Abstract
Dmrt1 (doublesex and mab-3 related transcription factor 1) is a conserved transcriptional regulator of male differentiation required for testicular development in vertebrates. Here, we show that in mice of the 129Sv strain, loss of Dmrt1 causes a high incidence of teratomas, whereas these tumors do not form in Dmrt1 mutant C57BL/6J mice. Conditional gene targeting indicates that Dmrt1 is required in fetal germ cells but not in Sertoli cells to prevent teratoma formation. Mutant 129Sv germ cells undergo apparently normal differentiation up to embryonic day 13.5 (E13.5), but some cells fail to arrest mitosis and ectopically express pluripotency markers. Expression analysis and chromatin immunoprecipitation identified DMRT1 target genes, whose missexpression may underlie teratoma formation. DMRT1 indirectly activates the GDNF coreceptor Ret, and it directly represses the pluripotency regulator Sox2. Analysis of human germ cell tumors reveals similar gene expression changes correlated to DMRT1 levels. Dmrt1 behaves genetically as a dose-sensitive tumor suppressor gene in 129Sv mice, and natural variation in Dmrt1 activity can confer teratoma susceptibility. This work reveals a genetic link between testicular dysgenesis, pluripotency regulation, and teratoma susceptibility that is highly sensitive to genetic background and to gene dosage.
Keywords: GDNF, testicular teratoma, germ cell tumor, embryonal carcinoma
Germ cells are unique in at least two respects. First, as the agents of genetic transmission they are the only intergenerational cell lineage. Second, although committed to form only one cell type, sperm or oocyte, they retain a latent pluripotency. Transplantation of early embryonic gonads into adult hosts can induce this pluripotency, resulting in teratomas containing complex mixtures of somatic cell types (1). Similarly, pluripotent stem cells can be derived from embryonic and postnatal germ cells of the testis, including presumptive spermatogonial stem cells (SSCs) (2–4). These cultured germ cells resemble embryonic stem (ES) cells, and like ES cells they are pluripotent when injected into mouse blastocysts (5, 6). Paracrine signaling is important to maintain germ line stem cells in vitro and in vivo. Extrinsic factors that support germ line stem cells in culture include LIF, bFGF, SCF, EGF, and GDNF (2, 4). Signals from Sertoli cells, including GDNF, also are required in vivo for SSC maintenance and proliferation (7).
Human disorders of germ cell development are common and can arise either from primary defects in gametogenesis or secondary to somatic gonadal dysgenesis (8, 9). Germ cell cancers also are common; indeed testicular germ cell tumors (TGCTs) are the most frequent cancer of young men (10). TGCTs can be grouped into distinct classes, based on time of incidence, histopathology, and presumptive cell type of origin (10). Type I tumors occur in neonates and infants and arise from fetal germ cells. Type II tumors also are thought to arise from fetal germ cells but occur mainly in young men. Type I and II TGCTs both can differentiate into diverse somatic cell types, suggesting a failure to control pluripotency may play a role in their formation (10). The rare type III tumors, or spermatocytic seminomas, occur in men older than 50, have distinct pathogenesis, and are thought to arise from postnatal spermatogonia or early spermatocytes (11).
Human TGCTs have a strong genetic component, and family history and ethnicity are significant risk factors (12). Despite this, linkage studies have failed to reveal strong associations in human TGCTs, suggesting that multiple genetic modifiers contribute to TGCT susceptibility (12, 13). In epidemiological studies, TGCTs are strongly associated with other gonadal abnormalities, and it has been proposed that these disorders share a common etiology as part of a “testicular dysgenesis syndrome” (14, 15).
Spontaneous teratomas do not occur in most inbred mouse strains, but in the 1950s, Stevens established a mouse substrain, 129Sv, in which spontaneous testicular teratomas arise at an incidence of 1% (16). Stevens also identified a spontaneous mutation, Ter (an allele of Dnd1), which causes further elevated teratoma incidence, but only on the 129Sv background (17–19). Backcross mapping between 129Sv and other inbred strains and analysis of chromosome substitutions indicated that teratoma susceptibility is multigenic and localized several genetic modifiers to chromosome 19 (20, 21).
Here, we examine the phenotype of Dmrt1 (doublesex and mab-3 related transcription factor 1) mutations on the 129Sv genetic background. Dmrt1 is a member of a widely conserved group of sexual regulators that share the DM domain DNA binding motif (22, 23) and is required for testis differentiation in vertebrates (24, 25). In mice, Dmrt1 is expressed only in the gonad and is essential for postnatal differentiation of germ cells and Sertoli cells (26). Human XY individuals missing one copy of the region containing DMRT1 have testicular dysgenesis closely resembling that of Dmrt1 mutant mice and in some cases are feminized (27, 28). Amplification of DMRT1 occurs in human type III TGCTs (29), but no involvement of DMRT1 has been reported in type I or II tumors. We find that loss of Dmrt1 in 129Sv mice results in a high incidence of teratomas and that Dmrt1 acts as a dose-sensitive tumor suppressor gene that directly controls transcription of the pluripotency regulator Sox2 in germ cells.
Results
Loss of Dmrt1 Causes Teratoma Formation in Mice of the 129Sv Strain.
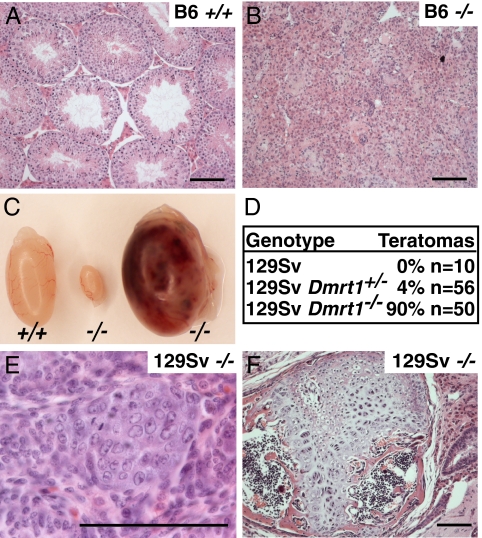
Previous analysis of Dmrt1 mutants used a mixed genetic background (26, 30). To ask whether the phenotype is sensitive to genetic background, we compared testes from Dmrt1−/− animals bred onto the C57BL6/J (“B6”) and 129SvEv/S6 (“129Sv”) backgrounds. B6 Dmrt1−/− animals had postnatal testicular dysgenesis (Fig. 1 A and B) closely resembling that described in ref. 26. In contrast, 129Sv Dmrt1−/− animals developed teratomas at a high incidence (Fig. 1 C–F). Clusters of embryonal carcinoma (EC) cells were present at birth (Fig. 1E; three to eight clusters per gonad; n = 3), and these differentiated into large mature teratomas within 3 weeks after birth (Fig. 1F). These tumors represent a transformation of germ line to somatic cell fates. Although Dmrt1 is expressed in the female genital ridge before sexual differentiation, we did not observe ovarian tumors in Dmrt1 mutant females (n = 50 ovaries). Except where indicated, the data described below are all from mice of the 129Sv background.
Fig. 1.
129Sv Dmrt1−/− mice have a high incidence of teratomas. (A and B) Hematoxylin and eosin (H&E) staining of testis sections from adult B6 mice. (A) Wild-type; (B) Dmrt1−/−; (C) testes from 129Sv adults. Left: Wild-type; middle: Dmrt1−/− dysgenic; right: Dmrt1−/− teratoma. (D) Teratoma incidence in 129Sv Dmrt1 mutant mice, tumors per testis. (E) H&E staining of embryonal carcinoma cells at day of birth in 129Sv Dmrt1−/−. (F) H&E staining of 4-week-old teratoma from 129Sv Dmrt1−/−. (Scale bars, 10 μm.)
Dmrt1 Is Required in Germ Cells to Prevent Teratoma Formation.
Dmrt1 is required in both Sertoli and germ cells for postnatal germ cell development (30), so the teratomas in Dmrt1−/− testes could result from lack of Dmrt1 in germ cells or in the surrounding Sertoli cells or in both. To define where Dmrt1 is required, we used conditional gene targeting (Table 1). Dmrt1flox/− controls had elevated teratoma incidence (see below), and deletion of Dmrt1 in embryonic Sertoli cells with Dhh-Cre (31) or Sf1-Cre (32) in Dmrt1flox/− mice caused no further increase. By contrast, deletion of Dmrt1 in migratory PGCs with TNP-Cre (33) did increase the incidence of teratomas. The incidence was lower than in Dmrt1−/− animals, possibly due to the relatively low efficiency of Dmrt1 deletion by TNAP-Cre (30). We conclude that loss of Dmrt1 in germ cells is sufficient to cause teratoma formation, whereas loss of Dmrt1 in Sertoli cells is not. It remains possible that loss of Dmrt1 in both cell types causes a higher tumor incidence than loss only in germ cells.
Table 1.
Dmrt1 is required in germ cells to prevent germ cell tumors
| Genotype | Teratomas | |
|---|---|---|
| Dmrt1flox/flox | 9% | n = 76 |
| Dmrt1flox/− | 42% | n = 124 |
| Dmrt1flox/−; Dhh-Cre | 42% | n = 62 |
| Dmrt1flox/−; Sf1-Cre | 39% | n = 36 |
| Dmrt1flox/−;TNAP-Cre | 63% | n = 46 |
Dmrt1flox/− is used as the control in all experiments. Removal of Dmrt1 in Sertoli cells using either Dhh-Cre or SF1-Cre does not increase the incidence of teratomas (P = 0.87 and P = 0.69, respectively). However, removal of Dmrt1 in germ cells using TNAP-Cre causes an elevated incidence of teratomas (P = 0.03). n = number of testes examined.
Normal Early Development of Dmrt1 Mutant Germ Cells.
Teratomas in Dmrt1 mutant mice could result from a general failure in some aspect of germ cell development or from more specific defects. Because DM domain genes in other species are involved in sex determination, we first asked whether mutant germ cells might be feminized, leading to embryonic rather than postnatal meiotic initiation. We examined expression of STRA8, a regulator of meiotic initiation expressed in premeiotic germ cells (34, 35), but observed no ectopic expression at embryonic day 16.5 (E16.5; n = 4). Likewise, the synaptonemal complex component SYCP3 was not expressed at E16.5 or at birth (n = 3). We conclude that fetal germ cells are unlikely to be feminized.
To ask whether other aspects of early germ cell differentiation are normal, we examined expression of several developmental markers and parental DNA imprint erasure (Figs. S1 and S2). Expression of MVH and GM114 at E13.5 was normal, and translocation of BLIMP1 to the cytoplasm at E13.5 occurred normally (Fig. S1). Erasure of parental imprinting occurred at the H19 (paternally imprinted) (36) and Lit1 (maternally imprinted) (37) loci by E13.5. Similarly the Xist locus underwent normal demethylation by E13.5 (Fig. S2) (38). In addition, we used microarray-based comparative genome hybridization to ask whether loss of Dmrt1 causes aneuploidy, but observed no copy number abnormalities in two tumors. From these results, we conclude that Dmrt1 mutant germ cells do not have a developmental block or major aneuploidies during embryonic development.
Dmrt1 Appears to Act in a Distinct Pathway from Dnd1 and Pten.
Mutations in Dnd1 and Pten cause teratomas in mice (39, 40), so we asked whether Dmrt1 might act in the same pathway as either gene. Dnd1 mutants have a severe loss of germ cells before E11.5 (39), whereas Dmrt1 mutants do not (26). Thus, Dmrt1 is unlikely to regulate Dnd1, but DND1 might promote DMRT1 expression. Germ cell death in Dnd1 mutants can be suppressed by mutation of the proapoptotic gene Bax (41). We found no difference in DMRT1 expression in germ cells of Dnd1Ter/Ter;Bax−/− versus Dnd1+/Ter;Bax+/− controls at E14.5 (Fig. S3). We also found that the DND1 target P27Kip1 is expressed normally in Dmrt1 mutant germ cells at E16.5 (Fig. S3). We conclude from these results that Dmrt1 is likely to act independently of Dnd1.
Because Pten mutants develop teratomas on non-129Sv genetic backgrounds and Dmrt1 mutants do not, reduced Dmrt1 activity cannot be the primary cause of teratomas in Pten mutants. We therefore asked whether Dmrt1 regulates the Pten pathway. Loss of PTEN in germ cells leads to elevated levels of AKT P-308 (40). However, PTEN levels were normal in mutant germ cells at E13.5 and E15.5, and PTEN and AKT P-308 levels were normal in EC cell clusters at P0 (Fig. S3). These results indicate that Dmrt1 acts either independently or downstream of the PTEN pathway.
Reduced Ret Expression at E13.5.
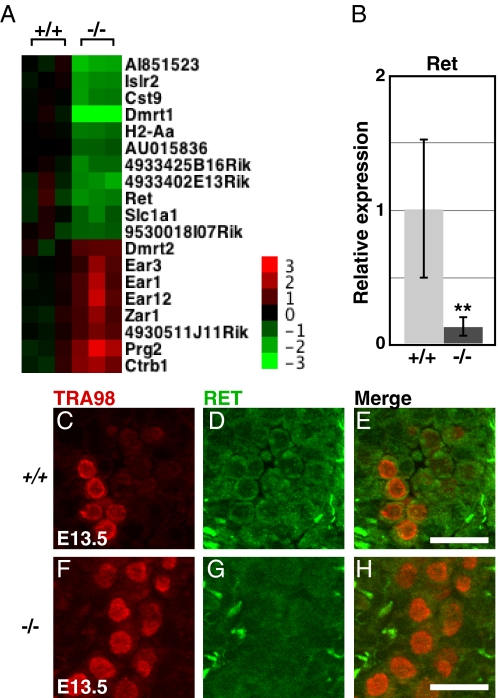
Tissue transplantation suggested that teratomas in 129Sv initiate between about E11 and E12.5 (1), coincident with the time of pluripotency restriction in cultured germ cells (42). We reasoned therefore that mRNA expression defects underlying the teratoma initiation might be apparent in Dmrt1 mutant testes at E13.5. Expression profiling of E13.5 testes identified 11 transcripts with >2-fold reduced expression and eight with >2-fold elevated expression in Dmrt1 mutants (Fig. 2A). Of these, the only one previously known to affect germ cell development is the GDNF receptor Ret (43), which also is regulated by Dmrt1 postnatally (44). Ret mRNA was reduced 8-fold (Fig. 2B), and RET protein was severely reduced based on immunofluorescence in mutant germ cells (Fig. 2 C–H). We conclude that GDNF signaling may be compromised in Dmrt1 mutant germ cells by reduced RET expression.
Fig. 2.
Reduced Ret expression in Dmrt1 mutant testes (A) Heat map showing 11 mRNAs reduced >2-fold, and eight mRNAs elevated >2-fold in Dmrt1−/− compared to wild-type. (B) qRT-PCR of Ret mRNA at E13.5, normalized to Hprt. Error bars: SD from testes of six animals of each genotype (**, P < 0.005). (C–H) Double staining for the germ cell marker TRA98 (C and F) and RET (D and G) in wild-type (C–E) and Dmrt1−/− (F–H) testis sections. (Scale bars, 20 μm.)
DMRT1 Controls Germ Cell Mitotic Proliferation.
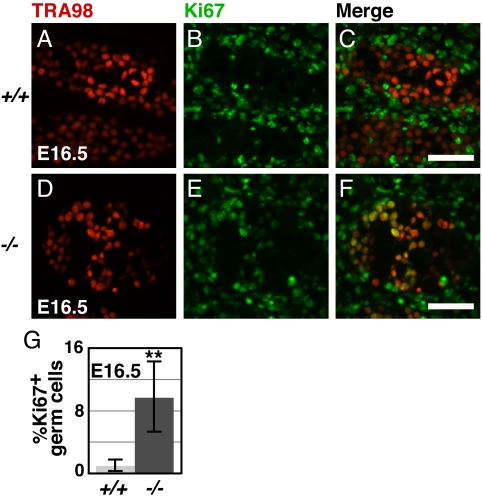
In neonates, Dmrt1 mutant germ cells form EC cell clusters with visible mitotic figures (Fig. 1E) and later form large tumors. It is likely, therefore, that Dmrt1 controls genes that regulate the cell cycle. To test whether Dmrt1 mutant germ cells escape the normal embryonic mitotic arrest, we used the active cell cycle marker Ki67 (45). Normally male germ cells are almost entirely arrested by E13.5, but 10-fold elevated numbers of mutant germ cells that escaped G0 arrest were present at E16.5 (Fig. 3 A–G).
Fig. 3.
DMRT1 controls mitotic proliferation in 129Sv germ cells. (A–F) Double staining for the germ cell marker TRA98 (A and D) and active cell cycle marker Ki67 (B and E) in wild-type (A–C) and Dmrt1−/− (D–F) testis sections. Green cells in merged images (C and F) are mitotically active somatic cells, whereas yellow cells are mitotically active germ cells. (Scale bars, 50 μm.) (G) Percentage Ki67 positive germ cells in wild-type vs. Dmrt1−/− (**, P < 0.005).
We investigated the cell cycle defect by assaying the expression and binding by DMRT1 to the promoters of three cell cycle inhibitors known to be expressed in the embryonic testis. We found that mRNA expression of P18INK4c (Cdkn2c) and P19INK4d (Cdkn2d) decreased at E15.5 in Dmrt1 mutant testes, consistent with a possible role in the proliferation defect (Fig. S4A). Expression of P15INK4b (Cdkn2b) increased in the mutant, suggesting it is not a factor in the excess proliferation. In the E13.5 testis, we found that DMRT1 binds the promoter of P19INK4d, suggesting that DMRT1 could directly affect mitotic proliferation (Fig. S4B). Loss of P18INK4c has been implicated in formation of invasive seminomas and EC cells in humans (46), suggesting a possible mechanistic link between mouse and human tumors.
DMRT1 Controls Expression of Pluripotency Regulators.
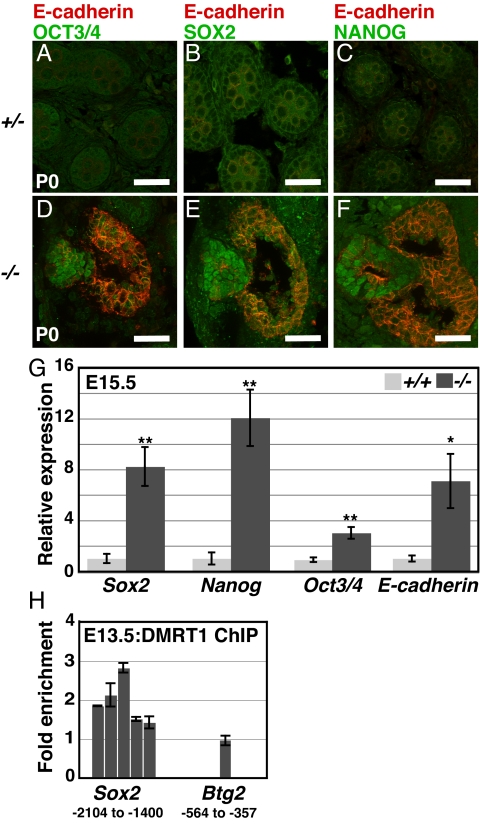
The inappropriate formation of EC cells and multiple somatic cell types (Fig. 1 E and F) by Dmrt1 mutant germ cells suggests a possible defect in restriction of pluripotency. In fetal germ cells, pluripotency markers normally are down-regulated by E15.5, but in 129Sv Dmrt1 mutant testes, we found that EC cell clusters expressing elevated levels of SOX2, NANOG, and OCT3/4 are present at birth (Fig. 4 A–F). By contrast, B6 Dmrt1 mutant testes do not develop EC cell clusters. E-cadherin is also expressed in early germ cells and normally diminishes before birth, but at birth, elevated E-cadherin was present in mutant cells both within and surrounding the EC cell clusters (Fig. 4 D–F). E-cadherin recently was shown to promote pluripotency and teratoma formation in FAB-derived mouse stem cell cultures (47), suggesting that its elevated expression may contribute to pluripotency of Dmrt1 mutant germ cells. ChIP and qRT-PCR demonstrated that the Sox2 promoter is bound by DMRT1 at E13.5 and mRNA expression of Sox2, Nanog, Oct3/4, and E-cadherin was elevated in mutant testes at E15.5 (Fig. 4 G and H). We conclude that DMRT1 controls expression of pluripotency regulators in the embryonic testis, in part via transcriptional repression.
Fig. 4.
DMRT1 represses pluripotency regulators (A–F) Expression of pluripotency regulators and E-cadherin (D–F). Clusters of OCT3/4, NANOG, and SOX2 expressing cells present in Dmrt1−/− testes at birth but not in Dmrt1+/− controls. Cells expressing elevated E-cadherin are within and adjacent to clusters of cells expressing pluripotency regulators. (Scale bars, 50 μm.) (G) qRT-PCR of Sox2, Nanog, Oct3/4, and E-cadherin mRNAs at E15.5 in wild-type vs. Dmrt1−/− using Hprt as a normalizer. Error bars: SD from three animals of each genotype. (*, P < 0.01, **, P < 0.005). (H) ChIP-qPCR of Sox2 and Btg2 regulatory regions, comparing enrichment in chromatin immunoprecipitated with DMRT1 relative to input chromatin. Numbering below gene names indicates region covered by amplicons tested, relative to start of transcription for each gene. The negative control promoter Btg2 was chosen because it is expressed at similar levels in germ cells and Sertoli cells and does not change expression in Dmrt1 mutant testes. Error bars: SD of duplicate qPCR of sample.
To ask whether the misexpression of pluripotency regulators has functional consequences, we assayed the expression of 10 OCT3/4 early response genes defined in ES cells (48). Seven out of the 10 direct targets of OCT3/4 are overexpressed in Dmrt1−/− compared to wild-type, suggesting that an active pluripotency gene network is ectopically expressed (Fig. S5).
We also examined expression of Eras (ES-expressing Ras), a gene shown to be important for teratoma formation in ES cells (49). We found that Eras is expressed at similar levels in wild-type 129Sv and B6 testes and in B6 mutant testes at E15.5, but has 7-fold elevated expression in 129Sv mutant testes (Fig. S6).
To ask whether DMRT1 may function similarly in human TGCTs, we examined expression of several genes in spermatocytic seminomas, seminomas, dysgerminomas, and EC cells. Spermatocytic seminomas have elevated expression of DMRT1 (29). We found a clear correlation between the level of DMRT1 and expression of RET, E-CADHERIN (CDH1), NANOG, CDKN2C (P18INK4C) and CDKN2D (P19INK4D), similar to that seen in the mouse (Fig. S7).
Dmrt11 As a Teratoma Susceptibility Locus.
We next investigated whether variation in Dmrt1 activity contributes to teratoma susceptibility. Mice with chromosome 19 from the MOLF strain replacing that of 129Sv (chromosome 19 substitution strain; “CSS”) have greatly elevated teratoma incidence (20). Analysis of consomic strains substituting smaller regions of MOLF chromosome 19 identified several susceptibility regions (50). One strain, 129.MOLF-Chr19 L1 (congenic L1; “L1”), substitutes a 7.6-Mb region containing the Dmrt1Dmrt3Dmrt2 gene cluster (51).
We hypothesized that low expression or function of MOLF Dmrt1 might contribute to teratomas in consomic strains. We performed a complementation test, crossing 129Sv Dmrt1+/− mice to L1 congenic mice. The resulting progeny were a mix of Dmrt1L1/129− and Dmrt1L1/129+ (Fig. S8). The Dmrt1L1/129− animals had many more teratomas than the controls or Dmrt1L1/L1 animals (Table 2). We conclude that Dmrt1 is likely to be one of the major teratoma susceptibility loci mapped to chromosome 19. Pten and Gfra1 map to critical regions of chromosome 19 outside the L1 interval, and Pten loss of function causes teratoma formation (40). It will be important to test the role of Ret and Gfra1 in teratoma susceptibility.
Table 2.
Reduced DMRT1 activity in MOLF L1 congenic testes
| Genotype | Teratomas | |
|---|---|---|
| L1/L1 | 21% | n = 164 |
| L1/129Sv Dmrt1+ | 4% | n = 46 |
| L1/129Sv Dmrt1− | 53% | n = 110 |
Complementation test comparing L1/L1 congenic mice to Dmrt1L1/129+ and Dmrt1L1/129−. Data for L1/L1 congenic mice are from ref. 51.
The high incidence of teratomas in Dmrt1L1/129− animals suggests that Dmrt1L1 is haploinsufficient in a 129Sv genomic context. We examined expression of the MOLF Dmrt1 allele and, at E13.5, detected no significant difference in mRNA or protein levels between CSS animals and controls (51). Sequencing the MOLF Dmrt1 coding region revealed one coding difference, an insertion of alanine at position 363 near the C terminus in the MOLF allele, and this may reduce DMRT1 function in germ cells.
Dmrt1 Acts as a Dose-Sensitive Tumor Suppressor in 129Sv Mice.
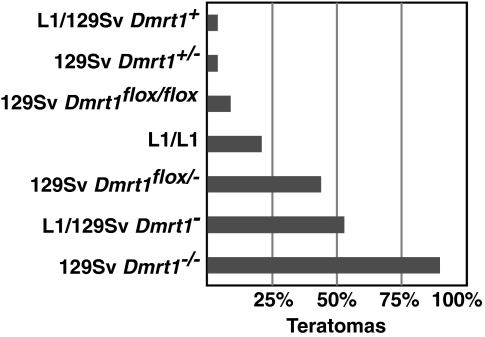
Comparison of Dmrt1 alleles shows that Dmrt1 is highly dose-sensitive in preventing teratomas. Homozygous Dmrt1 null mutants have a teratoma incidence of 90% and this drops to 4% in heterozygotes. The Dmrt1flox allele behaves as a hypomorph, and changing one allele has major effects: Dmrt1flox/− animals have a 4-fold higher teratoma incidence than Dmrt1flox/flox animals and a 10-fold higher incidence than Dmrt1flox/+ animals (Fig. 5). The MOLF region in L1 conferred a teratoma susceptibility intermediate between the floxed and null Dmrt1 alleles, consistent with Dmrt1L1 acting as a hypomorphic allele.
Fig. 5.
Dmrt1 dose sensitivity in teratoma formation. Percentage of testes with teratomas is indicated for each genotype.
Discussion
Loss of Dmrt1 results in testicular teratomas in mice. These tumors occur at a very high incidence in the 129Sv strain, but not in B6 mice, and reflect a requirement for Dmrt1 in fetal germ cells. Landmarks of early germ cell development are normal in Dmrt1 mutants, but expression analysis suggests that GDNF signaling, cell cycle control, and pluripotency regulation are disrupted. Analysis of L1 congenics suggests that variation in DMRT1 activity can cause teratoma susceptibility.
Although DMRT1 is expressed in the early ovary, mutant females did not develop tumors. Either DMRT1 does not function in females or the testicular environment is more permissive for germ cell tumor formation. This latter idea is supported by the observation that XX Dnd1Ter/Ter germ cells form tumors in XX Sry transgenic testes but not in XX ovaries (41).
Dmrt1 loss of function causes teratomas only in 129Sv mice. Dnd1 mutations also cause 129-specific teratomas, but increasing the number of surviving germ cells leads to tumors on mixed genetic backgrounds (41). In contrast, teratoma susceptibility in Dmrt1 mutants seems unrelated to cell death, as germ cell numbers are normal and no abnormal apoptosis was observed (Fig. S1). In Dmrt1 mutant germ cells, loss of repression of pluripotency genes is likely to contribute to transformation. In addition, genes like Eras, which are expressed at similar levels in 129Sv and B6 but respond differently to loss of Dmrt1 in the two strains, are likely to be critical in teratoma formation.
Dmrt1 mutant germ cells should be genetically uniform, but only a small fraction form EC cells. This may reflect a requirement for additional genetic or epigenetic lesions, or it may indicate that microenvironments in the embryonic testis are not uniform. To gain insight into potential 129Sv-specific modifiers of teratoma susceptibility, we performed a backcross with B6. We found no teratomas in 129Sv/B6 Dmrt1−/− F1 animals (n = 10), and teratomas were present in only 12% of Dmrt1−/− N2 progeny from 129/B6 animals backcrossed to 129Sv (9/77). These results are consistent with multiple recessive modifiers, likely three or more.
It is unknown whether loss-of-function mutations in DMRT1 cause human TGCTs. Sequencing of 240 human TGCT samples (types I, II, and III) uncovered four nonconservative missense changes in conserved amino acids in type II tumors; all were absent from 180 control genomes and from existing SNP databases. This suggests that DMRT1 mutations are uncommon in TGCTs, but our analysis would not have detected deletions, noncoding changes, or epigenetic defects.
Teratoma formation in Dmrt1 mutants involves a failure of germ cells to repress pluripotency regulators. DMRT1 represses expression of three core regulators of pluripotency, Nanog, Sox2, Oct3/4, plus many of their downstream targets, and this inappropriate expression is correlated with the inappropriate differentiation of the mutant cells. In Dmrt1 mutants, pluripotency gene overexpression and EC cell clusters precede teratoma formation; a similar progression may underlie development of human type I TGCTs. Genome-wide ChIP studies should identify additional DMRT1 targets with potential roles in teratoma formation.
RET, along with GFRα1, is a coreceptor for the TGFβ family ligand GDNF (43). GDNF signaling is essential for SSC maintenance and can support pluripotent germ cell cultures. Forced overexpression of GDNF in mouse spermatogonia causes tumors similar in some respects to human spermatocytic seminomas, in which premeiotic germ cells fail to differentiate and continue to proliferate (7, 52). Human spermatocytic seminomas overexpress DMRT1 (29), and we find that this is associated with overexpression of RET (Fig. S7). Thus, loss of Dmrt1 and reduced Ret expression in fetal germ cells is associated with testicular teratomas, while elevated DMRT1 and GDNF signaling in postnatal germ cells is associated with spermatocytic seminomas. Our data suggest that reduced GDNF signaling, together with elevated expression of pluripotency regulators, permits execution of somatic differentiation programs and teratoma formation. Overactivation of the GDNF pathway postnatally may have the opposite effect, blocking differentiation of germ cells and leading to spermatocytic tumors.
Transplantation and cell culture indicate that germ cell pluripotency is suppressed between E13.5 and birth in the testis. At E13.5, male germ cells also become mitotically arrested, resuming proliferation at birth, and expression of pluripotency regulators is reduced between E15.5 and birth. We suggest that the late embryonic testis provides a permissive environment for cellular differentiation and that germ cells must therefore suppress pluripotency and mitosis as they transit this stage. In this model, Dmrt1 is an essential component of a mechanism that normally achieves this suppression. The teratoma formation of Dmrt1 mutants can be viewed as a heterochronic defect, in which germ cells with immature character are present in a more mature and permissive environment. Similar models have been proposed for human type II germ cell tumors in which carcinoma in situ (CIS) cells with embryonic character and expression of pluripotency markers are present in the postnatal testis (10).
The work described here identifies a number of genes whose inappropriate expression correlates with teratoma formation in the mouse. These genes also are expressed during human germ cell development, and our gene expression analysis in human TGCTs suggests mechanistic links between mouse and human germ cell tumorigenesis that will be important to explore further.
Materials and Methods
Mouse Breeding.
Mixed background Dmrt1flox/flox and Dmrt1+/− mice (26) were out-crossed to 129S6/SvEvTac (Taconic Labs) at least seven times unless otherwise noted. For experiments on the B6 background, Dmrt1+/− mice were out-crossed 10 times to C57BL/6J (Jackson Labs). Oct4ΔPE:GFP on a 129S1/SvImJ background and 129.MOLF-Chr19 L1 mice have been described in refs. 51 and 53. For conditional targeting of Dmrt1 in embryonic Sertoli cells, Dmrt1−/− females carrying Dhh-Cre (31) or SF1-Cre (32) were crossed to 129Sv Dmr1flox/flox males. For conditional targeting in fetal germ cells 129Sv Dmrt1+/− mice carrying TNAP-Cre (33) were crossed to 129Sv Dmrt1flox/flox females. Cre transgenes were out-crossed at least 4 times to 129Sv before breeding with Dmrt1 mutants. Presence of a copulation plug in the morning was recorded as E0.5. For ChIP experiments, mixed background males hemizygous for an X-linked GFP (54) were crossed to B6 females. Genotyping of Dmrt1, Cre, and Oct4ΔPE:GFP was as described in refs. 30 and 53.
Immunofluorescence and Immunohistochemistry.
Immunofluorescence was performed as described in ref. 41, except secondary antibody was incubated for 4 h at RT and mounted with Permafluor (Lab Vision). The Mouse On Mouse kit (Vector Labs) was used for all primary antibodies (Table S1) raised in mouse per manufacturer's instructions. Immunohistochemistry was performed by using the ABC kit (Vector Labs).
mRNA Expression Profiling.
Gonad pairs were dissected from E13.5 embryos and placed in RNAlater (Qiagen), and RNA was prepared by using Allprep Micro kit (Qiagen). Total RNA (100 ng) was amplified and labeled using NuGen Ovation labeling kit and hybridized to Affymetrix 430 2.0 microarrays. Data analysis is described in SI Text.
qRT-PCR.
Gonads were stored in RNAlater until RNA was prepared by using RNeasy Micro kit (Qiagen). RNA was reverse-transcribed by using SuperScript III Reverse Transcriptase. cDNA was amplified by using FastStart SYBR green (Roche). qRT-PCR primers are in Tables S2.
ChIP.
Mixed background embryos were collected at E13.5 and sexed by X-linked GFP expression. Gonads from 83 males were harvested in ice-cold PBS and fixed 10 min in 1% PFA. Fixation was stopped and washed as described in ref. 55 in the presence of 1× Complete proteinase inhibitors (Roche). After final wash, buffer was removed, and gonads were stored at −70 °C until all were collected. Gonads were pooled, and ChIP was performed essentially as described in EZ ChIP kit (Upstate). Chromatin was sonicated for 7.5 min by using a Biorupter (Diagenode). ChIP DNA was amplified using LM-PCR (56) modified to use DNATerminator (Lucigen Corp). qPCR was performed on immunoprecipitated DNA using gene specific primers (Table S3).
Supplementary Material
Acknowledgments.
We thank Dr. James Amatruda and members of the Zarkower and Bardwell laboratories for helpful discussions, Dr. David Greenstein for critical reading of the manuscript, and the Minnesota Supercomputing Institute. We thank A.J. Gillis, LeAnn Oseth, Nisha Shan, and Chris Small for technical assistance. Antibodies and mice were generously provided by Drs. Pierre Chambon, Andras Nagy, and Dies Meijer. This work was supported by University of Minnesota Masonic Cancer Center, Minnesota Medical Foundation, the National Institutes of Health Grants GM59152, T32HD007480, CA093754, and the Norman Hackerman Advanced Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905431106/DCSupplemental.
References
- 1.Stevens LC. Experimental production of testicular teratomas in mice. Proc Natl Acad Sci USA. 1964;52:654–661. doi: 10.1073/pnas.52.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan K, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 3.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 4.Kanatsu-Shinohara M, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: Transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- 6.Stewart CL, Gadi I, Bhatt H. Stem cells from primordial germ cells can reenter the germ line. Dev Biol. 1994;161:626–628. doi: 10.1006/dbio.1994.1058. [DOI] [PubMed] [Google Scholar]
- 7.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 8.Matzuk MM, Lamb DJ. The biology of infertility: Research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 10.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 11.Looijenga LH, Stoop H, Hersmus R, Gillis AJ, Oosterhuis J Wolter. Genomic and expression profiling of human spermatocytic seminomas: Pathogenetic implications. Int J Androl. 2007;30:328–335. doi: 10.1111/j.1365-2605.2007.00779.x. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- 12.Krausz C, Looijenga LH. Genetic aspects of testicular germ cell tumors. Cell Cycle. 2008;7:3519–3524. doi: 10.4161/cc.7.22.6980. [DOI] [PubMed] [Google Scholar]
- 13.Rapley E. Susceptibility alleles for testicular germ cell tumour: A review. Int J Androl. 2007;30:242–250. doi: 10.1111/j.1365-2605.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 14.Skakkebaek NE. Testicular dysgenesis syndrome. Horm Res. 2003;60(Suppl 3):49. doi: 10.1159/000074499. [DOI] [PubMed] [Google Scholar]
- 15.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LC, Little CC. Spontaneous testicular teratomas in an inbred strain of mice. Proc Natl Acad Sci USA. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LC. Genetic influences on teratocarcinogenesis and parthenogenesis. Prog Clin Biol Res. 1981;45:93–104. [PubMed] [Google Scholar]
- 18.Stevens LC. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973;50:235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- 19.Youngren KK, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matin A, Collin GB, Asada Y, Varnum D, Nadeau JH. Susceptibility to testicular germ-cell tumours in a 129. MOLF-Chr 19 chromosome substitution strain. Nat Genet. 1999;23:237–240. doi: 10.1038/13874. [DOI] [PubMed] [Google Scholar]
- 21.Collin GB, Asada Y, Varnum DS, Nadeau JH. DNA pooling as a quick method for finding candidate linkages in multigenic trait analysis: An example involving susceptibility to germ cell tumors. Mamm Genome. 1996;7:68–70. doi: 10.1007/s003359900017. [DOI] [PubMed] [Google Scholar]
- 22.Raymond CS, et al. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- 23.Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarkower D. Establishing sexual dimorphism: Conservation amidst diversity? Nat Rev Genet. 2001;2:175–185. doi: 10.1038/35056032. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 26.Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogata T, et al. Impaired male sex development in an infant with molecularly defined partial 9p monosomy: Implication for a testis forming gene(s) on 9p. J Med Genet. 1997;34:331–334. doi: 10.1136/jmg.34.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flejter WL, Fergestad J, Gorski J, Varvill T, Chandrasekharappa S. A gene involved in XY sex reversal is located on chromosome 9, distal to marker D9S1779. Am J Hum Genet. 1998;63:794–802. doi: 10.1086/302016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Looijenga LH, et al. Genomic and expression profiling of human spermatocytic seminomas: Primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 2006;66:290–302. doi: 10.1158/0008-5472.CAN-05-2936. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeboom F, et al. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 2003;31:5405–5412. doi: 10.1093/nar/gkg723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 33.Lomeli H, Ramos-Mejia V, Gertsenstein M, Lobe CG, Nagy A. Targeted insertion of Cre recombinase into the TNAP gene: Excision in primordial germ cells. Genesis. 2000;26:116–117. [PubMed] [Google Scholar]
- 34.Oulad-Abdelghani M, et al. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 36.Olek A, Walter J. The pre-implantation ontogeny of the H19 methylation imprint. Nat Genet. 1997;17:275–276. doi: 10.1038/ng1197-275. [DOI] [PubMed] [Google Scholar]
- 37.Mitsuya K, et al. LIT1, an imprinted antisense RNA in the human KvLQT1 locus identified by screening for differentially expressed transcripts using monochromosomal hybrids. Hum Mol Genet. 1999;8:1209–1217. doi: 10.1093/hmg/8.7.1209. [DOI] [PubMed] [Google Scholar]
- 38.Monk M, McLaren A. X-chromosome activity in foetal germ cells of the mouse. J Embryol Exp Morphol. 1981;63:75–84. [PubMed] [Google Scholar]
- 39.Noguchi T, Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985;75:385–392. [PubMed] [Google Scholar]
- 40.Kimura T, et al. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- 41.Cook MS, Coveney D, Batchvarov I, Nadeau JH, Capel B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev Biol. 2009;328:377–383. doi: 10.1016/j.ydbio.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labosky PA, Barlow DP, Hogan BL. Embryonic germ cell lines and their derivation from mouse primordial germ cells. Ciba Found Symp. 1994;182:157–168. doi: 10.1002/9780470514573.ch9. discussion 168–178. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fahrioglu U, Murphy MW, Zarkower D, Bardwell VJ. mRNA expression analysis and the molecular basis of neonatal testis defects in Dmrt1 mutant mice. Sex Dev. 2007;1:42–58. doi: 10.1159/000096238. [DOI] [PubMed] [Google Scholar]
- 45.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 46.Bartkova J, Thullberg M, Rajpert-De Meyts E, Skakkebaek NE, Bartek J. Cell cycle regulators in testicular cancer: Loss of p18INK4C marks progression from carcinoma in situ to invasive germ cell tumours. Int J Cancer. 2000;85:370–375. doi: 10.1002/(sici)1097-0215(20000201)85:3<370::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Chou YF, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharov AA, et al. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008;9:269. doi: 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 50.Youngren KK, Nadeau JH, Matin A. Testicular cancer susceptibility in the 129.MOLF-Chr19 mouse strain: Additive effects, gene interactions and epigenetic modifications. Hum Mol Genet. 2003;12:389–398. doi: 10.1093/hmg/ddg036. [DOI] [PubMed] [Google Scholar]
- 51.Zhu R, Ji Y, Xiao L, Matin A. Testicular germ cell tumor susceptibility genes from the consomic 129. MOLF-Chr19 mouse strain. Mamm Genome. 2007;18:584–595. doi: 10.1007/s00335-007-9036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X, de Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- 53.Anderson R, et al. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- 54.Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Non-invasive sexing of preimplantation stage mammalian embryos. Nat Genet. 1998;19:220–222. doi: 10.1038/893. [DOI] [PubMed] [Google Scholar]
- 55.Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- 56.Oberley MJ, Tsao J, Yau P, Farnham PJ. High-throughput screening of chromatin immunoprecipitates using CpG-island microarrays. Methods Enzymol. 2004;376:315–334. doi: 10.1016/S0076-6879(03)76021-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.