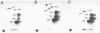Abstract
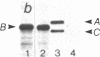
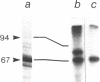
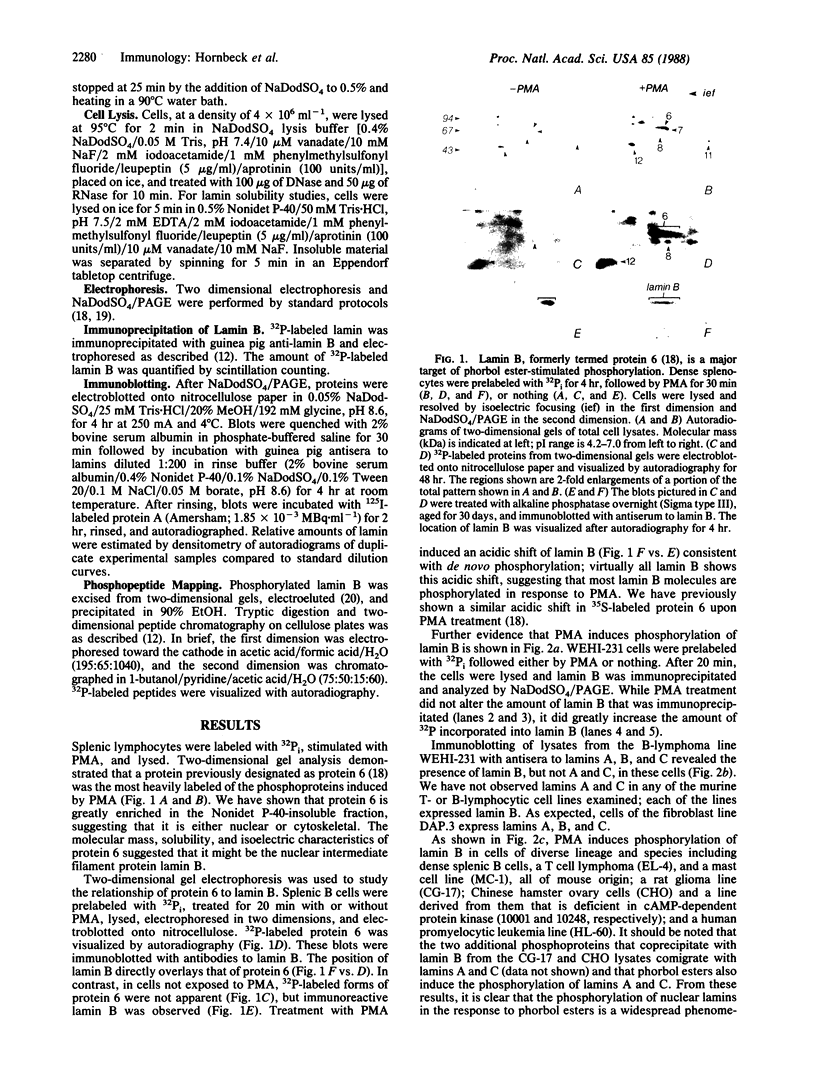
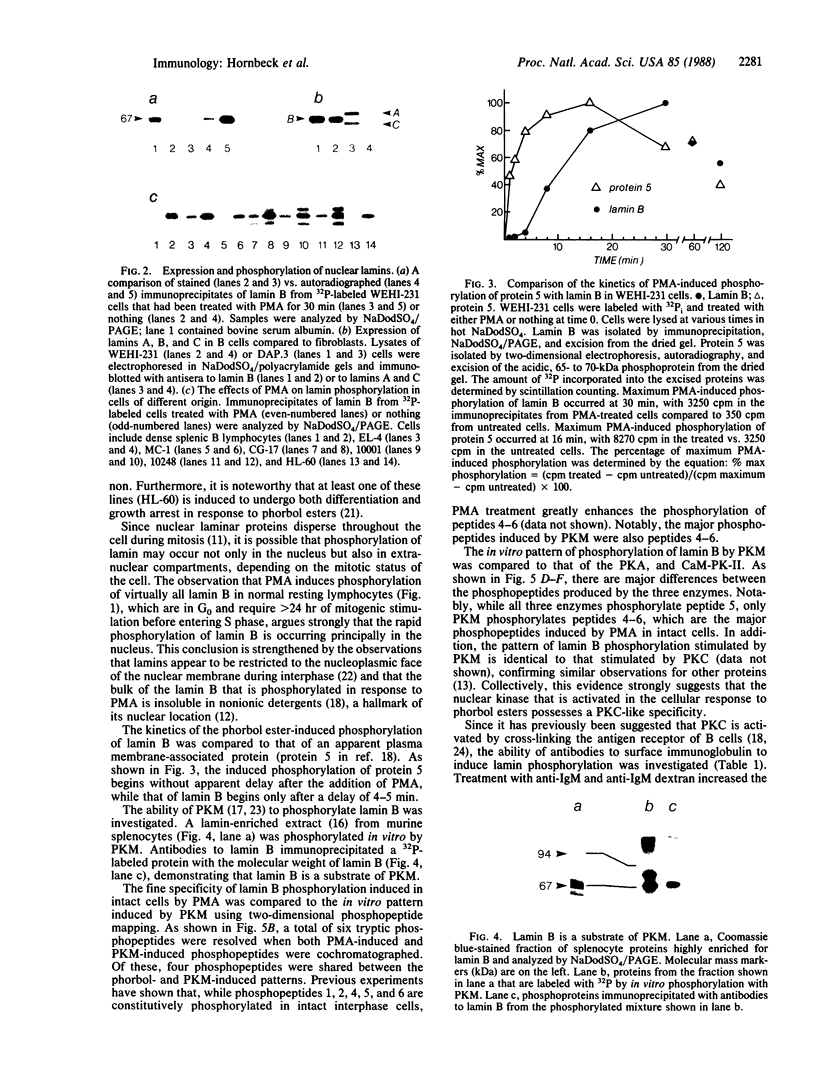
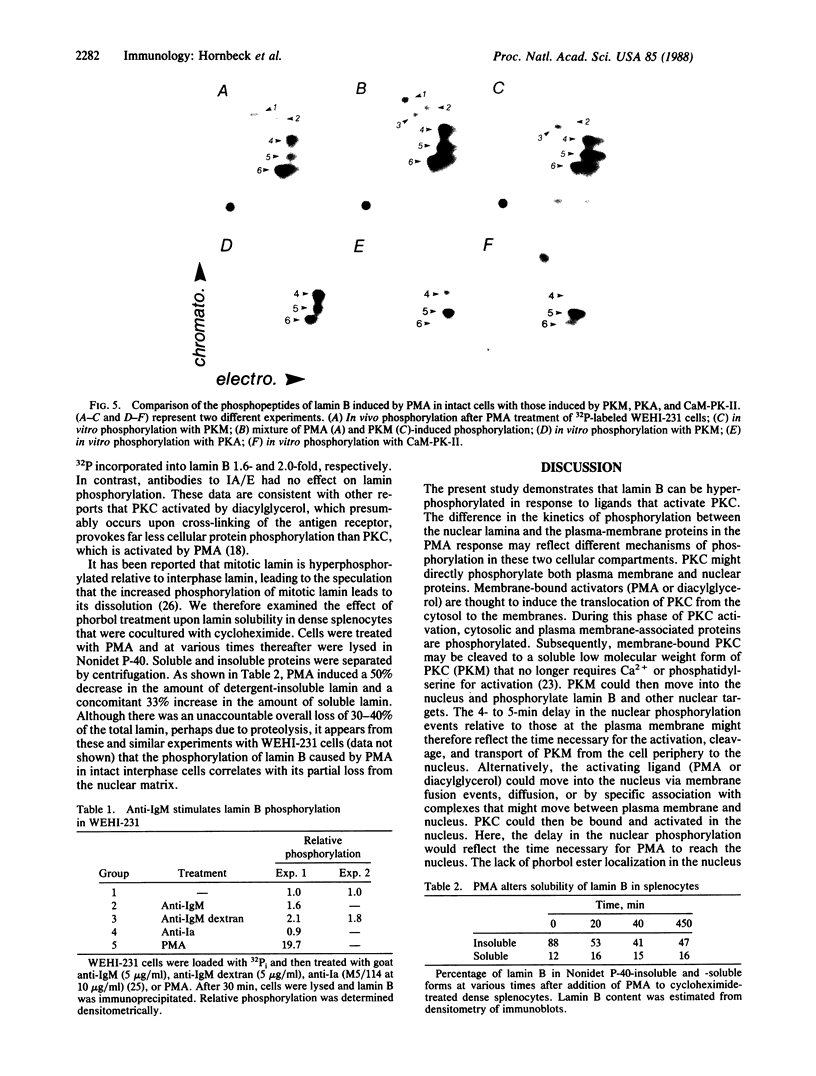
Lamin B was shown to be a major substrate of cellular phosphorylation in the response of lymphocytes to phorbol esters. Lamins A and C, which were not observed in lymphocytes, were also substrates of phorbol-stimulated phosphorylation in those cell types that express them. Lamin B phosphopeptides labeled with 32P in intact cells treated with phorbol 12-myristate 13-acetate were compared to those produced by in vitro phosphorylation with protein kinase M, cAMP-dependent protein kinase, and Ca2+/calmodulin-dependent protein kinase II. The phosphopeptides labeled by in vivo stimulation with phorbol esters are very similar to those phosphorylated in vitro by protein kinase M, a catalytic domain of protein kinase C. Phorbol treatment of interphase cells significantly reduces the amount of detergent-insoluble lamin B, suggesting that phosphorylation of lamin may alter the architecture of the nuclear lamina. In addition, we have shown that treatment of a B-cell line with antibodies to IgM induces a modest increase in lamin B phosphorylation. These results strongly suggest that ligands that are known to activate protein kinase C at the cell surface or in the cytosol also lead to the activation of a nuclear kinase activity with a protein kinase C-type specificity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahm J., Rovera G. The effect of tumor-promoting phorbol diesters on terminal differentiation of cells in culture. Mol Cell Biochem. 1980 Aug 16;31(3):165–175. doi: 10.1007/BF00225849. [DOI] [PubMed] [Google Scholar]
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Hornbeck P., Paul W. E. Anti-immunoglobulin and phorbol ester induce phosphorylation of proteins associated with the plasma membrane and cytoskeleton in murine B lymphocytes. J Biol Chem. 1986 Nov 5;261(31):14817–14824. [PubMed] [Google Scholar]
- Huang K. P., Huang F. L. Conversion of protein kinase C from a Ca2+-dependent to an independent form of phorbol ester-binding protein by digestion with trypsin. Biochem Biophys Res Commun. 1986 Aug 29;139(1):320–326. doi: 10.1016/s0006-291x(86)80116-4. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Phorbol ester induces the transcriptional stimulatory activity of the SV40 enhancer. Nature. 1986 Oct 9;323(6088):555–558. doi: 10.1038/323555a0. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Ganong B. R., Vandenbark G. R., Shirley J. E., Bell R. M. Role of protein kinase C in diacylglycerol-mediated induction of ornithine decarboxylase and reduction of epidermal growth factor binding. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1941–1945. doi: 10.1073/pnas.82.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Krauss S. W., Mochly-Rosen D., Koshland D. E., Jr, Linn S. Exposure of HeLa DNA polymerase alpha to protein kinase C affects its catalytic properties. J Biol Chem. 1987 Mar 15;262(8):3432–3435. [PubMed] [Google Scholar]
- Liskamp R. M., Brothman A. R., Arcoleo J. P., Miller O. J., Weinstein I. B. Cellular uptake and localization of fluorescent derivatives of phorbol ester tumor promoters. Biochem Biophys Res Commun. 1985 Sep 16;131(2):920–927. doi: 10.1016/0006-291x(85)91327-0. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Niedel J. E., Cambier J. C. B cell activation. IV. Induction of cell membrane depolarization and hyper-I-A expression by phorbol diesters suggests a role for protein kinase C in murine B lymphocyte activation. J Immunol. 1984 Mar;132(3):1472–1478. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Rabin M. S., Doherty P. J., Gottesman M. M. The tumor promoter phorbol 12-myristate 13-acetate induces a program of altered gene expression similar to that induced by platelet-derived growth factor and transforming oncogenes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):357–360. doi: 10.1073/pnas.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., Wolf M., Besterman J., Hsieh T., Sander M., LeVine H., 3rd, Chang K. J., Cuatrecasas P. Protein kinase C phosphorylates topoisomerase II: topoisomerase activation and its possible role in phorbol ester-induced differentiation of HL-60 cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1603–1607. doi: 10.1073/pnas.83.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Evidence that treatment of platelets with phorbol ester causes proteolytic activation of Ca2+-activated, phospholipid-dependent protein kinase. Eur J Biochem. 1985 Sep 2;151(2):419–423. doi: 10.1111/j.1432-1033.1985.tb09118.x. [DOI] [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]