaphragm. Aneurysmal dilatations involving ascending aorta (AA) and pulmonary trunk (PT) are noted in 3-year-old girl with Loeys-Dietz syndrome.
B. Stair-step artifacts on prospective ECG-triggered sequential coronal CT image are remarkably decreased by applying combined ECG and respiratory triggering for same patient who underwent valve sparing aortic root replacement surgery. In contrast, difference in cardiovascular enhancement is frequently observed between adjacent slabs (arrows) due to long examination time that is further lengthened by combined triggering.
Combo CT Scan
For free breathing young children, there is no single scan mode that can satisfactorily evaluate all the cardiovascular structures and the airway. A non-ECG-synchronized spiral scan is subject to cardiac and respiratory motion artifacts. In particular, cardiac motion artifacts on the scan mode commonly make the anatomic evaluation of the coronary arteries, proximal great arteries and intracardiac structures difficult. On the other hand, the prolonged examination time of a prospective ECG-triggered sequential scan using the currently available first-generation dual-source CT system may cause motion artifacts other than cardiac pulsation, and there is a difficulty for maintaining peak vascular enhancement. A temporary solution to this matter may be a combination of both scan modes, the so-called 'Combo' scan. In this scan mode, two or three slabs of a prospective ECG-triggered sequential scan confined to the conotruncal area of the heart follow a non-ECG-synchronized spiral scan at the expense of a small additional radiation dose (Fig. 9). As a result, all the cardiovascular and airway structures related to various congenital heart diseases can be much more satisfactorily evaluated with this 'Combo' scan than with using either a non-ECG-synchronized spiral scan or a prospective ECG-triggered sequential scan alone. The average CT dose of this scan mode is approximately 1.4 mSv. It is speculated that this 'Combo' scan for free-breathing young children with congenital heart disease will be replaced by an advanced prospective ECG-triggered sequential scan with second-generation dual-source CT or with a combined (ECG and respiration) triggering method (Fig. 8B).
Fig. 9.
Combo CT scan comprised of non-ECG-synchronized spiral scan with usual scan range (A, B) and prospective ECG-triggered sequential scan with narrow scan range confined to conotruncal area of heart (C, D) in 9-months-old boy with Williams syndrome. Supravalvular aortic stenosis (arrows on C) and combined valvar (arrow on D) and subvalvar (arrowheads on D) pulmonary stenoses are clearly shown on prospective ECG-triggered sequential CT images (C, D). Dose estimates are 1.6 mSv for non-ECG-synchronized spiral scan and 0.2 mSv for prospective ECG-triggered sequential scan.
CINE CT FOR AIRWAYS
Vascular or non-vascular airway narrowing is a frequent complication in patients with congenital heart disease, and this may result in respiratory difficulty or failure to be weaned from a ventilator (24). The airway compression may be a fixed form of narrowing, a dynamic form of narrowing or a combination of the two. Dynamic airway obstruction, termed tracheobronchomalacia, may develop as a result of longstanding vascular airway compression, recurrent airway infection/inflammation or a congenital anomaly. The other CT scan techniques previously mentioned in this article allow us to make the diagnosis of fixed airway narrowing, but only the cine CT technique enables us to identify tracheobronchomalacia by demonstrating the excessive expiratory collapsibility of airways throughout the respiratory cycle (Fig. 10; Electronic supplementary material, animation 2). Cine CT is a non-spiral, sequential scan that is acquired without table movement. In order to minimize the radiation dose related to cine CT, the scan time should be limited to the minimum that is slightly longer than one respiratory cycle. The multi-segment CT images are retrospectively reconstructed by means of a partial reconstruction algorithm (180° of gantry rotation + the fan beam angle) (25). The estimated dose of cine CT is low, for example, in the range of 0.2-0.3 mSv when the images are obtained at six slice levels (25). In addition to the evaluation of tracheobronchomalacia, air trapping in the lung can also be detected with cine CT (25).
Fig. 10.
Cine CT (A, inspiratory phase; B, expiratory phase) for diagnosis of tracheomalacia in 16-day-old female newborn with tracheoesophageal fistula, esophageal atresia and tetralogy of Fallot. Excessive expiratory collapsibility of trachea (arrows) is displayed on cine CT images. Distended proximal esophagus is also seen posterior to trachea.
STRATEGIES FOR CT DOSE REDUCTION
The importance of tailoring the CT scan techniques to minimize the radiation dose, while the diagnostic image quality is preserved, cannot be overemphasized and particularly in children. It goes without saying that clear and sound clinical indications are the premise. Various useful strategies to reduce the cardiac CT dose are described in this section of the paper. Another important thing for the cardiac radiologists who are involved in CT imaging of congenital heart disease is having a concrete knowledge about how to estimate the CT dose in both children and adults. Among the several dose estimation methods, the dose-length product-based method is the most simple and practical. The dose estimate is easily calculated by multiplying the dose-length product on the CT dose report by an age, gender and scanner-specific conversion factor (5). The only thing to be careful about is that a dose-length product for children must be derived from a 16-cm CT dose index phantom.
Body-Size Adaptive Low Dose CT Protocols
Establishing a body-size adaptive CT protocol is a first step for CT dose minimization because body-size adaptation is critical for children because they have greater radiosensitivity, a longer life expectancy and a smaller body size (4, 5). Various indices that reflect body size, including the body weight, the body height, the body mass index, the cross-sectional dimensions and the attenuation, can be used for the adaptive CT protocol. The weight-based protocol is easy to use and practical, and so body weight is the most commonly used body-size parameter. Because the benefits of low kV for thin adults and children are well established, a weight-based heart CT protocol using variable kV and variable mA should be used (5). On the other hand, the cross-sectional dimension-based protocol is better for adapting the CT dose to an individual body habitus, as compared with the weight-based protocol (26). Nevertheless, the cross-sectional dimension-based CT protocol has not been commonly used because a practical method for this is currently not available.
Minimization of Z-Axis Coverage and Z-Overranging
Minimization of the scan range along the z-axis according to the individual clinical indications is a simple, effective way to minimize the CT dose. This is attributed to the fact that the CT dose is directly proportional to the scan range when the other CT parameters are the same. A longer scan range may be necessary for evaluating the infracardiac type of total anomalous pulmonary venous return or the major aortopulmonary collateral arteries. Otherwise, the longitudinal coverage usually ranges from the aortic arch to the most caudal margin of the heart. Because the safety margins at both ends are very narrow at a minimal z-axis, the scout image should be updated whenever there is any doubt as to whether or not a patient moved during a preparation period after the scout imaging. In fact, reexamination following a failed study is the worst scenario for minimizing the CT dose. It should be noted that contribution of 'z-overranging' to the total CT dose is greater for a shorter z-axis range. This relationship is derived from the physical features of 'z-overranging': the effect is proportional to the beam collimation, the reconstructed slice width and the pitch, while it is irrespective of the planned scan length (27). For the maximal dose saving effect of minimal z-axis coverage, it is highly recommended to use the adaptive collimation technology for eliminating 'z-overranging'.
Tube Current Modulation
Tube current modulation or automatic exposure control is a useful dose-saving technique that reduces the CT dose according to the body size and shape, and the attenuation. In order to correctly use the technique, we should clearly understand the various methods of tube current modulation (28): it may be applied along the x-y plane (angular mode), the z-axis or a combination of both; a reference for modulation is based on the standard deviation, the noise index, a reference mA or a reference image. The degree of dose reduction is variable (it is 9-26% in pediatric heart CT [29]), depending on the anatomic region and the individual body features. In addition, it should be kept in mind that dose modulation is influenced by many factors such as the distance from the gantry isocenter, the kV level, scan direction and so on (30). Yet the technique is limited by the fact that there is no guideline for how to determine a suitable reference value for each anatomic region, for individual clinical tasks and for a different level of kV, and so the best possible decision remains vague and difficult.
ECG-Controlled Tube Current Modulation and Low KV
With this ECG-based tube current modulation technique or ECG pulsing for a retrospective ECG-gated spiral scan, the tube current outside a predefined window in the cardiac cycle can be reduced by 20% of the full tube current in the conventional mode or by 4% in the 'MinDose' mode (Fig. 3B). The former can be used for functional evaluation of the heart at a lower dose, whereas the latter is only for anatomic evaluation of the cardiovascular structures. Optimal ECG pulsing windows that depend on three ranges of heart rates were reported in adults with coronary artery disease: 60-76% at low heart rates (≤65 bpm), 30-77% at intermediate heart rates (66-79 bpm) and 31-47% at high heart rates (≥80 bpm) (31). This optimized use of ECG pulsing can reduce the radiation dose of retrospective ECG-gated spiral CT by up to 64% (31). Combined use of this ECG pulsing and low kV further reduces the radiation dose of retrospective ECG-gated spiral CT (21) (Fig. 11).
Fig. 11.
Dose reduction by applying low kV and ECG-controlled tube current modulation to retrospective ECG-gated spiral CT scan in 9-year-old girl with pulmonary atresia, ventricular septal defect and major aortopulmonary collateral arteries and who underwent Rastelli operation. Dose estimate of CT scan acquired with 120 kV and conventional ECG pulsing (A, B) is 9.2 mSv (heart rate: 88 bpm). On other hand, dose estimate can be reduced to 3.5 mSv by applying 100 kV and MinDose for follow-up CT (heart rate: 86 bpm). Of interest, image quality of CT images is much better and degree of cardiovascular enhancement appears much higher on follow-up CT with lower radiation dose.
A three-dimensional noise reduction reconstruction algorithm and a cardiac bow-tie filter are also used to reduce the radiation dose of ECG-synchronized CT (19).
INTRAVENOUS CONTRAST INJECTION METHODS
Optimal enhancement of the cardiovascular structures is essential for cardiac CT. The amount of iodinated contrast agent may be reduced to 1.0-1.5 ml/kg, thanks to the shorter scan time of the current CT systems, in the usual cases of congenital heart disease. A larger amount up to 2.0 ml/kg is necessary for the cases with a large intracardiac or extracardiac shunt, substantial valvular regurgitation and/or a severely enlarged heart. A high iodine delivery rate (mgI/ml/sec), a unit comprising both the iodine concentration and the injection rate, is more important than an high iodine concentration of the contrast agent itself to achieve higher cardiovascular enhancement. The injection rate has to be appropriately adjusted depending on the size of the placed angiocatheter and the body size. In my practice, the amount of contrast agent is first determined and the injection rate is then calculated to meet an approximately 15-second injection time of the contrast agent in most of cases. For instance, the injection rate would be 1.0 ml/sec when 1.5 ml/kg of contrast agent is used for a 10-kg child. Other investigators have suggested a simplified method (32), the so-called 'contrast-covering time', which is basically similar to the injection methods advocated by others. The intravenous injection site is important and it should be properly selected in advance: the leg vein is preferred for the evaluation of the aortic arch and the presence of the left superior vena cava; simultaneous injection of 50% diluted contrast agent through the arm and leg veins is preferred for the evaluation of Fontan pathway (Fig. 12); the leg vein should not be used in patients who have undergone bidirectional cavopulmonary connection (1, 2).
Fig. 12.
Non-ECG-synchronized multiplanar reformatted (A-C) and volume-rendered (D) CT images clearly show patent Fontan pathway and pulmonary vessels in 5-year-old boy with functional single ventricle. Simultaneous intravenous injection of 50% diluted contrast agent through arm and leg veins was used to obtain homogeneously high enhancement of cardiovascular structures.
Three methods are used to determine the appropriate scan delay. They include the empirical method, the test injection method and the bolus tracking method. A bolus tracking method is the most commonly used method among them (1, 2). In order to minimize the radiation dose, a monitoring delay should be slightly earlier than the actual peak enhancement of a target cardiovascular structure: 13 seconds for visualizing the right heart and the pulmonary circulation, and 15 seconds for visualizing the left heart and the systemic circulation. In contrast, other researchers have recommended beginning the bolus tracking series before the contrast injection starts (33). However, a substantial radiation dose might be accumulated simply by the bolus tracking series rather than the actual scan. In fact, the dose from the bolus tracking series turned out to be obviously too much, that is, 2.7 mSv on the average (34). The threshold of the CT density to trigger a scan is usually between 100 HU and 150 HU. A delay between this trigger and the actual scan ranges from a minimal value (2-5 seconds depending on a CT model) to 7 seconds, and the latter is necessary for breath-hold instructions. A region of interest for bolus tracking should be appropriately placed depending on the clinical indications in the cardiac chambers (the right ventricle, the left atrium and the left ventricle) or vessels (the internal jugular vein in the patients with a Fontan operation to target the second circulation of contrast agent). It should be noted that the right atrium is not used because this location is subject to artifacts from the undiluted contrast agent. As a rule of thumb, the scan delay for bolus tracking is targeted at the peak aortic enhancement, which occurs approximately 5 seconds after the completion of contrast injection (35). A test injection method is more accurate than a bolus tracking method for achieving peak vascular enhancement. In general, the scan delay is increased by 3-5 seconds from the calculated transit time of the contrast agent because a larger volume is used at the main CT scan. Therefore, I use the method usually for the evaluation of pulmonary thromboembolism for which it is important to have peak enhancement of the pulmonary circulation precisely during the CT scan. I use the peak enhancement time of the pulmonary vein for the scan delay for the evaluation of pulmonary thromboembolism. Power injection is preferred to manual injection because the results of the former are more consistent and reproducible.
As has happened for the CT techniques, the contrast injection protocol has also evolved over time. As for CT imaging of congenital heart disease, its goal is to simultaneously achieve adequate vascular enhancement and minimal perivenous artifacts. In addition, the injection protocol should delineate all the cardiovascular structures on CT. A protocol employing a saline chaser with using a single- or dual-head power injector allows us to reduce the amount of contrast agent and the perivenous artifacts for thoracic CT (36). We do not have to worry about the amount of the saline flush because the intravenous injection would be stopped immediately after the end of the main scan. A triphasic intravenous injection protocol, in which undiluted contrast agent is followed by 50-60% diluted contrast agent and then by a saline chaser, was recently developed to improve the visualization of the right heart, to circumvent poor enhancement of the pulmonary artery and to reduce the perivenous artifacts (37). In my practice, 5-10% diluted contrast agent, instead of a saline chaser, is used at the third phase of the injection protocol when simultaneous visualization of the systemic veins is required.
CLINICAL APPLICATIONS
The clinical applications of CT for patients with congenital heart disease are rapidly changing and growing in keeping with the technical developments of CT. Classically, the extracardiac great vessels, including the aortic arch and the pulmonary vessels, have been evaluated with CT (1-3, 9). Evaluation of the patency of vascular shunts, conduits or stents is another traditional indication of CT (38). A dedicated reconstruction algorithm for vascular stents has recently been introduced for better evaluation of in-stent stenosis. Evaluation of airways is an exclusive merit of CT over the other imaging modalities. Vascular or non-vascular airway narrowing, airway anomalies and dynamic airway obstruction can all be accurately evaluated with CT (24, 39, 40). The introduction of the ECG-synchronized CT scan improves the visibility of those cardiovascular structures that are greatly affected by cardiac pulsation. They include the ascending aorta, the pulmonary trunk, the coronary artery and the heart. Thus, various congenital heart diseases that involve the above mentioned anatomic structures, such as Williams syndrome and Marfan syndrome, can be accurately evaluated with ECG-synchronized CT scanning (Figs. 8, 9) (2, 3, 10, 11). ECG-synchronized CT scanning has become a reality in our clinical practice thanks to the increased flexibility and the reduced radiation dose of the scan technique. In place of cardiac MRI, CT may be alternatively used for the evaluation of cardiac function and ventricle volumetry, for instance, in a patient with repaired tetralogy of Fallot and with or without a permanent cardiac pacemaker (Fig. 13).
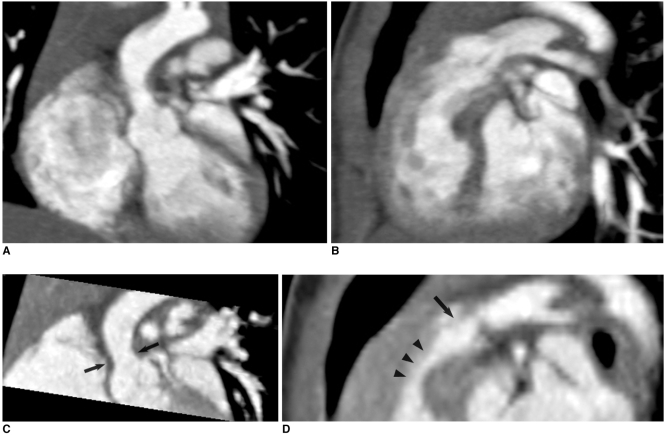
Fig. 13.
Right ventricle CT volumetry in 8-year-old boy with repaired tetralogy of Fallot. Retrospective ECG-gated spiral CT was performed because of inconsistent right ventricle volumes on serial cardiac MR examinations (end-systolic volume of right ventricle was normalized to body surface area, 86 → 128 ml/m2).
A. Crisp margin of right ventricle is shown with high spatial resolution on volume-rendered CT image.
B, C. Consequently, right ventricular cavity can be accurately segmented with three-dimensional region growing method. End-systolic volume of right ventricle that's normalized to body surface area calculated with CT is 86 ml/m2, which indicates that volume at second cardiac MR examination is inaccurate.
FUTURE DEVELOPMENTS
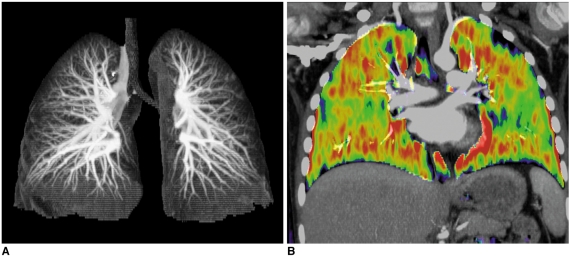
CT techniques are continuously evolving. For cardiac applications, second-generation dual-source CT and large-coverage volume CT (256-320 slices) have come into the spotlight (14, 23). On top of the higher spatial resolution and longer coverage, other innovative technical developments are waiting for the initial clinical results. They include 1) a high-pitch (up to 3.4) dual-source spiral scan, 2) a dual-energy lung perfusion and ventilation scan (Fig. 14), 3) a new type of tube current modulation to reduce the radiation dose to superficial radiation-sensitive tissue such as the breast, the thyroid gland or the eyeball, 4) automatic suggestions for the CT dose parameters according to the individual clinical task and the anatomic region, and (5) combined triggering of the ECG and respiration (Fig. 8).
Fig. 14.
Dual-energy lung perfusion CT. In addition to pulmonary CT angiography (A), lung perfusion status (B) can be obtained without additional radiation dose by means of dual-energy CT technology when pulmonary thromboembolism is suspected in patients with congenital heart disease.
CONCLUSION
The state-of-the-art CT imaging techniques for congenital heart disease are useful to evaluate diverse cardiovascular and airway abnormalities with improved accuracy and patient- and user-friendliness. Therefore, CT is steadily becoming an invaluable imaging modality to fill the gap among echocardiography, cardiac catheterization and cardiac MRI. Moreover, cardiac CT is not a radiation-intensive study any more when the appropriate dose-saving strategies are used. Last but not least, general anesthesia or controlled ventilation is virtually unnecessary for the uncooperative children to undergo CT examination. To get all the benefits of the currently available cardiac CT, we as cardiac radiologists, should aware of the different types of CT scans in detail and we should keep an eye open for future technical developments.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material
Electronic Supplementary Material
References
- 1.Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Yun TJ, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147–S165. doi: 10.1148/rg.23si035501. [DOI] [PubMed] [Google Scholar]
- 2.Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Park JJ. Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients. Int J Cardiovasc Imaging. 2005;21:347–365. doi: 10.1007/s10554-004-4015-0. [DOI] [PubMed] [Google Scholar]
- 3.Leschka S, Oechslin E, Husmann L, Desbiolles L, Marincek B, Genoni M, et al. Pre- and postoperative evaluation of congenital heart disease in children and adults with 64-section CT. Radiographics. 2007;27:829–846. doi: 10.1148/rg.273065713. [DOI] [PubMed] [Google Scholar]
- 4.Goo HW. Pediatric CT: understanding of radiation dose and optimization of imaging techniques. J Korean Radiol Soc. 2005;52:1–5. [Korean] [Google Scholar]
- 5.Yang DH, Goo HW. Pediatric 16-slice CT protocol: radiation dose and image quality. J Korean Radiol Soc. 2008;59:333–347. [Google Scholar]
- 6.Alkadhi H. Radiation dose of cardiac CT--what is the evidence? Eur Radiol. 2009;19:1311–1315. doi: 10.1007/s00330-009-1312-y. [DOI] [PubMed] [Google Scholar]
- 7.Mayo JR, Leipsic JA. Radiation dose in cardiac CT. AJR Am J Roentgenol. 2009;192:646–653. doi: 10.2214/AJR.08.2066. [DOI] [PubMed] [Google Scholar]
- 8.Ha HI, Goo HW, Seo JB, Song JW, Lee JS. Effects of high-resolution CT of the lung using partial versus full reconstruction on motion artifacts and image noise. AJR Am J Roentgenol. 2006;187:618–622. doi: 10.2214/AJR.05.0852. [DOI] [PubMed] [Google Scholar]
- 9.Yang DH, Goo HW, Seo DM, Yun TJ, Park JJ, Park IS, et al. Multi-slice CT angiography of interrupted aortic arch. Pediatr Radiol. 2008;38:89–100. doi: 10.1007/s00247-007-0662-3. [DOI] [PubMed] [Google Scholar]
- 10.Goo HW, Seo DM, Yun TJ, Park JJ, Park IS, Ko JK, et al. Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: multislice CT findings. Pediatr Radiol. 2009;39:265–273. doi: 10.1007/s00247-008-1111-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsai IC, Lee T, Chen MC, Fu YC, Jan SL, Wang CC, et al. Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr Radiol. 2007;37:818–825. doi: 10.1007/s00247-007-0512-3. [DOI] [PubMed] [Google Scholar]
- 12.Busch S, Johnson TR, Wintersperger BJ, Minaifar N, Bhargava A, Rist C, et al. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. Eur Radiol. 2008;18:570–575. doi: 10.1007/s00330-007-0767-y. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TR, Nikolaou K, Wintersperger BJ, Leber AW, von Ziegler F, Rist C, et al. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006;16:1409–1415. doi: 10.1007/s00330-006-0298-y. [DOI] [PubMed] [Google Scholar]
- 14.Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG. Technical principles of dual source CT. Eur J Radiol. 2008;68:362–368. doi: 10.1016/j.ejrad.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakou Y, Kachelriess M, Knaup M, Krause JU, Kalender WA. Impact of the z-flying focal spot on resolution and artifact behavior for a 64-slice spiral CT scanner. Eur Radiol. 2006;16:1206–1215. doi: 10.1007/s00330-005-0118-9. [DOI] [PubMed] [Google Scholar]
- 16.Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Yun TJ, et al. Visibility of the origin and proximal course of coronary arteries on non-ECG-gated heart CT in patients with congenital heart disease. Pediatr Radiol. 2005;35:792–798. doi: 10.1007/s00247-005-1482-y. [DOI] [PubMed] [Google Scholar]
- 17.Tzedakis A, Damilakis J, Perisinakis K, Karantanas A, Karabekios S, Gourtsoyiannis N. Influence of z overscanning on normalized effective doses calculated for pediatric patients undergoing multidetector CT examinations. Med Phys. 2007;34:1163–1175. doi: 10.1118/1.2710331. [DOI] [PubMed] [Google Scholar]
- 18.Deak PD, Langner O, Lell M, Kalender WA. Effects of adaptive section collimation on patient radiation dose in multisection spiral CT. Radiology. 2009;252:140–147. doi: 10.1148/radiol.2522081845. [DOI] [PubMed] [Google Scholar]
- 19.McCollough CH, Primak AN, Saba O, Bruder H, Stierstorfer K, Raupach R, et al. Dose performance of a 64-channel dual-source CT scanner. Radiology. 2007;243:775–784. doi: 10.1148/radiol.2433061165. [DOI] [PubMed] [Google Scholar]
- 20.Ruzsics B, Gebregziabher M, Lee H, Brothers RL, Allmendinger T, Vogt S, et al. Coronary CT angiography: automatic cardiac-phase selection for image reconstruction. Eur Radiol. 2009;19:1906–1913. doi: 10.1007/s00330-009-1368-8. [DOI] [PubMed] [Google Scholar]
- 21.Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol. 2008;18:1809–1817. doi: 10.1007/s00330-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 22.Stolzmann P, Leschka S, Scheffel H, Krauss T, Desbiolles L, Plass A, et al. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008;249:71–80. doi: 10.1148/radiol.2483072032. [DOI] [PubMed] [Google Scholar]
- 23.Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 24.Goo HW. Evaluation of the airways in patients with congenital heart disease using multislice CT. J Korean Pediatr Cardiol Soc. 2004;8:37–43. [Google Scholar]
- 25.Goo HW, Kim HJ. Detection of air trapping on inspiratory and expiratory phase images obtained by 0.3-second cine CT in the lungs of free-breathing young children. AJR Am J Roentgenol. 2006;187:1019–1023. doi: 10.2214/AJR.05.0895. [DOI] [PubMed] [Google Scholar]
- 26.Jung YY, Goo HW. The optimal parameter for radiation dose in pediatric low dose abdominal CT: cross-sectional dimensions versus body weight. J Korean Radiol Soc. 2008;58:169–175. [Google Scholar]
- 27.van der Molen AJ, Geleijns J. Overranging in multisection CT: quantification and relative contribution to dose--comparison of four 16-section CT scanners. Radiology. 2007;242:208–216. doi: 10.1148/radiol.2421051350. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, et al. Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics. 2008;28:1451–1459. doi: 10.1148/rg.285075075. [DOI] [PubMed] [Google Scholar]
- 29.Goo HW, Suh DS. Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol. 2006;36:344–351. doi: 10.1007/s00247-005-0105-y. [DOI] [PubMed] [Google Scholar]
- 30.Goo HW, Suh DS. The influences of tube voltage and scan direction on combined tube current modulation: a phantom study. Pediatr Radiol. 2006;36:833–840. doi: 10.1007/s00247-006-0177-3. [DOI] [PubMed] [Google Scholar]
- 31.Weustink AC, Mollet NR, Pugliese F, Meijboom WB, Nieman K, Heijenbrok-Kal MH, et al. Optimal electrocardiographic pulsing windows and heart rate: effect on image quality and radiation exposure at dual-source coronary CT angiography. Radiology. 2008;248:792–798. doi: 10.1148/radiol.2483072098. [DOI] [PubMed] [Google Scholar]
- 32.Lee T, Tsai IC, Fu YC, Jan SL, Wang CC, Chang Y, et al. MDCT evaluation after closure of atrial septal defect with an Amplatzer septal occluder. AJR Am J Roentgenol. 2007;188:W431–W439. doi: 10.2214/AJR.06.0709. [DOI] [PubMed] [Google Scholar]
- 33.Frush DP. Technique of pediatric thoracic CT angiography. Radiol Clin North Am. 2005;43:419–433. doi: 10.1016/j.rcl.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth CL, Yoshizumi TT, Frush DP, Chan FP, Toncheva G, Nguyen G, et al. Pediatric cardiac-gated CT angiography: assessment of radiation dose. AJR Am J Roentgenol. 2007;189:12–18. doi: 10.2214/AJR.06.1507. [DOI] [PubMed] [Google Scholar]
- 35.Bae KT. Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology. 2003;227:809–816. doi: 10.1148/radiol.2273020102. [DOI] [PubMed] [Google Scholar]
- 36.Hopper KD, Mosher TJ, Kasales CJ, TenHave TR, Tully DA, Weaver JS. Thoracic spiral CT: delivery of contrast material pushed with injectable saline solution in a power injector. Radiology. 1997;205:269–271. doi: 10.1148/radiology.205.1.9314997. [DOI] [PubMed] [Google Scholar]
- 37.Litmanovitch D, Zamboni GA, Hauser TH, Lin PJ, Clouse ME, Raptopoulos V. ECG-gated chest CT angiography with 64-MDCT and tri-phasic IV contrast administration regimen in patients with acute non-specific chest pain. Eur Radiol. 2008;18:308–317. doi: 10.1007/s00330-007-0739-2. [DOI] [PubMed] [Google Scholar]
- 38.Eichhorn JG, Jourdan C, Hill SL, Raman SV, Cheatham JP, Long FR. CT of pediatric vascular stents used to treat congenital heart disease. AJR Am J Roentgenol. 2008;190:1241–1246. doi: 10.2214/AJR.07.3194. [DOI] [PubMed] [Google Scholar]
- 39.Jhang WK, Park JJ, Seo DM, Goo HW, Gwak M. Perioperative evaluation of airways in patients with arch obstruction and intracardiac defects. Ann Thorac Surg. 2008;85:1753–1758. doi: 10.1016/j.athoracsur.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 40.Goo HW, Jhang WK, Kim YH, Ko JK, Park IS, Park JJ, et al. CT findings of plastic bronchitis in children after Fontan operation. Pediatr Radiol. 2008;38:989–993. doi: 10.1007/s00247-008-0937-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Supplementary Material