Abstract
The means by which a polypeptide chain acquires its unique 3-D structure is a fundamental question in biology. During its synthesis on the ribosome, a nascent chain (NC) emerges vectorially and will begin to fold in a cotranslational fashion. The complex environment of the cell, coupled with the gradual emergence of the ribosome-tethered NC during its synthesis, imposes conformational restraints on its folding landscape that differ from those placed on an isolated protein when stimulated to fold following denaturation in solution. To begin to examine cotranslational folding as it would occur within a cell, we produce highly selective, isotopically labeled NCs bound to isotopically silent ribosomes in vivo. We then apply NMR spectroscopy to study, at a residue specific level, the conformation of NCs consisting of different fractional lengths of the polypeptide chain corresponding to a given protein. This combined approach provides a powerful means of generating a series of snapshots of the folding of the NC as it emerges from the ribosome. Application of this strategy to the NMR analysis of the progressive synthesis of an Ig-like domain reveals the existence of a partially folded ribosome-bound species that is likely to represent an intermediate species populated during the cotranslational folding process.
Keywords: cotranslational folding, ribosome, SecM
The ribosome consists of approximately 50 proteins and three large RNA molecules and is the molecular machine that generates protein molecules in vivo from information encoded within the genome sequences of all organisms. Studies of ribosome structure and function have made dramatic progress over recent years (1), revealing (2–4) many aspects of the organization of these remarkable structures in atomic detail.
A description of the manner in which an emerging nascent chain (NC) attains its 3-D structure is crucial to the development of an understanding of the principles of in vivo protein folding. Evidence from epitope recognition (5–7), enzymatic activity (8, 9), and biophysics (10, 11) indicates that during synthesis, which occurs in an N- to C-terminal vectorial fashion, the emerging NC can acquire conformations that assist in the achievement of the native architecture, a process referred to as cotranslational folding (CTF) (12–14). These favorable interactions, likely to be driven, at least in part, by the necessity of burying hydrophobic residues, would help to avoid some forms of partially structured intermediates that can readily lead to misfolding and aggregation.
Biophysical experiments using FRET (15) reveal that the ribosomal tunnel has the capacity to accommodate the formation of helical secondary structure in much of the sequence of an NC (16) and, at least in the vicinity of the exit site, elementary tertiary interactions such as hairpins (17), while cryoEM (18) and pegylation (19) approaches indicate some compaction of the polypeptide chain is likely to occur within the tunnel. Its dimensions [≈80-Å long and 10- to 20-Å wide at its narrowest point (20)], together with its conservative global flexibility (21), suggests that complete NC folding can only take place once it has emerged from the tunnel (22).
An ability to probe the growth and acquisition of structure in NCs, and their interactions with cellular components associated with the promotion of efficient folding such as protein disulfide isomerases, and with the reduction of aberrant intermolecular interactions, such as molecular chaperones, would enable fundamental questions relating to protein folding in the cell to be addressed. At present, in vitro and in silico studies of polypeptide chains in isolation have been used to define the fundamental principles of folding (23). Although these principles are undoubtedly universal, an NC is under conformational restraints that differ from those of a polypeptide chain in isolation, not least as a result of the restraints imposed on its conformational freedom while tethered to the ribosome, and may lead to differences in at least some of the specific events that lead to the fully folded structure.
We have recently shown that high-resolution NMR of ribosome-NC complexes (RNCs), in this case prepared using cell-free methods (15), can be used to probe stalled NCs (24). NMR is a powerful tool for providing detailed structural information on dynamic systems at the atomic level, and the ability to derive residue-specific structural information related to the folding of an NC, and particularly of the inter- and intra-molecular interactions that develop as these chains emerge, would dramatically enhance our understanding of protein folding. The study of RNCs generated in vivo, however, would provide an additional dimension to these studies by enabling CTF to be examined as it occurs in this environment.
The production of RNCs in vivo has so far been developed largely for use in conventional biophysical and biochemical studies (25–27), but to apply NMR successfully requires isotopically labeled NCs attached to isotopically silent ribosomes. In the growing cell, however, ribosomes are continuously generated and under appropriate growth conditions, can themselves be isotopically labeled along with the NC. Achieving selective isotopic labeling while an NC is bound to its ribosome thus presents a significant technical challenge in cellular regulation and manipulation. Additionally there is the requirement for the generation of large quantities of material for NMR. NMR experiments also require long accumulation times, typically several hours and sometimes days, making it important to generate highly stable samples. To overcome these very significant challenges we have developed a powerful and robust expression system based upon the principles of autoinduction (28). The result enables us to manipulate selectively the growth characteristics of E. coli cells to generate, in vivo, significant quantities of homogeneous, translationally arrested ribosomes with isotopically labeled NCs attached to them. We here describe this technique and show how it has enabled a powerful strategy to be developed to probe CTF as it occurs within the cellular environment, by using NMR methods to examine (13C and 15N) isotopically labeled NCs of varying lengths produced in vivo, and thus to obtain snapshots of the folding of the NC as it emerges from the exit tunnel.
Results
An Expression System for Production of Stalled RNCs in E. coli.
We use a robust E. coli expression system that we have developed with the aim of enabling the NC to fold within the natural cellular milieu. We chose as the NC for this investigation the truncated sequence corresponding to a pair of Ig domains from the multidomain gelation factor protein, ddFLN, from Dictyostelium discoideum that we have studied previously (24). The construct consists of 104 residues of domain 5 (Dom5) and 90 residues of domain 6 (Dom6), designated ddFLN646–839.
We selected a T7-driven vector backbone for high-level protein expression both in in vivo and in vitro (cell-free) conditions, and introduced several features to create a vector, pLDC-17 (Fig. 1). The vector (see Methods) includes a multiple cloning site to allow for the ready introduction of the polypeptide sequence of interest, and a purification tag, here a hexa-His sequence, fused to the N terminus of the NC to permit purification of ribosomal complexes from the cellular milieu to be carried out rapidly under mild conditions. The SecM motif (25, 26, 29) was incorporated at the C terminus to cause the ribosome to stall at a specific position in the sequence (P166 at the A site).
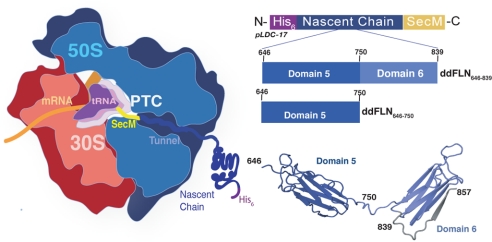
Fig. 1.
The 70S ribosome and RNC constructs. Left: schematic diagram of the 70S ribosome with the 30S and 50S subunits highlighted in red and blue, respectively. The PTC is shown occupied by tRNA (purple). Mapped onto the ribosome is the NC construct as produced via the pLDC vectors. From the C terminus at the PTC is the 17-residue SecM motif, (yellow), followed by the selected NC (blue). The NC is shown fused to a hexa-His tag; Center: the general features of the pLDC-17 vector are shown (above) in the N to C direction. Schematics of the two RNCs described in this study are shown below: ddFLN646–839-RNC is Dom5 plus a 90 residue sequence derived from Dom6 fused to SecM; ddFLN646–750-RNC is Dom5 alone fused to SecM; Right: representation of the two domain structure (from 1QFH.pdb) of Dom 5 and Dom 6 (ddFLN646–857). The ddFLN646–839-RNC vector comprises an NC construct designed such that the final strand in Dom6 (G-strand, shaded gray) is absent.
For the production of RNCs in vivo we used an adaptation of the principles of autoinduction for protein expression, which relies on the ability of E. coli to metabolize carbon sources selectively within the medium, and which therefore facilitates high cell density growth and enables the automatic induction (autoinduction) of protein expression in the absence of inducers such as IPTG (28). We used a variation of the autoinduction procedure and adopted a medium (Methods and SI Text), which allowed us to stimulate the growth of E. coli to reach stationary phase at high cell densities (OD600 5–10) in the absence of NC expression, followed by re-introduction of the cells into a M9 medium for expression. As a result, despite the high cell density, we found that by replenishing essential nutrients, in particular by adding glucose as the carbon source, and maintaining high shaking speeds, the pH remained stable (6.8–7.0) and the OD600 continued to increase, indicating that the cells remained viable.
We then devised a modification of this strategy to introduce 15N-labeling (and subsequently 15N, 13C-labeling) selectively into the NCs and not the ribosomes to which they are attached. After the initial high cell density growth step described above, the cells were transferred into M9 in the absence of a nitrogen source to enable the cells to deplete their endogenous supply of nitrogen. At induction, the medium was replenished with isotopically (15N) labeled ammonium chloride; this ensured that the added 15N would be the sole source of nitrogen present during expression (Fig. 2). At the end of the expression period, the increase in OD600 was typically 5–10%; this low value is important as it suggests that the majority of cells were engaged in the energy-expending process of overexpression and thus limiting the extent of cell growth (Fig. 2). Alternatively, the environmental conditions (high cell density) may slow the overall growth of the cells thus limiting the production of new ribosomes; regulation and biogenesis of ribosomes in the context of cellular growth and conditions of stress is a complex process, and the details of how the cell regulates the production of ribosomes remain unclear. Nevertheless, the modest increase in OD600 indicates that the cells are viable under these environmental conditions. Moreover, the slow growth of the cells is highly beneficial as it results in a reduced production of new ribosomes that could take up the isotope, thus minimizing the possible background signal arising from such labeled ribosomes in the subsequent NMR spectra.
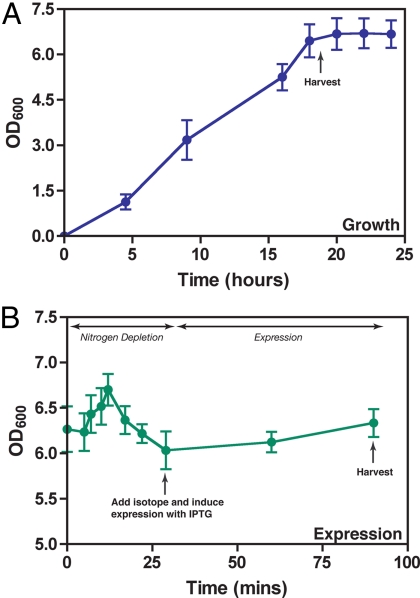
Fig. 2.
Generation of RNCs in vivo using E. coli expression. After transformation of the NC-containing pLDC-17 vector into E. coli, cell growth to high cell densities in MDG medium was allowed for up to 20 h, 37 °C, 280 rpm. Cells were then harvested and resuspended in M9 expression medium in the absence of a nitrogen source. The nitrogen source was depleted before the addition of 15N ammonium chloride and expression induced at 25 °C. Cells were harvested 45 min after induction.
RNC purification from the E. coli lysate was carried out using metal affinity chromatography followed by sucrose gradient ultracentrifugation (Fig. 3). Analysis of the NMR spectrum of the sample indicates that the level of isotopic labeling in the NC was typically over 95% with negligible background signals (see below).
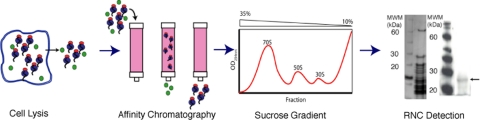
Fig. 3.
Purification scheme for RNCs. RNCs were purified using a two-step purification process. After expression, the E. coli cells were lysed using a French press, the lysate applied to a Co2+ affinity column, and the eluate subjected to a 10–35% (vol/vol) sucrose gradient. After fractionation the RNCs were analyzed on SDS/PAGE (silver stain shown) and probed with an anti-His antibody to confirm the presence of the NC (shown by the arrow).
Viability of an in Vivo Approach for the Production of Selectively Labeled RNCs.
Using this dual strategy, we have been able to generate very pure RNCs at yields of 50–200 pmol/L, comparable to those of an equivalent in vitro approach (26). Importantly the combined introduction of both the His tag and the SecM sequence enabled samples to be prepared where more than 90% of the ribosomes have an NC attached (as judged by correlating cross-peak intensity in 2D spectra, 1D 1H NMR, and OD260 values).
Although mid-log induction of expression is commonplace in procedures for protein production, expression in high yields at the late log/stationary phase has also been achieved (30). After sustained growth in a non-inducing medium, not only do the cells reach stationary phase but additionally the ribosomes are capable of entering a ‘hibernating’ state where the intact 70S particles self-associate to form a 100S dimeric species, presumably to help maintain cell viability under conditions of stress (31). Thus, the re-introduction of the cells into a new medium where nutrients are replenished and the aeration is sufficient reactivates the dormant state of ribosomes ensuring that the cells remain viable. This successful approach to manipulating growth conditions and exploiting ribosome biogenesis under these conditions suggests great promise for further labeling schemes within the growing cell. Moreover the high adaptability of E. coli means that there are numerous specialized cell lines that can address issues of toxicity, codon usage and posttranslational modifications, such as disulfide bond formation that impact on protein folding and indeed deletion strains of E. coli can be readily generated (32).
Structural Properties of ddFLN646–839 as a Ribosome-Bound NC.
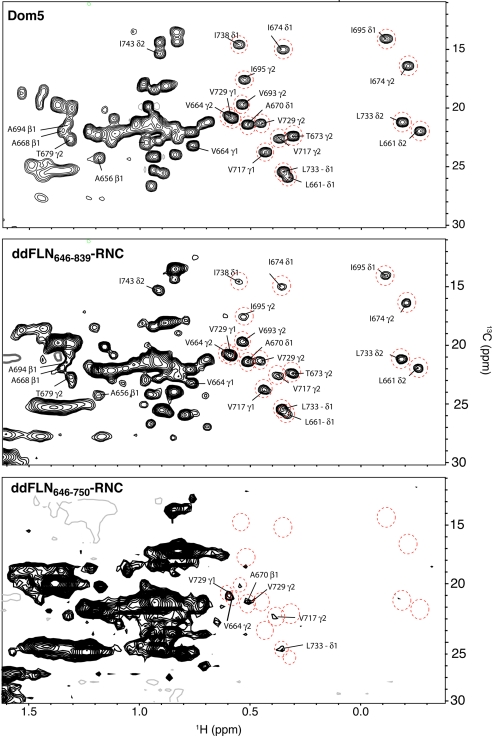
The 2D 1H-15N NMR correlation spectra of 15N-labeled ddFLN646–839-RNC (Fig. 4) prepared using the in vivo method described above, were found to be of very high quality. By using SOFAST-HMQC spectroscopy (33), a technique with an ability to give an approximately 3-fold sensitivity increase over conventional correlation spectroscopy, we could obtain such spectra in approximately 6 h, over which time no trace of sample degradation or release of the NC was observed. The spectrum contains a large number (≈50) of well dispersed cross-peaks surrounding a region of overlapping signals (Fig. 4) and is essentially identical to the analogous spectra of samples prepared using cell-free methods (24). This result indicates that the folding of this NC in the cell-free and in vivo systems is not detectably different. The 1H-13C methyl correlation spectrum of an independent sample of the same construct (15N, 13C-labeled ddFLN646–839-RNC) is of exceptional quality (Fig. 5). The well resolved cross-peaks in both the 13C and the 15N spectra can be attributed to the folded Dom5 region of ddFLN646–839 (Figs. 4 and 5) (24, 34).
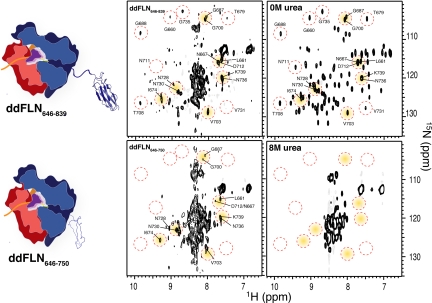
Fig. 4.
Comparison of 15N-1H correlation NMR spectra of ddFLN646–839-RNC and ddFLN646–750-RNC with that of isolated Dom5. A selection of cross-peaks has been highlighted (red circles) in each of the three 15N-1H correlation spectra for ready comparison. Cross-peaks in the spectrum of ddFLN646–839-RNC show similarities to cross-peaks present in spectra of the isolated Dom5 protein under native conditions (0 M urea), indicating that, on the ribosome, the in vivo generated NC is able to acquire native-like structure. ddFLN646–750-RNC, by contrast, shows a predominantly unfolded spectrum, as compared to isolated Dom5 in denaturing conditions (8 M urea) although there are nine well-resolved cross-peaks corresponding to the signals of several residues present in the isolated Dom5 spectrum under native conditions (0 M urea); the latter residues are indicative of the presence of native-like structure. The spectrum of ddFLN646–750-RNC therefore is neither that of a fully folded or of a fully unfolded protein.
Fig. 5.
Comparison of 13C-1H NMR spectra of ddFLN646–839-RNC and ddFLN646–750-RNC with that of isolated Dom5 (ddFLN646–839). The 1H-13C methyl region spectra of the two RNCs, ddFLN646–839 (Middle) and ddFLN646–750 (Bottom), are compared to that (Top) of native ddFLN646–839 (0 M urea). Highlighted in each spectrum is a selection of well resolved cross-peaks whose chemical shifts coincide with native-like structure (within 0.05 ppm in the 1H dimension in isolated Dom5). The long RNC (ddFLN646–839-RNC) shows the equivalent cross-peak dispersion as seen for Dom5, while the short RNC, ddFLN646–750-RNC, shows several dispersed cross-peaks (corresponding to V664, V717, L733, and V729 in the fully folded domain and clearly separated from random coil chemical shift values), coinciding with native-like structure but the absence of the most high field shifted methyl groups (those between −0.1 and −0.5 ppm in the 1H dimension) indicates the presence of a partially folded structure.
Despite the ability to resolve a substantial number of cross-peaks in both the 15N and 13C spectra, there are peaks in the spectra of the recombinant protein that are not detectable in the spectra of the RNC. In addition, selective broadening is observed for many of the resolved signals indicating the presence of dynamical fluctuations associated with attachment to the ribosome (24, 34). The cross-peak clusters visible between 8.0–8.5ppm in the 1H dimension of the 1H-15N spectrum, and between 0.8–1.3 ppm in the 1H dimension of the 1H-13C spectrum, are indicative of a substantial degree of disorder and can be attributed to the presence of Dom6 in this construct (24) (Fig. 5B). Dom6 is partially located in the tunnel and in addition lacks the terminal β-strand of the native structure; it is therefore unable to fold completely to give the characteristic dispersed signals of a natively folded protein and so can act as a flexible linker connecting the folded Dom5 to the ribosome at the PTC (Figs. 1, 4, and 5).
The very highly selective labeling of the NC relative to the ribosome itself is evident from the fact that the signals arising from the L7/L12 proteins, which have sufficient motional narrowing to be observed in NMR spectra of isotopically labeled ribosomes (35, 36), have negligible, indeed undetectable, intensity in the spectra of the samples prepared in the present work. Evidence to support this conclusion comes from the examination of non-translating ribosomes that are recovered from the purification process (collected in the flow-through of the Co2+-column, Fig. 3) and whose 15N spectra reveal labeled species (Fig. S1) with cross-peak dispersions characteristic of L7/L12, the structure and dynamics of which we have described previously (35). 15N-labeled L7/L12 must be associated with newly synthesized ribosomes generated during the expression phase, which therefore incorporate isotopic labels from the medium. Intriguingly, despite the production of new ribosomes during expression, there appears to be no mixed labeling, where both the L7/L12 proteins and the NC are labeled on the same ribosome particle. Regardless of its explanation, however, this important result illustrates the highly regulated conditions under which expression takes place and the intrinsic complexity and delicate balances within the cellular environment that influence cell viability, ribosome biogenesis, and heterologous protein expression and which are responsible for the success of our production strategy.
Structural Properties of ddFLN646–750 as a Ribosome Bound Nascent Chain.
To initiate studies of CTF in vivo, additional RNC samples were produced where the N-terminal 104-amino acid segment of the folding competent Dom5 was fused to the 17-residue SecM, to produce the ddFLN646–750-RNC. In this RNC vector, no linking sequence was added and only the SecM motif (Fig. 1) separates the NC from the PTC. The construct was uniformly 15N-labeled (and subsequently 15N, 13C-labeled) and purified using the strategy described above for ddFLN646–839-RNC. NMR spectra of this shorter construct, ddFLN646–750-RNC, reveal substantially fewer resolved cross-peaks than observed in spectra of ddFLN646–839-RNC, with a large number of the peaks between 8.0–8.5 ppm in the 15N spectrum (Fig. 4) and 0.8–1.5 ppm in the 13C spectrum (Fig. 5); the narrow lines that enable these cross-peaks to be visible indicate, as with the longer construct, that the NC is highly dynamic relative to the ribosome itself. Remarkably, while the pattern of these cross-peaks in this domain reveals a significant degree of disordered structure, the spectra also show a number of dispersed cross-peaks that are not observed in comparative NMR spectra of isolated Dom5 denatured in vitro in 8 M urea to generate spectra of the completely unfolded polypeptide (44) (Fig. 4 and SI Text). Although the intensities of these peaks are relatively low, these are reproducible in the 15N spectra, and this result indicates unambiguously that persistent non-random structure exists in the NC relative to the fully unfolded state, despite the fact that not all of the chain that is folded in the native state has emerged from the ribosomal tunnel as we discuss below.
When compared to the spectra of ddFLN646–839-RNC and that of isolated Dom5, the 1H-15N (Fig. 4) and 1H-13C correlation spectra (Fig. 5) of ddFLN646–750-RNC show marked differences in the degree of dispersion of the observed cross-peaks (Fig. 4). It is also evident that much more extensive broadening is present in the spectra of this shorter construct. A comparison between isolated Dom5, ddFLN646–839-RNC, and ddFLN646–750-RNC, shows, however that in some cases there are dispersed cross-peaks at very similar positions (Figs. 4 and 5, circled resonances) in the two sets of spectra, while many other cross-peaks do not have such apparent analogues. The absence of the majority of the most highly shifted resonances is particularly noticeable; it is possible that this arises from the fact that the fluctuations in global structure will affect the most highly shifted resonances to a greater extent than those with smaller shifts. The line broadening is also likely to contain contributions from local fluctuations as we also previously observed for the ddFLN646–839-RNC (24, 34).
Although it is not possible to give definitive assignments for the dispersed resonances in the spectra of ddFLN646–750-RNC at present, we have examined the spectra using the preliminary assumption that when resonances coincide within approximately 0.1 ppm in the 1H dimension of the 1H-15N spectra and within 0.05 ppm in 1H-13C spectra, the cross-peaks could correspond to those observed and assigned in the spectrum of the isolated protein. The exit tunnel is thought to hold between 30–40 amino acids of an NC sequence attached to the PTC (16), and it is therefore expected that, along with the 17 residue SecM motif, between 10–20 residues of the C terminus of Dom5 will be occluded within the tunnel; this sequence includes the entire G-strand and some of the adjacent loop region, here spanning residues 742–750, (residue 742 is 30 residues from the PTC and thus at the lower limit of residues that would be occluded) of a canonical Ig fold (38, 39). In accord with this conclusion, inspection of the cross-peaks in spectra of the ddFLN684–750-RNC that correlate closely in chemical shifts with those observed in the isolated Dom5 and of the ddFLN646–839-RNC reveals no peaks that correspond to those of the native state in this entire region of the sequence (Fig. 4). Cross-peaks are, however, observed that could correspond to native-like structure in other regions of the spectrum (Figs. 4 and 5, details in Fig. 6). Interestingly, corresponding signals from dispersed resonances of the same residues are often absent when the 15N and 13C spectra are compared (Fig. 6). A similar low correlation is seen between the observation of the backbone and mainchain resonances even for the well-folded emerged domain 5 within ddFLN646–839-RNC (Fig. 6A) and also in the cell-free derived analogue (24, 34), suggesting that this phenomenon is due to the differential response of the 13C and 15N probes to the dynamics and exchange properties of an NC when attached to the ribosome.
Fig. 6.
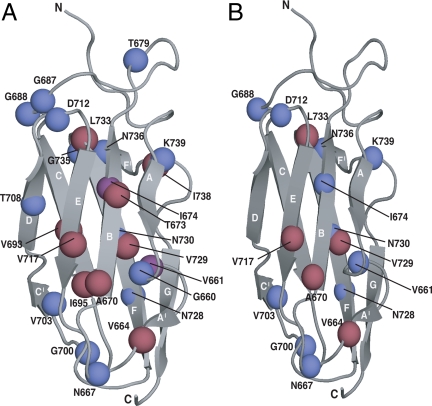
The structure of Dom5 with residues labeled that appear to have crosspeaks showing native-like structure in ddFLN646–750. Mapped on to structural models (from 1QFH.pdb) of Dom5 are residues with cross-peaks in the 15N-1H spectra (blue spheres) and cross-peaks in the 13C-1H (red spheres) spectra of Dom5 that are within 0.1 and 0.05 ppm (1H dimension) respectively of cross-peaks in both (A) ddFLN646–839-RNCand (B) ddFLN646–750-RNC. Shown in purple spheres are cross-peaks that are visible in both the 15N-1H and 13C-1H spectra. In the case of ddFLN646–750-RNC, the residues of the final G-strand of Dom5 remains within the exit tunnel although cross-peaks are, however, observed in the NMR spectrum of ddFLN646–750-RNC (lower left spectrum in Fig. 4 and lower spectrum in Fig. 5) that could correspond to native-like structure in other regions including strand B (the peak attributed to I674 is visible in 15N spectra and that attributed to A670 in 13C spectra, respectively), strand E (V717 visible in 13C spectrum), and strand F (peaks for N728 and N730 are visible in 15N spectra, and those for V729 and L733 can be seen in13C spectra) and also several connecting loop regions.
These results, therefore, show that the NC in ddFLN646–750-RNC is highly dynamic but has characteristics of a low population of partially folded species with native-like structure in at least some regions of the chain that has emerged from the tunnel. Although further experiments are required to examine the extent of such structure, the very existence of a number of well-resolved dispersed signals (depicted in Fig. 6) is by itself of great significance, regardless of their assignments, as intermediates with elements of highly persistent structure are only very rarely observed in vitro in the folding and unfolding of small protein domains. These studies, however, are almost invariably carried out in the presence of denaturants or at extremes of pH or temperature; it is quite possible therefore that the in vivo folding of the same systems taking place under physiological conditions differs in detail from that defined from such in vitro studies.
These findings are of particular interest when compared to force-induced mechanical unfolding experiments, which provide an in vitro representation of one form of vectorial folding that could be somewhat analogous to the behavior of an NC as it emerges from the ribosomal tunnel. It has been shown for a structurally related protein, titin-I27, that the G-strand makes important contacts with the N-terminal A-strand within the native structure, and that this interaction is disrupted during force-induced unfolding (39). As discussed above, in ddFLN646–750-RNC the G-strand is constrained within the tunnel and so it is unavailable for forming interactions with the A-strand (Fig. 6). Although the characteristics of the partially folded state of the NC remain to be defined, it is interesting that the loss of contacts through the deletion of the A-strand in titin-I27 results in the formation of an intermediate ensemble, which has been shown to adopt a significant degree of native-like structure (39). Similar behavior has also been observed for the Ig-like proteins of the PapD/K chaperone system, where the absence of the C-terminal G-strand permits ‘donor strand complementation,’ a potent assembly mechanism required in pilus biogenesis (40). Both of these examples suggest that Ig-like proteins have the propensity to adopt a significant degree of native-like structure in the absence of either the N- or C-terminal strands and give credence to the tentative conclusions drawn here concerning the ddFLN646–750-RNC.
Discussion
The ability to study the structure and dynamics of RNCs in atomic detail by NMR has the potential to provide unprecedented insights into protein folding in the context of the cellular environment. In the present study, we have substantially expanded the scope of our previous approaches for defining the structure and dynamics of an NC attached to the ribosome (24) by developing a strategy that allows RNCs to be synthesized and to undergo folding in vivo before isolation and characterization. In the present case we have carried out our studies in E. coli, but in principle, this approach can be extended to other organisms. This development provides an opportunity to generate large quantities of material using well-established techniques and in addition provides a basis for comparing the conformations of NCs generated as a result of the folding of an RNC in vitro, with that resulting from folding within the living cell, albeit following extraction before detailed structural studies.
In the present study, we have applied this approach to begin to probe the nature of cotranslational folding by addressing the characteristics of NCs corresponding to different stages of ribosomal synthesis and folding. The ddFLN646–839-RNC construct generates an Ig domain, Dom5, that extends through the ribosomal tunnel, is tethered through a linker to the ribosome, and displays NMR characteristics of a folded N-terminal domain and a largely disordered C terminus. We then prepared and examined the ddFLN684–750-RNC species in which, only about 80–90 out of the 104 residues of the complete sequence of Dom5 are likely to be located outside the exit tunnel. From the NMR spectra at least a significant region of the NC is highly mobile, as in the ddFLN646–839-RNC construct, as the line widths are again dramatically narrower than would be the case for a rigid structure of 2.5-MDa molecular weight. Of particular interest however, is the identification of a partially folded intermediate state whose spectral properties are indicative of native-like persistent structure in the Ig domain. This finding strongly suggests that extension of the present types of study should be able to provide the answers to questions such as the minimal length of an NC that must emerge from the ribosome before the formation of any persistent secondary or tertiary structure, the extent to which this phenomenon is sequence dependent, and the stages of the synthesis of a protein sequence at which different elements of structure in a protein are attained.
The in vivo strategy described in this paper offers a major step forward in our ability to study, at a residue specific level, protein folding that has taken place within a cellular environment. The nature of the partially folded structures observed for the RNCs should in the future be amenable to detailed structural analysis; for example, recently developed techniques that require only NMR chemical shift data as restraints in structure determination procedures (41, 42). Such techniques hold out the prospect of eliminating the major challenge of carrying out extensive measurements of additional NMR parameters, such as NOEs, to determine high-resolution structures (41, 42). In addition to investigations of the role of the ribosome itself, this general approach offers the exciting prospect of examining by NMR, and indeed by using a battery of other complementary biophysical techniques, the interactions of an NC with chaperones and other individual cellular factors associated with the regulatory mechanisms that impact on folding in a living system.
Methods
Plasmid Construction.
The pLDC-17 vector is based upon the pET21b(+) backbone. Using standard techniques, a multiple cloning site was introduced, which allowed for the subsequent introduction of a purification tag (here a hexa-His) as well as the SecM motif (SecM: 149–166) to create pLDC-17. To create the ddFLN-RNC variants, the ddFLN646–839 gene and the ddFLN646–750 fragment were amplified from pT7–7 (37) and subcloned into pLDC-17.
Expression of Uniformly 15N-Labeled RNCs.
After transformation of the ddFLN constructs into BL21(DE3):ssra E. coli, the cells were used to inoculate flasks containing 500 mL MDG medium (Table S1 and SI Text). After growth (16–20 h, 37 °C, 280 rpm), the cells were harvested and then gently (i.e., 280 rpm shaking at 25 °C, to avoid shearing or lysis) resuspended in M9 (without a nitrogen source). The OD600 was monitored until it fell by 10–15%, approximately 15–45min after resuspension was complete. The sole nitrogen source, 15N-ammonium chloride (1g/L), and 1 mM IPTG were added and the cells harvested after a 45-min induction. For 13C, 15N labeling after the initial growth, the cells were resuspended in expression media in the absence of both nitrogen and carbon sources, and after the exhaustion period, 15NH4Cl (1g/L) and 13C-glucose (2g/L) were added with IPTG. Cells were harvested 45 min after induction. For unlabeled RNCs, the procedure was similar to that described above, except that unlabelled ammonium chloride was present (50 mM) in the expression medium (M9), and expression was induced when the OD600 began to increase (30–45 min after resuspension, where OD600 values increased by 20–30%).
RNC Purification.
E. coli cells were resuspended in Tico buffer (10 mM HEPES, 30 mM NH4Cl, 10 mM MgCl2, and 1 mM BME, pH 7.6) containing RNase-free DNase I followed by lysis using a homogenizer. The lysate was purified using a His-Select column and RNCs were subject to 10–35% (vol/vol) sucrose gradient ultracentrifugation (18 h at 21,000 rpm, 4 °C, SW40Ti rotor, Beckman). Gradients were fractionated and analyzed via SDS/PAGE. The purest 70S fractions were pooled, buffer exchanged and concentrated into a ‘binding’ buffer (10 mM HEPES, 140 mM NH4Cl, 6 mM MgCl2, 2 mM BME, 1 mM spermine, and 0.5 mM spermidine, pH 7.6). For the 15N, 13C-labelled RNC samples, the spermine and spermidine were omitted from the buffer.
Detection of RNCs.
NCs were probed by immunodetection using the HisProbe Anti-His antibody in conjunction with the SuperSignal Pico kit (Pierce).
NMR Spectroscopy.
RNC samples (3–10 μM in 70S) were prepared in the binding buffer described above and NMR spectra were recorded at 25 °C using a Bruker Avance 700 MHz spectrometer: 1H-15N and 1H-13C SOFAST-HMQC spectra (33) and 15N-edited X-STE diffusion experiments (43) were recorded as we described previously (24). X-STE spectroscopy was used to assess ribosomal attachment of the NC by a comparison with low Mr proteins in the presence of ribosomes.
Supplementary Material
Acknowledgments.
We thank Dr. David Waugh (National Cancer Institute) for the BL21(DE3):ssra E.coli strain and the pNH52 plasmid was a gift from Professor Koreaki Ito (Institute of Virus Research, Kyoto University, Japan) and the Department of Chemistry at the University of Cambridge, UCL, and the NMR Centre at the National Institute for Medical Research (MRC) London for the use of their Biomolecular NMR facilities. L.D.C is a NH&MRC C. J. Martin Fellow. This work was supported by Human Frontier Long-Term Fellowship LT0798/2005 and National Science Council of the Republic of China, Taiwan Grant NSC97-2917-1-564-102 (to S.-T.D.H.); the Wellcome and Leverhulme Trusts (to C.M.D and J.C.); HFSP Young Investigators Award HFSP67/07 (to J.C.); New Investigators Award BBSRC BBG0156511 (to J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903750106/DCSupplemental.
References
- 1.Steitz TA. A structural understanding of the dynamic ribosome machine. Nat Rev Mol Cell Biol. 2008;9:242–253. doi: 10.1038/nrm2352. [DOI] [PubMed] [Google Scholar]
- 2.Bashan A, Yonath A. Correlating ribosome function with high-resolution structures. Trends Microbiol. 2008;16:326–335. doi: 10.1016/j.tim.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Mitra K, Frank J. Ribosome dynamics: Insights from atomic structure modeling into cryo-electron microscopy maps. Ann Rev Biophys Biomol Struc. 2006;35:299–317. doi: 10.1146/annurev.biophys.35.040405.101950. [DOI] [PubMed] [Google Scholar]
- 5.Evans MS, Sander IM, Clark PL. Cotranslational folding promotes beta-helix formation and avoids aggregation in vivo. J Mol Biol. 2008;383:683–692. doi: 10.1016/j.jmb.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark PL, King J. A newly synthesized, ribosome-bound polypeptide chain adopts conformations dissimilar from early in vitro refolding intermediates. J Biol Chem. 2001;276:25411–25420. doi: 10.1074/jbc.M008490200. [DOI] [PubMed] [Google Scholar]
- 7.Fedorov AN, et al. Folding on the ribosome of Escherichia coli tryptophan synthase beta subunit nascent chains probed with a conformation-dependent monoclonal antibody. J Mol Biol. 1992;228:351–358. doi: 10.1016/0022-2836(92)90825-5. [DOI] [PubMed] [Google Scholar]
- 8.Netzer WJ, Hartl FU. Recombination of protein domains facilitated by co-translational folding in eukaryotes. Nature. 1997;388:343–349. doi: 10.1038/41024. [DOI] [PubMed] [Google Scholar]
- 9.Nicola AV, Chen W, Helenius A. Co-translational folding of an alphavirus capsid protein in the cytosol of living cells. Nat Cell Biol. 1999;1:341–345. doi: 10.1038/14032. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JP, et al. Chain dynamics of nascent polypeptides emerging from the ribosome. ACS Chem Biol. 2008;3:555–566. doi: 10.1021/cb800059u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alekhina OM, Vassilenko KS, Spirin AS. Translation of non-capped mRNAs in a eukaryotic cell-free system: Acceleration of initiation rate in the course of polysome formation. Nucleic Acids Res. 2007;35:6547–6559. doi: 10.1093/nar/gkm725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorov AN, Baldwin TO. Contribution of cotranslational folding to the rate of formation of native protein structure. Proc Natl Acad Sci USA. 1995;92:1227–1231. doi: 10.1073/pnas.92.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 14.Evans MS, Clarke TF, 4th, Clark PL. Conformations of co-translational folding intermediates. Protein Pept Lett. 2005;12:189–195. doi: 10.2174/0929866053005908. [DOI] [PubMed] [Google Scholar]
- 15.Woolhead CA, McCormick PJ, Johnson AE. Nascent membrane and secretory proteins differ in FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell. 2004;116:725–736. doi: 10.1016/s0092-8674(04)00169-2. [DOI] [PubMed] [Google Scholar]
- 16.Voss NR, Gerstein M, Steitz TA, Moore PB. The geometry of the ribosomal polypeptide exit tunnel. J Mol Biol. 2006;360:893–906. doi: 10.1016/j.jmb.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Kosolapov A, Deutsch C. Tertiary interactions within the ribosomal exit tunnel. Nat Struct Mol Biol. 2009;16:405–411. doi: 10.1038/nsmb.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert RJC, et al. 3D structures of translating ribosomes by cryo-EM. Mol Cell. 2004;14:57–66. doi: 10.1016/s1097-2765(04)00163-7. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Deutsch C. Folding zones inside the ribosomal exit tunnel. Nat Struct Mol Biol. 2005;12:1123–1129. doi: 10.1038/nsmb1021. [DOI] [PubMed] [Google Scholar]
- 20.Ban N, et al. The complete atomic structure of the large ribosomal subunit at 2.4 angstrom resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 21.Fulle S, Gohlke H. Statics of the ribosomal exit tunnel: Implications for cotranslational peptide folding, elongation regulation, and antibiotics binding. J Mol Biol. 2009;387:502–517. doi: 10.1016/j.jmb.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Elcock. A Molecular simulations of cotranslational protein folding: Fragment stabilities, folding cooperativity and trapping in the ribosome tunnel. PLoS Comput Biol. 2006;2:e98. doi: 10.1371/journal.pcbi.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 24.Hsu ST, et al. Structure and dynamics of a ribosome-bound nascent chain by NMR spectroscopy. Proc Natl Acad Sci USA. 2007;104:16516–16521. doi: 10.1073/pnas.0704664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans MS, Ugrinov KG, Frese MA, Clark PL. Homogeneous stalled ribosome nascent chain complexes produced in vivo or in vitro. Nat Methods. 2005;2:757–762. doi: 10.1038/nmeth790. [DOI] [PubMed] [Google Scholar]
- 26.Schaffitzel C, Ban N. Generation of ribosome nascent chain complexes for structural and functional studies. J Struct Biol. 2007;159:302–310. doi: 10.1016/S1047-8477(07)00167-0. [DOI] [PubMed] [Google Scholar]
- 27.Rutkowska A, et al. Dynamics of trigger factor interaction with translating ribosomes. J Biol Chem. 2008;283:4124–4132. doi: 10.1074/jbc.M708294200. [DOI] [PubMed] [Google Scholar]
- 28.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Nakatogawa H, Ito K. The ribosomal exit tunnel functions as a discriminating gate. Cell. 2002;108:629–636. doi: 10.1016/s0092-8674(02)00649-9. [DOI] [PubMed] [Google Scholar]
- 30.Ou J, et al. Stationary phase protein overproduction is a fundamental capability of Escherichia coli. Biochem Biophys Res Commun. 2004;314:174–180. doi: 10.1016/j.bbrc.2003.12.077. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H, Yamamoto H, Uchiumi T, Wada A. RMF inactivates ribosomes by covering the peptidyl transferase centre and entrance of peptide exit tunnel. Genes Cells. 2004;9:271–278. doi: 10.1111/j.1356-9597.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 32.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schanda P, Kupce E, Brutscher B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J Biomol NMR. 2005;33:199–211. doi: 10.1007/s10858-005-4425-x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu ST, et al. Probing side-chain dynamics of a ribosome-bound nascent chain using methyl NMR spectroscopy. J Am Chem Soc. 2009;131:8366–8367. doi: 10.1021/ja902778n. [DOI] [PubMed] [Google Scholar]
- 35.Christodoulou J, et al. Heteronuclear NMR investigations of dynamic regions of intact E. coli ribosomes. Proc Natl Acad Sci USA. 2004;101:10949–10954. doi: 10.1073/pnas.0400928101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulder FA, et al. Conformation and dynamics of ribosomal stalk protein L12 in solution and on the ribosome. Biochemistry. 2004;43:5930–5936. doi: 10.1021/bi0495331. [DOI] [PubMed] [Google Scholar]
- 37.McCoy AJ, Fucini P, Noegel AA, Stewart M. Structural basis for dimerization of the Dictyostelium gelation factor (ABP120) rod. Nat Struct Biol. 1999;6:836–841. doi: 10.1038/12296. [DOI] [PubMed] [Google Scholar]
- 38.Harpaz Y, Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994;238:528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- 39.Fowler SB, et al. Mechanical unfolding of a titin Ig domain: Structure of unfolding intermediate revealed by combining AFM, molecular dynamics simulations, NMR and protein engineering. J Mol Biol. 2002;322:841–849. doi: 10.1016/s0022-2836(02)00805-7. [DOI] [PubMed] [Google Scholar]
- 40.Sauer FG, et al. Structural basis of chaperone function and pilus biogenesis. Science. 1999;285:1058–1061. doi: 10.1126/science.285.5430.1058. [DOI] [PubMed] [Google Scholar]
- 41.Cavalli A, Salvatella X, Dobson CM, Vendruscolo M. Protein structure determination from NMR chemical shifts. Proc Natl Acad Sci USA. 2007;104:9615–9620. doi: 10.1073/pnas.0610313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y, et al. Consistent blind protein structure generation from NMR chemical shift data. Proc Natl Acad Sci USA. 2008;105:4685–4690. doi: 10.1073/pnas.0800256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrage F, et al. Slow diffusion of macromolecular assemblies by a new pulsed field gradient NMR method. J Am Chem Soc. 2003;125:2541–2545. doi: 10.1021/ja0211407. [DOI] [PubMed] [Google Scholar]
- 44.Hsu S-TD, et al. Structure, dynamics and folding of an immunoglobulin domain of the gelation factor (ABP-120) from Dictyostelium discoideum. J Mol Biol. 2009;388:865–879. doi: 10.1016/j.jmb.2009.02.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.