Abstract
Altered intestinal barrier function is postulated to be a central predisposing factor to intestinal diseases, including inflammatory bowel diseases and food allergies. However, the mechanisms involved in maintaining homeostatic intestinal barrier integrity remain undefined. In this study, we demonstrate that mice deficient in mast cells (KitW-sh/W-sh [Wsh]) or mast cell chymase (Mcpt4−/−) have significantly decreased basal small intestinal permeability compared with wild-type (WT) mice. Altered intestinal barrier function was linked to decreased intestinal epithelial cell migration along the villus/crypt axis, altered intestinal morphology, and dysregulated claudin-3 crypt expression. Remarkably, engraftment of Wsh mice with WT but not Mcpt4−/− mast cells restored intestinal epithelial cell migration, morphology, and intestinal epithelial barrier function. Collectively, these findings identify a mechanism by which mast cells regulate homeostatic intestinal epithelial migration and barrier function.
Keywords: homeostasis, intestinal permeability, mast cell protease-4
The intestinal epithelium constitutes the largest and most important barrier between our internal and external environments, forming a selectively permeable barrier that permits the absorption of nutrients, electrolytes, and water, while maintaining an effective defense against intraluminal bacteria, toxins, and potentially antigenic material. Disruption of the intestinal barrier is associated with bacterial, viral, and parasitic infestation (1, 2), as well as autoimmune and inflammatory conditions, including inflammatory bowel disease (IBD), food allergy, celiac disease, and diabetes (2). While altered intestinal barrier function (increased intestinal epithelial permeability) can be a consequence of disease exacerbation, clinical evidence suggests it may also be a primary etiologic factor predisposing to disease development. For example, increased intestinal permeability is found not only in patients with IBD, celiac disease, and type I diabetes, but also their healthy first-degree relatives (3–7).
Mast cells contribute to innate and acquired immunity and are important effector cells in host defense. Mast cell activation induces degranulation and release of inflammatory mediators, including histamine, cytokines, proteoglycans, and proteases. Mast cell-specific neutral proteases, including chymases, tryptases, and carboxypeptidase A, are the major constituents of mast cell secretory granules (8). Chymases are serine proteases with chymotrypsin-like specificity that cleave target peptides and proteins after aromatic amino acid residues. Human mast cells express only one chymase, whereas there are 13 known murine chymase genes (9). Based on molecular phylogenetics, the murine α-chymase Mcpt5 is the phylogenetic homologue to human chymase; however, an amino acid substitution in Mcpt5 confers elastase-like, rather than chymotrypsin-like substrate specificity (10). Tissue distribution, heparin-binding properties and substrate and cleavage specificity identify the β-chymase Mcpt4 as the functional homologue to human chymase (11, 12). Mcpt4 cleaves several physiological substrates important for tissue remodeling and extracellular matrix (ECM) degradation both directly and by activating ECM-degrading proteases, including matrix metalloproteases (MMPs) (13).
Previous studies have demonstrated that mast cells regulate intestinal epithelial permeability during the effector phase of intestinal inflammatory responses (2). This raises the question whether mast cells may also regulate homeostatic intestinal barrier function, a process that is poorly understood. In the present study, we examine the molecular regulation of homeostatic intestinal barrier function. Employing mast cell-deficient (KitW-sh/W-sh [Wsh]) Mcpt4−/− and C57BL/6 wild-type (WT) mice, and engrafting Wsh mice with bone marrow-derived mast cells (BMMCs), we demonstrate a mechanism by which intestinal mast cells regulate basal homeostatic intestinal morphology, epithelial migration, and barrier function.
Results
Mast Cells Regulate Homeostatic Intestinal Epithelial Barrier Function.
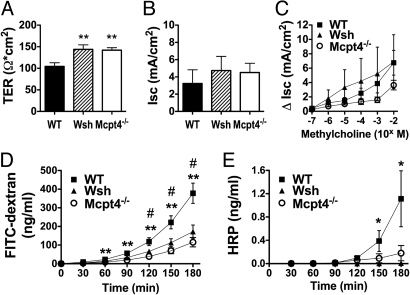
To begin delineating mast cell contribution to homeostatic small intestinal barrier function, we compared the transepithelial resistance (TER) and permeability of ex vivo jejunum from Wsh and WT mice. The TER was significantly increased in Wsh mice compared with WT (Fig. 1A). Altered TER can be explained by modification of either transepithelial ion conductance and/or epithelial permeability, both of which are modulated by intestinal mast cells during inflammatory conditions (2). To distinguish whether mast cells influence homeostatic intestinal permeability and/or ion conductance, we examined ion channel flux. We found no significant difference between Wsh and WT mice in baseline short-circuit current (Isc) or Isc responses to β-methylcholine stimulation (Fig. 1 B and C). We next examined intestinal paracellular and transcellular permeability by luminal-to-serosal flux of FITC-dextran and horseradish peroxidase (HRP) across ex vivo jejunum segments. Notably, we found a significant decrease in permeability to both molecules (Fig. 1 D and E) in Wsh mice. Collectively, these results suggest that mast cells regulate homeostatic small intestinal barrier function through effects on paracellular and transcellular epithelial permeability, but not ion conductance.
Fig. 1.
Decreased basal intestinal permeability in Wsh and Mcpt4−/− mice. Segments of jejunum from WT, Wsh, and Mcpt4−/− mice were mounted in Ussing chambers and the baseline (A) transepithelial resistance (TER) and (B) short-circuit current (Isc), and (C) β-methylcholine-stimulated changes in Isc were measured. Ex vivo intestinal permeability was measured as luminal-to-serosal flux of (D) FITC-dextran and (E) HRP. Values represent mean± SEM; n = 12–18 mice per group. Statistical significance is: (A) **, P < 0.01 vs. WT; (D) #, P < 0.01 Wsh vs. WT, **, P < 0.01 Mcpt4−/− vs. WT; (E) *, P < 0.05 Wsh vs. WT.
Human Chymase Induces Intestinal Epithelial Permeability.
Studies in endotoxemic rats showed that mast cell proteases increased colonic permeability and that this effect was dependent on chymase activity (14). We hypothesized that mast cells regulate homeostatic intestinal epithelial permeability through their expression of chymase/Mcpt4. To test this hypothesis, we first assessed the effect of chymase on intestinal epithelial permeability in vitro. Monolayers of the colonic epithelial adenocarcinoma cell line, Caco2bbe, were stimulated basolaterally with recombinant human chymase and the TER and apical-to-basolateral flux of FITC-dextran were measured. Chymase significantly reduced TER in 2 h, with maximal reduction after 8 h (Fig. S1A). The chymase-induced decrease in TER was concentration-dependent and inhibited by chymostatin, a chymotryptic serine protease inhibitor (Fig. S1B), indicating chymase specificity. Consistent with the decrease in TER, FITC-dextran permeability was elevated after chymase stimulation (Fig. S1 C and D). To confirm that chymase-mediated intestinal barrier dysfunction was not due to epithelial damage or detachment, we evaluated expression of the adherens junction (AJ) protein E-cadherin and the intercellular tight junction (TJ) protein ZO-1 by immunofluorescence. Caco2bbe monolayers remained intact with preserved junctional integrity despite up to 24 h of chymase stimulation (Fig. S1E). Thus, human chymase increases intestinal epithelial paracellular permeability without destroying junctional integrity.
Mcpt4-Deficient Mice Have Altered Homeostatic Intestinal Barrier Function.
Following our in vitro observations, we examined the role of mast cell-derived chymase in intestinal epithelial barrier function in vivo by evaluating the TER and permeability of jejunum from mice deficient in Mcpt4, the murine functional homologue of human mast cell chymase. TER was significantly increased in Mcpt4−/− mice compared with WT mice (Fig. 1A), with no significant differences in ion conductance (Fig. 1 B and C). Consistent with increased TER, the luminal-to-serosal flux of FITC-dextran and HRP across ex vivo jejunum segments was significantly decreased in Mcpt4−/− mice (Fig. 1 D and E). Notably, the TER and permeability were comparable in Wsh and Mcpt4−/− mice, suggesting that mast cells regulate homeostatic intestinal permeability primarily through Mcpt4-dependent mechanisms. To confirm the observations were not a consequence of experimental manipulation, we also measured intestinal permeability using the everted gut sac method (15). Consistent with our Ussing chamber measurements, luminal-to-serosal clearance of FITC-dextran across jejunum sacs was significantly decreased in Wsh and Mcpt4−/− mice (Fig. S2). Collectively, these studies identify a role for mast cells and Mcpt4 in the regulation of basal paracellular intestinal permeability.
To assess whether Mcpt4 gene deletion induced intrinsic defects in intestinal mast cells, we compared mast cell numbers, distribution and protease expression in the jejunum to WT mice. Mcpt4−/− and WT mice exhibited no significant difference in intestinal mast cell numbers or distribution (Fig. S3A). Immunofluorescence analysis revealed equivalent levels of Mcpt1+ cells, which localized primarily to the villus/crypt junctions and villi of both Mcpt4−/− and WT mice (Fig. S3B). Mcpt4 expression was confirmed in WT jejunum with positive cells primarily in the villus/crypt junction, while no positive cells were found in Mcpt4−/− mice (Fig. S3B). Additionally, we performed IgE-mediated passive anaphylaxis to confirm mast cell functionality in Mcpt4−/− mice. Administration of anti-IgE antibody (EM95) induced systemic anaphylaxis in both WT and Mcpt4−/− mice as evidenced by similar decreases in rectal temperature (Fig. S3D). Additionally, we detected similar levels of serum Mcpt1 in anti-IgE-treated WT and Mcpt4−/− mice, indicating no global defect in mast cell protease secretion in Mcpt4−/− animals (Fig. S3C).
Mast Cells/Mcpt4 Regulate Intestinal Epithelial Architecture and Migration.
To begin defining the mechanistic basis of mast cell/Mcpt-4-mediated regulation of homeostatic intestinal barrier function, we examined intestinal architecture and epithelial cell turnover in the jejunum. Analysis of H&E stained jejunum displayed architectural differences in the intestine of Wsh and Mcpt-4−/− mice compared with WT (Fig. 2A). Morphometric quantification revealed no difference in jejunum villus length (Fig. 2B), but a significant increase in crypt depth in Wsh and Mcpt-4−/− mice compared with WT (Fig. 2C). To determine whether modification of intestinal epithelial cell architecture was a consequence of altered epithelial apoptosis or proliferation, we examined caspase-3 activation and in vivo BRDU incorporation. Immunohistochemical analysis of jejunum for cleaved caspase-3 revealed no significant differences in epithelial apoptosis between Wsh, Mcpt-4−/− and WT mice (Fig. S4A). Quantification of BRDU+ cells in the crypts, villi and lamina propria showed no significant differences in epithelial proliferation (Fig. S4B). Additionally, we measured epithelial cell migration in the jejunum as the distance from the base of the crypt to the furthest BRDU+ epithelial cell up the villus. Morphometric analysis revealed an approximately 20% reduction in the rate of epithelial cell migration up the villus-crypt axis of jejunum from Wsh and Mcpt-4−/− mice compared with WT (Fig. 2D). Notably, the modification in intestinal architecture (increased crypt depth) and intestinal migration of Mcpt4−/− mice was comparable to Wsh mice, suggesting that mast cell regulation of homeostatic intestinal epithelial migration and barrier function is primarily mediated by Mcpt4. These analyses identify a link between mast cell/Mcpt-4 deficiency and altered jejunal epithelial migration, morphology and barrier function.
Fig. 2.
Altered intestinal architecture and epithelial migration in Wsh and Mcpt4−/− mice. (A) H&E stained jejunum from WT, Wsh, and Mcpt4−/− mice was examined for (B) villus length, measured from villus-crypt junction to villus tip and (C) crypt depth, measured from crypt base to villus-crypt junction. (A) Arrows demonstrate approximate crypt measurements and highlight the difference between mice. (D) Epithelial migration was measured as the distance (μm) from crypt base to the farthest migrated BRDU+ cell. Values represent mean± SEM; n = 5–7 mice per group. Statistical significance is: (C) *, P < 0.01 and ***, P < 0.001 vs. WT; (D) **, P < 0.01 vs. WT.
Previous studies have implicated Mcpt4 in ECM degradation, demonstrating increased collagen deposition in the ear and fibronectin expression in the ear and lung of Mcpt4−/− mice (13). To determine if ECM modification in the jejunum could explain the altered barrier function, we examined intestinal collagen and fibronectin expression. There were no significant differences in collagen in the jejunum of Wsh, Mcpt4−/−, and WT mice by trichrome staining (Fig. S5A) and hydroxyproline quantification (Fig. S5B). Additionally, no difference in intestinal fibronectin expression was found by immunofluorescence (Fig. S5A). Thus, mast cell/Mcpt4 regulation of homeostatic intestinal barrier function is likely to be independent of changes in the collagen and fibronectin ECM.
Mast Cells/Mcpt4 Regulate Claudin-3 Expression and Distribution.
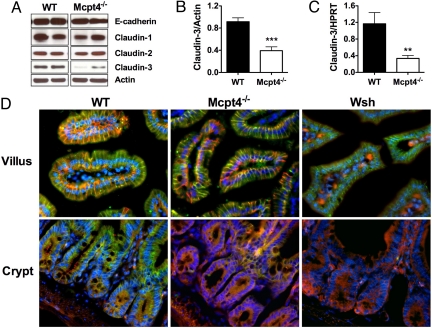
The intestinal epithelium maintains its selective barrier function through the formation of intercellular protein–protein networks involving desmosomes, AJs, and TJs that mechanically link adjacent epithelial cells and seal the intercellular space (2). Dysregulated expression of claudin-3, a TJ protein expressed in the junctional and basolateral membranes of both crypts and villi in rat intestine, can cause abnormal intestinal barrier function and ovarian epithelial cell motility (16). To assess the possible involvement of claudin-3 in mast cell/Mcpt-4-mediated intestinal epithelial migration, we examined claudin-3 expression in the jejunum (Fig. 3). Western blot (Fig. 3 A and B) and quantitative PCR analyses (Fig. 3C) revealed decreased claudin-3 in the jejunum of Mcpt4−/− mice compared with WT. Notably, we did not observe a decrease in other junctional proteins, including E-cadherin, claudin-1, and claudin-2 (Fig. 3A). We next examined claudin-3 distribution by immunofluorescence (Fig. 3D). We show that within the jejunum of WT mice, claudin-3 is predominantly expressed along the lateral membranes of both villus and crypt epithelium and colocalized with E-cadherin (Fig. 3D). In Mcpt4−/− and Wsh mice, claudin-3 villus expression was comparable to that observed in WT mice, predominantly localized along the lateral membranes (Fig. 3D). However, in crypt epithelium, lateral membrane expression of claudin-3 was reduced in both Mcpt4−/− and Wsh mice (Fig. 3D). Notably, the expression of E-cadherin in the crypt epithelium was comparable to WT mice.
Fig. 3.
Decreased claudin-3 expression in jejunal crypt epithelium of Mcpt4−/− and Wsh mice. (A) E-cadherin, claudin-1, -2, and -3 and actin were evaluated by Western blot. (B) Densitometric analysis of claudin-3 expression normalized to β-actin. (C) Quantitative real-time PCR analysis of claudin-3 normalized to HPRT. (D) Claudin-3 (green) and E-cadherin (red) immunofluorescence of the villi (Top) and crypts (Bottom) of jejunum from WT (Left), Mcpt4−/− (Middle), and Wsh (Right) mice; nuclei stained by DAPI (blue). Western representative of three individual experiments. Densitometry represents mean ± SEM; n = 10 mice. PCR represents n = 6–8 mice per group. Statistical significance is: **, P < 0.01, ***, P < 0.001.
Engraftment of Wsh Mice with WT but Not Mcpt4−/− Bone Marrow-Derived Mast Cells Restores Homeostatic Intestinal Barrier Function.
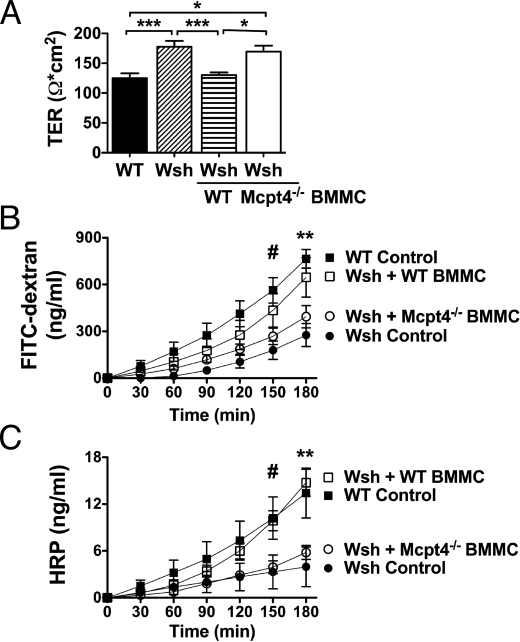
To delineate whether Mcpt4+ mast cells are required for homeostatic barrier function, we engrafted Wsh mice by i.p. injection of 5 × 106 (>95% c-kit+FcεRI+) WT or Mcpt4−/− BMMCs. Mast cell engraftment of the jejunum was confirmed 12 weeks after transfer by chloroacetate esterase staining. Notably, analysis of intestinal mast cell numbers and distribution in Wsh mice after engraftment with either WT or Mcpt4−/− BMMCs revealed that intestinal mast cell numbers were only approximately 50% of WT levels, and the mast cells were predominantly localized to the submucosa. Nevertheless, engraftment of Wsh mice with WT BMMCs significantly decreased TER (Fig. 4A) and significantly increased intestinal permeability to levels equivalent to WT mice (Fig. 4 B and C). In contrast, engraftment with Mcpt4−/− BMMCs failed to restore homeostatic intestinal barrier function and the TER and flux of FITC-dextran and HRP remained equivalent to those of non-engrafted Wsh mice (Fig. 4).
Fig. 4.
Mast cell engraftment of Wsh mice with WT, but not Mcpt4−/− BMMCs restores basal intestinal permeability. Wsh mice were engrafted with BMMCs from WT or Mcpt4−/− mice. Twelve weeks later, jejunum was examined ex vivo for (A) TER and luminal-to-serosal flux of (B) FITC-dextran and (C) HRP. Values represent mean± SEM; n = 4–8 mice per group. Representative of three independent experiments. Statistical significance by two-way ANOVA is: (A) *, P < 0.05, ***, P < 0.001; (B) #, P < 0.01 WT control vs. Wsh control; **, P < 0.001 WT control vs. Wsh control, P < 0.05 WT control vs. Mcpt4−/− BMMC and P < 0.01 Wsh control vs. WT BMMC; (C) #, P < 0.05 WT control vs. Wsh control, WT control vs. KO BMMC, WT BMMC vs. Wsh control and WT BMMC vs. KO BMMC; **, P < 0.001 WT control vs. Wsh control, WT BMMC vs. Wsh control and WT BMMC vs. KO BMMC, and P < 0.01 WT control vs. KO BMMC.
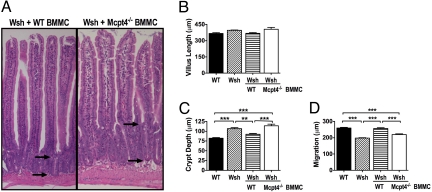
The restoration of intestinal barrier function was associated with restored epithelial architecture and migration. Remarkably, intestinal crypt depth and epithelial migration in Wsh mice were restored to WT levels after engraftment with WT but not Mcpt4−/− BMMCs (Fig. 5). These results identify a role for mast cells in the regulation of homeostatic intestinal epithelial migration. Furthermore, these studies reveal a link between mast cell/chymase-mediated epithelial migration and the regulation of homeostatic intestinal barrier function.
Fig. 5.
Mast cell engraftment of Wsh mice with WT, but not Mcpt4−/− BMMCs restores intestinal architecture and epithelial migration. Wsh mice were engrafted with BMMCs from WT or Mcpt4−/− mice and examined after 12 weeks. H&E-stained jejunum from (A) Wsh mice engrafted with either WT or Mcpt4−/− BMMCs were examined for (B) villus length, measured from villus-crypt junction to villus tip and (C) crypt depth, measured from crypt base to villus-crypt junction. Arrows demonstrate approximate location of crypt measurements and highlight the difference between mice. (D) Mice were injected with 0.2 mg/g body weight BRDU and after 24 h, epithelial migration was measured as the distance from the crypt base to the farthest migrated BRDU+ cell. Values represent mean± SEM; n = 4–8 mice per group. Statistical significance is: **, P < 0.01, ***, P < 0.001.
Discussion
We identify a role for mast cells and the murine functional homologue to human chymase, Mcpt4, in homeostatic regulation of the intestinal barrier. We demonstrate that mast cell-deficient Wsh mice have altered intestinal barrier structure and function, with decreased intestinal epithelial migration and permeability, and changes in intestinal architecture. We also show that Mcpt4 regulates intestinal barrier function and that Mcpt4−/− mice have intestinal barrier alterations similar to Wsh mice. Mechanistically, we demonstrate that Mcpt-4 regulation of intestinal epithelial function is linked with decreased claudin-3 expression in the crypts. Furthermore, we show that engraftment of Wsh mice with WT but not Mcpt4−/− BMMCs reconstitutes intestinal epithelial architecture, migration and barrier function. Collectively, these studies reveal a fundamental function for mast cells and chymase in the homeostatic regulation of intestinal epithelial anatomy and function.
Previous studies have examined the role of mast cells in intestinal barrier function during oral antigen- or helminth-induced intestinal inflammation (17–20). Repeated oral administration of ovalbumin to ovalbumin-immunized mice induced an IgE/FcεRI/mast cell-dependent increase in intestinal epithelial permeability and secretory diarrhea (18, 19). Furthermore, T. spiralis infection induced intestinal mastocytosis and intestinal permeability through a protease-dependent process (17). Genetic deletion of the mast cell protease Mcpt1 prevented the helminth-induced decrease in intestinal barrier function and inhibited T. spiralis expulsion (17). In contrast to the important role of Mcpt1 during an inflammatory response, uninfected Mcpt1−/− mice had normal intestinal Mcpt4 expression and intestinal barrier function (17). These findings suggest that Mcpt1 contributes to helminth-induced changes in intestinal barrier function, but not to intestinal barrier homeostasis. Conversely, we have found that Mcpt4 does not contribute to T. spiralis expulsion from the intestine or to parasite- or oral antigen-induced intestinal permeability, but is critical for the homeostatic regulation of intestinal barrier function.
The contribution of Mcpt4 to homeostatic barrier function, but not inflammation-induced changes in barrier function, likely reflects differences in intestinal mast cell subpopulations during homeostasis versus inflammation. Mast cells can be broadly categorized into mucosal and connective tissue mast cells (for review see ref. 21). Mucosal mast cells predominantly localize to the mucosal epithelium and lamina propria and express the chymases Mcpt1 and Mcpt2; whereas, the connective tissue subtype localizes primarily within the submucosa and expresses the chymases Mcpt4 and Mcpt5 and the tryptases, Mcpt6 and Mcpt7 and CPA3. Under homeostatic conditions the jejunum contains low numbers of mast cells (1–2 per mm2) with an approximate mucosal to connective tissue mast cell ratio of 1:1 (22). In contrast, during inflammatory conditions mast cell numbers increase ≈20–25-fold and are characterized by a 5:1 ratio of mucosal to connective tissue mast cells (22). Consistent with these observations, significantly increased Mcpt1 levels are observed in mice after T. spiralis infection or oral ovalbumin challenge (17).
Mast cells are classically associated with helminth- or allergen-induced IgE-mediated responses (23). Antigen-cross-linking of IgE/FcεRI complex on mast cells evokes mast cell degranulation and release of preformed mediators within seconds. In contrast, our data suggest that constant or intermittent release of mast cell-derived chymase regulates paracellular permeability, epithelial migration and intestinal barrier function. To determine if murine mast cells constitutively release Mcpt-4, we examined chymase release by cultures of IL-3- and SCF-generated BMMCs that received no additional stimulation and detected 0.71 ± 0.1 ng chymase/106 BMMC in culture supernatants (n = 3 experiments; mean ± SD). Increased chymase release could be induced after 3 h of LPS (10 ng/mL) stimulation [1.93 ± 0.7 ng chymase/106 BMMC (n = 3 experiments; mean ± SD)]. Consistent with these observations, murine β-chymase (Mcpt-1) and rat β-chymase (rMCP-II) are constitutively secreted by jejunal mast cells in uninfected normal rodents (24, 25) and piecemeal (nonanaphylactic) intestinal mast cell degranulation has been shown in nonlesional human intestine (26).
Importantly, ultrastructural and immunohistochemical characterization of normal mast cells allowed the identification of mast cell subsets, including chymase+/tryptase+, chymase−/tryptase+, and chymase+/tryptase− cells (27). Notably, the bowel submucosa contained the largest numbers of chymase+/tryptase− mast cells (27). Defining the stimuli and molecular mechanisms associated with constitutive intestinal mast cell release of granule proteins, in particular chymase, will be important to understanding regulation of homeostatic intestinal barrier function.
We demonstrate that altered intestinal barrier function in mast cell- and Mcpt4−/− mice is linked to altered intestinal architecture. Remarkably, we were able to transfer the WT phenotype by engraftment of Wsh mice with WT BMMCs, but not with Mcpt4−/− BMMCs. These studies link modified intestinal architecture with mast cell-derived chymase activity. Furthermore, we found that the altered architecture was not associated with changes in intestinal epithelial proliferation or apoptosis. Although the molecular mechanisms regulating intestinal architecture and epithelial migration along the villus-crypt axis are not yet elucidated, we demonstrate a link between altered intestinal epithelial architecture, decreased epithelial migration and claudin-3 crypt expression. Claudin-3 has also been implicated in both ovarian epithelial cell invasion and motility and barrier function (16). Overexpression of claudins-3 and -4 in immortalized normal human ovarian surface epithelial cells, which lack endogenous expression of these proteins, increased epithelial cell survival and enhanced invasion and motility (16). Conversely, siRNA-mediated knockdown of claudin-3 reduced invasion (16). Notably, dysregulated claudin-3 expression and function in ovarian epithelial cells was associated with matrix metalloproteinase-2 (MMP-2) activity (16). Intriguingly, MMP-2 activation is diminished in Mcpt4−/− mice (13). The involvement of MMP in Mcpt-4-mediated intestinal epithelial barrier function is yet to be determined.
In contrast to other species, including humans, where mast cells comprise 2–3% of total lamina propria cells, mast cells in the murine small intestine are infrequent, with approximately five mast cells per villus localized within the lamina propria (18, 21, 28). Despite this, mast cell-derived chymase impacts intestinal TJ protein expression, epithelial migration and barrier function. Intestinal epithelial barrier function is also regulated by epithelial cells (mucus production and secretion), innate and adaptive immunity and the enteric nervous system (ENS); dysregulation of any of these alters the barrier (29). Consequently, mast cell chymase may affect barrier function through effects on any of these systems. Chymase directly alters corneal epithelial barrier function through proteolytic cleavage of fibronectin and the TJ protein occludin (30). Alternatively, mast cell/chymase may regulate epithelial barrier function indirectly via interactions with the enteric nervous system (ENS). Intestinal mast cells are in close proximity to neuronal components of the ENS and functional studies demonstrate cross-talk between mast cells and the ENS (29). Enteric neurons express G protein-coupled protease-activated receptors (PAR), including PAR-1, PAR-2, and PAR-4 (31) and chymase cleavage of at least one PAR can increase permeability (32). Other mast cell-derived mediators, including histamine, serotonin, arachidonic acid metabolites and mast cell proteases, also communicate with the ENS and regulate intestinal epithelial function, including ion channel conductance and activation/inhibition of secretion of electrolytes and mucus (23, 29). It is likely that chymase regulates homeostatic epithelial barrier function in concert with these other mast cell-produced mediators.
By inducing a limited increase in basal intestinal permeability and promoting cellular migration within the villus, mast cell chymase may optimize intestinal absorption of nutrients and antigens and facilitate cellular interactions important for both digestive and immune system function. In contrast, pathological decreases in intestinal barrier function are postulated to be a primary etiologic factor in the development of several diseases including IBD, food allergy, celiac disease, and type I diabetes. Increased intestinal permeability is found in 10–25% of healthy first-degree relatives of IBD, celiac disease or Type I diabetes patients, in addition to the patients themselves, indicating that increased intestinal permeability likely precedes the onset of clinical disease (3–6). Cow's milk allergic patients also have increased intestinal permeability at baseline (33), and a link between increased intestinal permeability and the development of new-onset food allergies has been identified in patients after organ transplantation (34). Notably, these changes in IBD patients have been associated with modified levels of intestinal chymase-positive mast cells (35).
Taken together, these observations and considerations demonstrate a role for mast cell-derived proteases in the maintenance of homeostatic intestinal barrier function, epithelial cell migration, and architecture. Delineation of the mast cell/chymase/intestinal epithelial cell axis in intestinal barrier function is required to understand how intestinal barrier function is regulated in the normal animal and how development of intestinal barrier dysfunction predisposes to inflammatory intestinal diseases.
Materials and Methods
Mice.
C57BL/6 and mast cell-deficient Wsh mice (Jackson Laboratories) and murine mast cell protease-4-deficient (Mcpt4−/−) mice on a C57BL/6 background were bred and maintained under specific pathogen-free conditions in our facility in accordance with CCHMC Institutional Animal Care and Use Guidelines.
Ex Vivo Intestinal Permeability.
Jejunum was mounted in Ussing chambers and after 15 min stabilization, baseline short-circuit current (Isc) and transepithelial resistance (TER) were measured. FITC-dextran (2.2 mb/mL, 4.4 kDa; Sigma) and 1 mg/mL HRP (40 kDa; Sigma) were added to the luminal bath and permeability measured as described in ref. 18. In additional experiments, β-methylcholine was added in increasing concentrations (10−7 to 10−2 M) to the serosal bath and Isc monitored.
In Vitro Permeability.
Detailed methods provided in SI Methods.
BMMC Engraftment of Wsh Mice.
Bone marrow harvested from femurs of WT and Mcpt4−/− mice was cultured in RPMI supplemented as described for DMEM above with the addition of 10 μg/mL gentamicin, 50 μM 2-mercaptoethanol, and 20 ng/mL murine IL-3 (Peprotech). Cells were passaged weekly for 4 weeks. Cells were >95% mast cells as assessed by toludine blue stain and flow cytometry for c-kit+/FcεRI+ expression. BMMCs (5 × 106) or 200 μL DMEM alone were injected i.p. into 4–6-week-old Wsh mice. Recipients were studied after 12 weeks for intestinal mast cell engraftment, permeability and morphometrics.
Histopathological Examination and Morphometric Analysis.
Jejunum 8–18 cm distal of the pyloric sphincter was fixed in 10% formalin and stained for mast cell chloroacetate esterase activity as described in ref. 18. Masson trichrome staining for collagen was performed using standard histological techniques. Additional sections were processed for routine H&E. Villous height and crypt depth were measured in at least 25 well-oriented, villus-crypt units per mouse using ImageProPlus. Crypt depth was measured from the base of the crypt to the villus-crypt junction in crypts with open lumens and a continuous cell column on each side. Villus height was measured from the villus-crypt junction to the villus tip, in villi with a single layer of epithelial cells cut through the nuclei all of the way around the villus.
Epithelial Cell Proliferation, Migration, and Apoptosis.
Detailed methods provided in SI Methods.
Immunofluorescence.
Detailed methods for jejunum immunofluoresence analysis is provided in SI Methods. Sections were incubated with the following antibodies: rabbit anti-fibronectin, rabbit anti-claudin-3, rat anti-E-cadherin, rabbit anti-Mcpt-1 or -4, or isotype control and positive signal detected by AlexaFluor 488-conjugated goat anti-rabbit or AlexaFluor 594 goat anti-rat secondary antibodies.
Caco2 Cell Immunofluorescence.
Detailed methods provided in SI Methods.
Protein and RNA Analysis.
Detailed methods for jejunum Western blot analysis are provided in SI Methods. Protein samples were incubated with the following: rabbit antibodies against actin, claudins-1, -2, and -3 and E-cadherin, and detected with peroxidase-conjugated goat anti-rabbit Ig secondary antibody and by chemiluminescence. Densitometric analysis was performed using Image J (National Institutes of Health). RNA analysis by quantitative real-time PCR was performed as described in ref. 18.
Everted Gut Sac Method.
Detailed methods provided in SI Methods.
IgE-Mediated Passive Anaphylaxis.
Detailed methods provided in SI Methods.
Hydroxyproline Assay.
Detailed methods provided in SI Methods.
Statistical Analysis.
Data are presented as mean ± SEM. Data were analyzed using either 2-tailed unpaired Student's t test, or one- or two-way ANOVA with Bonferroni post-hoc test as appropriate (GraphPad Prism). Statistical significance considered when P < 0.05.
Supplementary Material
Acknowledgments.
We thank Drs. Marc Rothenberg, Marsha Wills-Karp, Nives Zimmermann, Rick Strait, and Ariel Munitz for helpful discussions and critical review of the manuscript. This work was supported by National Institutes of Health Grants R01-AI 073553 (to S.P.H.), F30-DK082113 (to K.R.G.), and T32-GM063483 (to K.R.G.) and a Crohn's Colitis Foundation of America Career Development Award, an American Heart Association Grant-in-Aid award, and by the Food Allergy and Anaphylaxis Network (S.P.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906372106/DCSupplemental.
References
- 1.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters M, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 4.Soderholm JD, et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: An inherited defect in mucosal defence? Gut. 1999;44:96–100. doi: 10.1136/gut.44.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with celiac disease and relatives of patients with celiac disease. Gut. 1993;34:354–357. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sapone A, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt J, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 8.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 9.Gallwitz M, Hellman L. Rapid lineage-specific diversification of the mast cell chymase locus during mammalian evolution. Immunogenetics. 2006;58:641–654. doi: 10.1007/s00251-006-0123-4. [DOI] [PubMed] [Google Scholar]
- 10.Kunori Y, et al. Rodent alpha-chymases are elastase-like proteases. Eur J Biochem. 2002;269:5921–5930. doi: 10.1046/j.1432-1033.2002.03316.x. [DOI] [PubMed] [Google Scholar]
- 11.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson MK, Karlson U, Hellman L. The extended cleavage specificity of the rodent beta-chymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol Immunol. 2008;45:766–775. doi: 10.1016/j.molimm.2007.06.360. [DOI] [PubMed] [Google Scholar]
- 13.Tchougounova E, et al. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 14.Moriez R, et al. Mucosal mast cell proteases are involved in colonic permeability alterations and subsequent bacterial translocation in endotoxemic rats. Shock. 2007;28:118–124. doi: 10.1097/SHK.0b013e3180315ba9. [DOI] [PubMed] [Google Scholar]
- 15.Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 1999;12:127–133. doi: 10.1097/00024382-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, D'Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378–7385. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- 17.McDermott JR, et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight PA, et al. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunol. 2002;105:375–390. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friend DS, et al. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff S. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009 Jun 17; doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 24.Scudamore CL, et al. Basal secretion and anaphylactic release of rat mast cell protease-II (RMCP-II) from ex vivo perfused rat jejunum: Translocation of RMCP-II into the gut lumen and its relation to mucosal histology. Gut. 1995;37:235–241. doi: 10.1136/gut.37.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wastling JM, et al. Constitutive expression of mouse mast cell protease-1 in normal BALB/c mice and its up-regulation during intestinal nematode infection. Immunol. 1997;90:308–313. doi: 10.1046/j.1365-2567.1997.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorak AM, Furitsu T, Kissell-Rainville S, Ishizaka T. Ultrastructural identification of human mast cells resembling skin mast cells stimulated to develop in long-term human cord blood mononuclear cells cultured with 3T3 murine skin fibroblasts. J Leukoc Biol. 1992;51:557–569. doi: 10.1002/jlb.51.6.557. [DOI] [PubMed] [Google Scholar]
- 27.Weidner N, Austen KF. Ultrastructural and Immunohistochemical characterization of normal mast cells at multiple body sites. J Invest Dermatol. 1991;96:26S–31S. [PubMed] [Google Scholar]
- 28.Miller HRP, et al. Granule proteases deinfe mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988;65:559–566. [PMC free article] [PubMed] [Google Scholar]
- 29.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastoenterol. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Ebihara N, et al. Mast cell chymase decreases the barrier function and inhibits the migration of corneal epithelial cells. Curr Eye Res. 2005;30:1061–1069. doi: 10.1080/02713680500346625. [DOI] [PubMed] [Google Scholar]
- 31.Gao C, et al. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterol. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, et al. Chymase increases glomerular albumin permeability via protease-activated receptor-2. Mol Cell Biochem. 2007;297:161–169. doi: 10.1007/s11010-006-9342-0. [DOI] [PubMed] [Google Scholar]
- 33.Ventura MT, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38:732–736. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Ozdemir O, Arrey-Mensah A, Sorensen RU. Development of multiple food allergies in children taking tacrolimus after heart and liver transplantation. Pediatr Transplant. 2006;10:380–383. doi: 10.1111/j.1399-3046.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 35.Andoh A, et al. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol Rep. 2006;16:103–107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.