Abstract
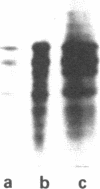
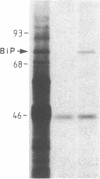
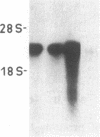
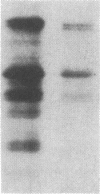
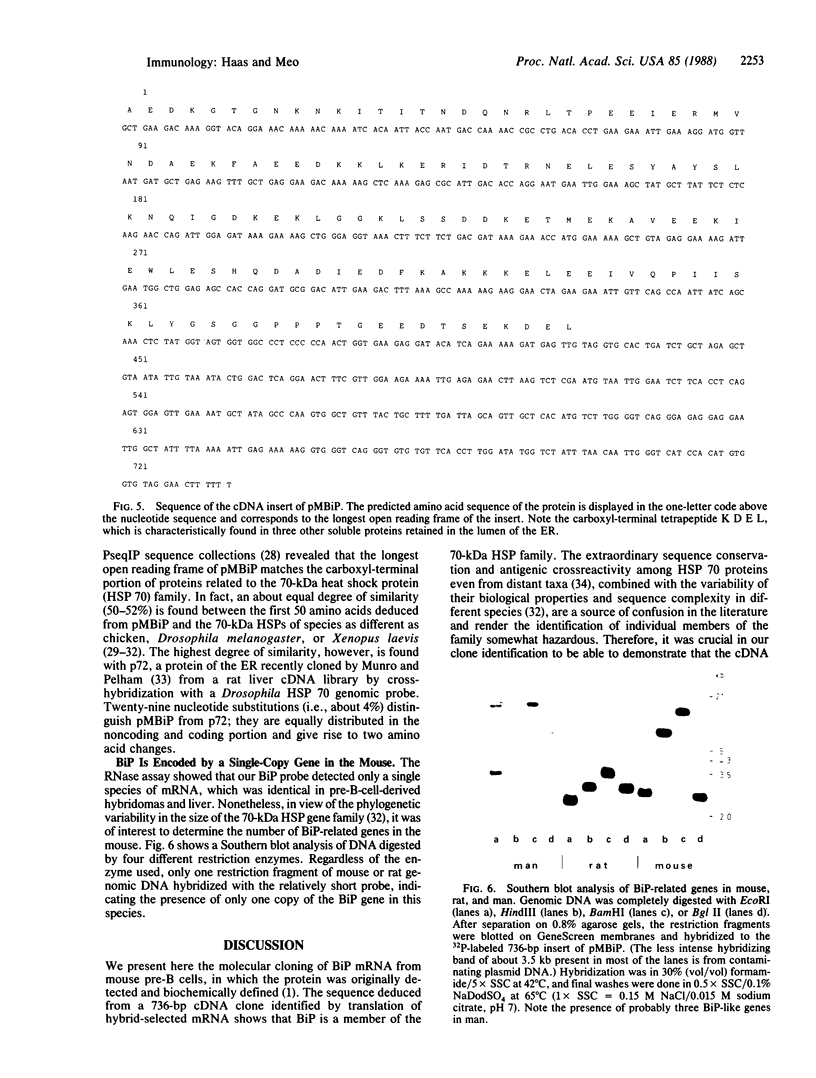
A cDNA library was constructed from size-fractionated poly(A)+ RNA prepared from a murine pre-B-cell hybridoma expressing high levels of immunoglobulin heavy chain binding protein (BiP) and mu heavy chains. Transformed bacterial colonies were screened for recombinant plasmids containing cDNA coding for BiP by hybrid-selected mRNA translation. A clone, pMBiP, containing a 736-base-pair insert was shown to encode the protein. Translation in vitro of hybridoma mRNA selected by hybridization to the pMBiP cDNA yielded a single polypeptide of BiP-like size. The authenticity of this mRNA was verified by comparing the peptides obtained by the limited proteolysis of its in vitro translation product with those obtained from the in vivo produced BiP. Likewise, the authenticity of the cDNA insert was verified by an RNase A protection assay of heteroduplex molecules obtained by annealing a uniformly labeled single-strand copy of the cDNA clone with the same mRNA selected by hybridization and tested by translation. The nucleotide sequence of this clone enabled us to deduce the carboxyl-terminal 142 amino acids of BiP and to establish its kinship with the 70-kDa heat shock protein family. The finding of a single copy of the BiP gene in DNA blots of mouse and rat implies that the BiP-related RNA transcripts constitutively expressed in various murine tissues and cell lines are indeed products of the same gene. These findings imply that BiP plays a more general role than previously anticipated on the basis of the discovery of its association with immunoglobulin heavy chains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows P. D., Beck G. B., Wabl M. R. Expression of mu and gamma immunoglobulin heavy chains in different cells of a cloned mouse lymphoid line. Proc Natl Acad Sci U S A. 1981 Jan;78(1):564–568. doi: 10.1073/pnas.78.1.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Bricault L. PseqIP: a nonredundant and exhaustive protein sequence data bank generated from 4 major existing collections. Proteins. 1986 Sep;1(1):60–65. doi: 10.1002/prot.340010110. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Franklin E. C., Frangione B. Molecular defect in a gamma-2 heavy chain. Science. 1972 Apr 14;176(4031):187–189. doi: 10.1126/science.176.4031.187. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. R. Immunoglobulin heavy chain toxicity in plasma cells is neutralized by fusion to pre-B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7185–7188. doi: 10.1073/pnas.81.22.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1987 May;87(Pt 4):491–492. doi: 10.1242/jcs.87.4.491. [DOI] [PubMed] [Google Scholar]
- Köhler G. Immunoglobulin chain loss in hybridoma lines. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2197–2199. doi: 10.1073/pnas.77.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lee A. S., Delegeane A., Scharff D. Highly conserved glucose-regulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4922–4925. doi: 10.1073/pnas.78.8.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lonberg N., Gilbert W. Primary structure of chicken muscle pyruvate kinase mRNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3661–3665. doi: 10.1073/pnas.80.12.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R. I., Hunt C., Huang S. Y., Berg K. L., Banerji S. S. Organization, nucleotide sequence, and transcription of the chicken HSP70 gene. J Biol Chem. 1986 Sep 25;261(27):12692–12699. [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Salerno G., Verde P., Nolli M. L., Corti A., Szöts H., Meo T., Johnson J., Bullock S., Cassani G., Blasi F. Monoclonal antibodies to human urokinase identify the single-chain pro-urokinase precursor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):110–114. doi: 10.1073/pnas.81.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Sharma S., Rodgers L., Brandsma J., Gething M. J., Sambrook J. SV40 T antigen and the exocytotic pathway. EMBO J. 1985 Jun;4(6):1479–1489. doi: 10.1002/j.1460-2075.1985.tb03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Hämmerling U. The role of glycosylation in secretion and membrane expression of immunoglobulins M and A. Mol Immunol. 1984 Aug;21(8):709–719. doi: 10.1016/0161-5890(84)90023-3. [DOI] [PubMed] [Google Scholar]
- Tosi M., Duponchel C., Bourgarel P., Colomb M., Meo T. Molecular cloning of human C1 inhibitor: sequence homologies with alpha 1-antitrypsin and other members of the serpins superfamily. Gene. 1986;42(3):265–272. doi: 10.1016/0378-1119(86)90230-1. [DOI] [PubMed] [Google Scholar]
- Tosi M., Lévi-Strauss M., Georgatsou E., Amor M., Meo T. Duplications of complement and non-complement genes of the H-2S region: evolutionary aspects of the C4 isotypes and molecular analysis of their expression variants. Immunol Rev. 1985 Oct;87:151–183. doi: 10.1111/j.1600-065x.1985.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M., Steinberg C. A theory of allelic and isotypic exclusion for immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6976–6978. doi: 10.1073/pnas.79.22.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]