Abstract
Von Recklinghausen neurofibromatosis is a common autosomal dominant genetic disorder characterized by benign and malignant tumors of neural crest origin. Significant progress in understanding the pathophysiology of this disease has occurred in recent years, largely aided by the development of relevant animal models. Von Recklinghausen neurofibromatosis is caused by mutations in the NF1 gene, which encodes neurofibromin, a large protein that modulates the activity of Ras. Here, we describe the identification and characterization of zebrafish nf1a and nf1b, orthologues of NF1, and show neural crest and cardiovascular defects resulting from morpholino knockdown, including vascular and cardiac valvular abnormalities. Development of a zebrafish model of von Recklinghausen neurofibromatosis will allow for structure-function analysis and genetic screens in this tractable vertebrate system.
Keywords: cardiovascular, neurofibromin
Von Recklinghausen neurofibromatosis (NF1) is caused by mutations in the NF1 gene that result in a wide variety of symptoms expressed with variable penetrance among affected individuals (1). NF1 patients inherit a single mutated copy of NF1 and may acquire additional somatic mutations in the wild-type allele that contribute to disease progression (2). Cutaneous neurofibromas, benign Schwann-cell tumors derived from the neural crest, are a pathogneumonic lesion of this disease and often appear in large numbers. More serious oncogenic lesions, including neurofibrosarcomas and malignant peripheral nerve sheath tumors, may also develop. Additional neural crest-related pathologies include pheochromocytomas and hyperpigmented lesions arising from melanocyte abnormalities known as café-au-lait spots. Non-neural-crest-related abnormalities are also common and include cognitive and learning deficits, skeletal abnormalities, and leukemia. Affected individuals also display an increased incidence of cardiovascular pathologies, including hypertension, renal artery stenosis, and congenital heart disease (3).
Neurofibromin, the product of the NF1 gene, is a protein of >2,800 aa. It contains a small region homologous to yeast IRA proteins that includes a Ras-GTPase-activating domain (GAP) capable of accelerating the hydrolysis of GTP-bound Ras, thus down-regulating the activity of Ras proto-oncogenes (4). Though additional extensive regions of neurofibromin have been conserved across evolution, other functions of this protein are largely unknown. Evidence from analysis of the Drosophila NF1 orthologue (5, 6) and mouse models of NF1 (7, 8) implicate a role for neurofibromin in modulating cAMP-dependent protein kinase A signaling. Unrelated work suggests that neurofibromin can associate with microtubules (9) as well as other molecules able to induce cytoskeletal changes (10).
The past 15 years have seen the emergence of increasingly sophisticated mouse models of NF1 (11–23). Genetic inactivation of murine Nf1 results in midgestation embryonic lethality with deficient embryos displaying congenital heart disease involving the cardiac outflow tract and endocardial cushions. Heterozygous Nf1-deficient mice are viable and fertile, exhibit learning defects (24, 25), and develop malignancies, albeit at a low rate over a period of several years. Genetic inactivation of the p53 tumor suppressor gene augments the disease phenotype in mouse models of NF1 and leads to a dramatic increase in the rate of malignancy (14, 15, 26, 27).
Tissue-specific Nf1 gene inactivation using Cre-lox technology has led to the development of mouse models that more accurately reproduce aspects of the human disease. For example, Schwann cell-specific inactivation of Nf1 results in the development of malignant peripheral nerve sheath tumors in all animals (19, 28). Nf1 inactivation in the developing neural crest using Pax3- or Wnt1-Cre results uniformly in early neonatal lethality, with affected animals exhibiting massive overgrowths of peripheral nervous tissue, including the dorsal root and sympathetic ganglia (11, 20). Related investigations have implicated a role for the heterozygous tumor microenvironment as a major contributor to the development of disease (19, 28). Tissue-specific inactivation studies have also identified a critical role for neurofibromin in endothelial cells as Tie2-Cre-mediated Nf1 inactivation reproduces the cardiac abnormalities associated with the Nf1−/− phenotype (11, 20). Nf1 inactivation in blood leads to the development of juvenile myelomonocytic leukemia (JMML), the specific form of leukemia found in NF1 patients (29–31). Nf1 function in mast cells and neurofibromin-mediated modulation of Ras/Erk signaling downstream of c-Kit have also been implicated in tumor progression (32).
Though mouse and Drosophila models of NF1 have proven to be informative, additional information will be obtained from alternative model systems. In particular, vertebrate models that allow for high-throughput in vivo screens and rapid, cost-effective phenotypic analysis may facilitate discovery of novel functions and therapeutic approaches. Hence, we sought to develop a zebrafish model of NF1 and to use this model system to elucidate novel developmental functions of neurofibromin. Here, we describe two closely related zebrafish orthologues: nf1a and nf1b. Transient knockdown of either gene, or both together, during embryogenesis results in developmental defects of cardiac and neural crest structures that closely resemble murine models and aspects of the human disease. In addition, we identify a previously unrecognized vascular defect apparent in zebrafish and mice.
Results
Identification of nf1a and nf1b.
We used a bioinformatics approach to identify the zebrafish orthologues of human NF1. Analysis of the eighth assembly (Zv8) of the zebrafish genome revealed two genes highly similar to NF1 at the amino acid level (90.4% and 90.7%, respectively), which we named nf1a and nf1b. These genes are highly related to one another (87.4% identical and 93.7% similar), with nf1a and nf1b sharing similar genomic structures and each containing 57 exons (Fig. 1 A and B). nf1a is located on chromosome 15 (Fig. 1A) and predicts a 311-kDa protein composed of 2,755 aa, whereas nf1b is located on chromosome 10 (Fig. 1B) and predicts a 310-kDa protein composed of 2,747 aa.
Fig. 1.
Zebrafish have two orthologues of human NF1. (A and B) Genomic and mRNA structures of the two orthologous zebrafish genes corresponding to human NF1. (C) Phylogenetic tree comparison of zebrafish, human, mouse, and Drosophila neurofibromin. (D) Analysis of syntenic relationships between human chromosome 17 (NF1) and zebrafish chromosomes 15 (nf1a) and 10 (nf1b). Relative genomic positions are to scale as indicated.
Comparison to Drosophila, murine, and human neurofibromin protein sequences reveals significant conservation in the GAP and IRA homology domains and also in extensive areas flanking these regions, suggesting additional functional motifs that have been conserved across evolution (Fig. S1). A phylogenetic tree (Fig. 1C) shows a tight clustering of the zebrafish neurofibromin orthologues with other mammalian neurofibromins and a divergence from the Drosophila neurofibromin orthologue. Human/zebrafish synteny maps and bioinformatics analyses suggest that nf1a and nf1b likely arose via gene duplication (Fig. 1D). Upstream of the human NF1 gene on chromosome 17 are genes encoding WD repeat and SOCS box-containing 1 (WSB1), kinase suppressor of Ras 1 (KSR1), and Galectin-9 (LGALS9), whereas A kinase anchor protein 1 (AKAP1) and RNA-binding protein Musashi homolog 2 (MSI2) both lie downstream of NF1. Similar genes flank nf1a, whereas nf1b is flanked only by orthologues of KSR1 and MSI2. The identification of duplicated genes is common in zebrafish and reflects the well-described chromosomal doubling event occurring early in teleost evolution (33).
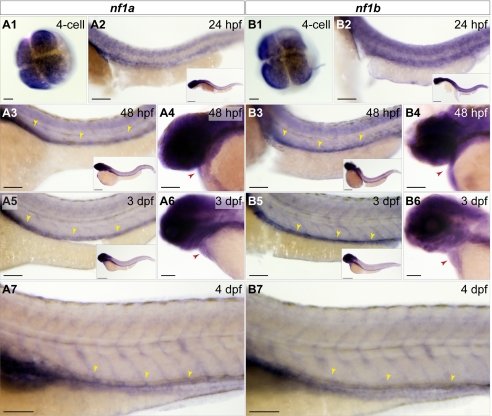
The GenBank EST database identified expressed sequence tags for both zebrafish nf1 genes in many tissues, including the heart (Fig. S2A and Tables S1 and S2), suggesting that neither gene is likely to be a pseudogene and that they are expressed in overlapping tissues. We examined the expression of both genes by whole-mount in situ hybridization between the four-cell stage and 4 days postfertilization (dpf) and found that both genes are expressed ubiquitously during early development with later restriction to regions of the head and anterior central nervous system (Fig. 2A1–A7 and B1–B7, and Fig. S2 C1–C7 and D1–D7). Notably, at 48 h postfertilization (hpf) and 3 dpf, both genes are expressed in the heart (Fig. 2 A4, B4, A6, and B6, and Fig. S2 C5, C6, D5, and D6) and in the dorsal vessel (Fig. 2 A3, B3, A5, and B5, and Fig. S2 C7 and D7). RT-PCR using RNA from wild-type 24-, 72-, and 84-hpf whole embryos or 3-dpf Tg(kdrl:GRCFP)zn1 GFP-positive sorted cells confirmed expression, particularly in the vascular endothelium (Fig. S2 B–G), whereas RNA from one-cell embryos indicate that both genes are expressed maternally (Fig. S2B). Queries of an expression database generated from sorted endothelial cells from Tg(fli1:egfp)y1 zebrafish identifies nf1a and nf1b in both GFP+ and GFP− cell populations, consistent with the expression of these genes in vascular endothelium (34).
Fig. 2.
nf1a and nf1b are expressed maternally and in the developing zebrafish cardiovascular system. Whole-mount in situ hybridization for nf1a and nf1b at the four-cell stage, 24 hpf, 48 hpf, 3 dpf, and 4 dpf. (A1 and B1) At the four-cell stage, nf1a and nf1b are expressed throughout the animal pole of the developing embryo. (A2 and B2) Both genes are expressed broadly at 24 hpf (Inset), with strong expression along the spinal cord. (A3 and B3) At 48 hpf, expression of nf1a and nf1b is noted in the head and regions of the anterior trunk (Inset). Spinal cord expression of both genes persists, and positive staining is observed along the dorsal vessel for nf1a and nf1b. (A4 and B4) Cardiac expression for both genes is observed at 48 hpf. (A5 and B5) Expression of nf1a and nf1b become progressively restricted to regions of the head at 3 dpf (Insets). nf1a and nf1b expression along the dorsal vessel (A5 and B5) and in the embryonic heart (A6 and B6) persist at 3 dpf. (A7 and B7) At 4 dpf, robust vascular staining is apparent for nf1a and nf1b. (Scale bars: 25 μm; 100 μm for insets.)
Morpholino Knockdown of nf1a and nf1b.
We used morpholino antisense oligonucleotides (MOs) to inhibit expression of nf1a and nf1b at early stages of development. Effectiveness of gene knockdown by translation blocking MO was confirmed by Western blot analysis (Fig. S3A). The ability of neurofibromin to function as a Ras-GAP, thereby down-regulating levels of active GTP-bound Ras, can result in decreased phosphorylation of downstream effectors, including Erk/MAPK. Western blots of 3.5-dpf whole-embryo extracts derived from nf1a, nf1b, or nf1a/nf1b morphants revealed a marked up-regulation of phospho-Erk in knockdown tissue, whereas levels of total Erk were unchanged (Fig. S3B). Efficacy of splice-blocking MOs was assessed by RT- and quantitative PCR using RNA collected from 24-hpf embryos (Fig. S3 C–G).
The phenotypes produced by MO treatment were compared in a blinded fashion to embryos injected with control MOs. We observed a marked increase in the intensity and domain of expression of glial fibrillary acidic protein (GFAP), a marker of Schwann and glial cells, by whole-mount in situ hybridization (Fig. S4). This finding is consistent with the increase in neural crest-derived tissue in Nf1−/− mice and the presence of neural crest-derived tumors in NF1 patients. We also examined the expression of myelin basic protein (mbp), sex-determining region Y-box 10 (sox10), forkhead box d3 (foxd3), and crestin, but did not observe changes. Therefore, the alterations observed in nf1a and nf1b morphant embryos appear to be restricted to the Schwann-glial lineages in the neural compartment.
Morpholino Knockdown of nf1a and nf1b Results in Cardiovascular Defects.
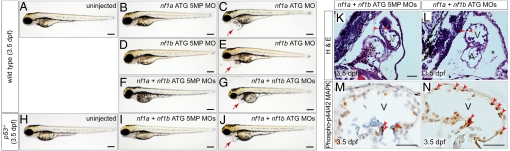
nf1a and nf1b morphant embryos displayed gross abnormalities of cardiovascular development appreciable to the blinded observer by 48 hpf. Frequently, blood was seen to move back and forth from atrium to ventricle in morphants, suggesting a malfunctioning atrioventricular valve (Movies S1 and S2). At the resolution afforded to us by histological analysis, we observed no readily apparent structural defects in the atrioventricular valves of morphants despite the observed functional deficits (Fig. S5). In addition, we observed pooling of blood in the common cardinal vein and a paucity of blood flow along the dorsal aorta and posterior cardinal vein. Valvular insufficiency and reduced blood flow were not seen in control morphants or wild-type embryos (Movie S3). Overall development of the embryos was relatively preserved through the first 3 days despite these cardiac defects. Histological analysis revealed a thinned ventricular myocardium and large pericardial effusions in MO-treated embryos (Fig. 3 K and L, and Fig. S6 A, B, F, and G). Immunohistochemical analysis of 3.5-dpf nf1a, nf1b, and nf1a/nf1b morphant zebrafish also reveals increases in phospho-Erk staining (Fig. 3 M and N, and Fig. S6 C–E and H–K). Gross morphological analysis showed an increased incidence of pericardial effusions beginning at 48 hpf, reflecting cardiac dysfunction, in nf1a and nf1b morhpants when compared with controls (Fig. 3 A–G, and Fig. S6 L and M). Nonspecific toxicity arising from MO exposure as a cause of the observed cardiovascular defects was unlikely because unrelated control or scrambled MOs failed to produce similar levels of abnormalities, defects were observed even at low doses of specific MOs, and similar defects were observed with several unrelated but specific MOs directed against nf1a and nf1b. In addition, injection of specific MOs in p53 mutant embryos also produced similar cardiovascular defects (Fig. 3 H–J), and off-target effects due to MO exposure are known to be partially mediated through p53 activation (35). Defects in cardiac valve morphogenesis and a thinning of the ventricular myocardium are also seen in Nf1−/− murine embryos.
Fig. 3.
MO knockdown of nf1a, nf1b, or both together results in pericardial effusions at 3.5 dpf and increased phospho-p44/42 MAPK in cardiac tissue. Analysis of 3.5-dpf wild-type embryos (A) or embryos injected with nf1a ATG 5-mispair (5MP) MO (B), nf1b ATG 5MP MO (D), or nf1a + nf1b ATG 5MP MO (F) reveal no apparent defects in gross morphology. Treatment with nf1a ATG MO (C), nf1b ATG MO (E), or a combination of both (G), however, results in a dilation of the pericardial space. (H–J) Injection of p53−/− embryos with nf1a + nf1b ATG MO results in a gross dilation of the pericardial space (J), whereas uninjected (H) and nf1a + nf1b ATG 5MP MO-injected p53−/− embryos (I) appear normal. (Scale bars: 0.25 mm.) (K and L) Transverse sections of 3.5-dpf nf1a/nf1b combined morphant embryos reveals a thinning of the ventricular myocardium and pericardial effusion (*) when compared with controls (A, atrium; V, ventricle). (M and N) Immunohistochemical analysis of transverse sections of 3.5-dpf nf1a/nf1b combined morphant embryos reveals an increase in the ratio of phospho-p44/42 MAPK-positive cardiac cells (arrows) to the total number of cardiac cells when compared with controls. (Scale bars: 25 μm.)
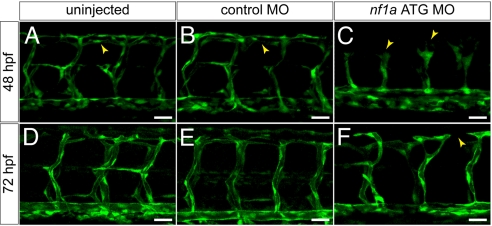
We performed knockdown experiments using zebrafish embryos in which endothelial cells are marked by expression of a cytoplasmic enhanced green fluorescent protein (GFP) to allow for a more detailed analysis of vascular development. Dramatic abnormalities of vascular patterning in the intersomitic vessels of morphant embryos were seen at 48 and 72 hpf (Fig. 4). In nf1a MO-treated embryos, the leading edge of the sprouting vessels displayed claw-like projections at 48 hpf (Fig. 4C) and failed to pattern normally such that the dorsal longitudinal anastomotic vessel (DLAV) did not form or developed in a rudimentary fashion (Fig. 4F). This occurred in embryos that were otherwise normal in overall size and maturity. These defects were also noted in nf1a/nf1b compound morphants, and were present but less severe in nf1b morphants. Vascular patterning defects did not appear to correlate directly with cardiac defects, as we observed embryos with vascular abnormalities that did not display pericardial effusion or valvular insufficiency as assessed by a to-and-fro movement of blood within the heart (36). Blood flow within the dorsal aorta and posterior cardinal vein appeared intact in these embryos (Movie S4).
Fig. 4.
MO knockdown of nf1a results in vascular patterning defects at 48 and 72 hpf. (A–C) At 48 hpf, nf1a ATG MO-treated Tg(fli:egfp)y1 (endothelial-specific GFP transgenic) zebrafish embryos display gross defects in vascular development compared with control MO-treated or uninjected samples. Morphant embryos (C) display abnormal claw-like projections at the leading edge of the developing intersomitic vessels and fail to develop the dorsal longitudinal anastomotic vessel (DLAV) present in both control MO-treated (B) and uninjected (A) samples. (D–F) At 72 hpf, nf1a ATG morphant embryos display only rudimentary DLAVs and a general disorganization of the trunk vasculature (F) when compared with control MO-treated (E) or uninjected (D) embryos. (Scale bars: 25 μm.)
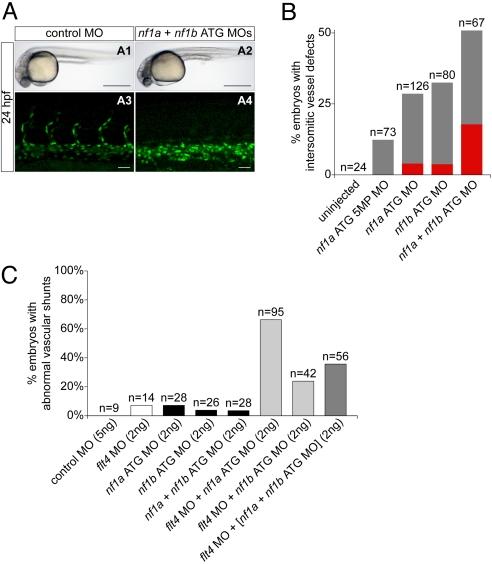
At 24 hpf, analysis using zebrafish embryos expressing a nuclear-localized GFP in endothelial cells indicated that morphants displayed a complete (Fig. 5A4) or partial absence of intersomitic vessels emanating from the dorsal aorta when compared with stage-matched controls (Fig. 5A3). Overall morphology of morphant and control embryos appeared equivalent (Fig. 5 A1 and A2), ruling out nonspecific developmental delay. These defects were apparent following MO-mediated knockdown of nf1a or nf1b, whereas knockdown of both together had an additive effect (Fig. 5B). The small percentage of embryos with defects produced by the 5MP MO may have been due to low-level knockdown of nf1a. Again, similar defects were observed with several unrelated but specific MOs directed against nf1a and nf1b (Fig. S7A). Our analysis of morphant embryos at 24 hpf also revealed a caudal vessel defect. Morphant embryos displayed a cystic expansion in the region of the caudal vein and exhibited inappropriate anastomoses between the caudal vein and artery (Fig. S7 D2–D4 and Movie S5) when compared with controls (Fig. S7D1). Identity of the expanded tissue as vascular was confirmed by expression of GFP (Fig. S7 D6–D8) and the observation of a pooling of red blood cells in the expanded region (Movie S5). This defect was present following knockdown of nf1a, nf1b, or both together (Fig. S7 C and D).
Fig. 5.
MO knockdown of nf1a, nf1b, or both together result in vascular defects at 24 at 48 hpf. (A and B) Analysis and quantification of vascular defects at 24 hpf in uninjected and morphant Tg(fli:negfp)y7 (endothelial-specific nuclear GFP transgenic) zebrafish embryos. Control MO- (A1) and combined nf1a/nf1b MO-treated (A2) zebrafish embryos appear similar by gross morphological analysis at 24 hpf. (Scale bars: 500 μm.) Development of intersomitic vessels is deficient at 24 hpf in nf1a/nf1b combined morphants (A4) when compared with controls (A3). (Scale bars: 25 μm.) (B) Intersomitic vessel formation between somites 17–30 at 24 hpf was scored as absent (red), intermediate (gray), or normal following administration of 2 ng of the indicated MO(s). (C) MO-mediated knockdown of flt4, providing a sensitized background for the detection of vascular defects, was combined with nf1a, nf1b, and nf1a + nf1b ATG MO knockdown. Twenty-four to 85% of combined flt4/(nf1a, nf1b, nf1a + nf1b) MO-treated embryos display abnormal vascular shunts compared with 3–8% of individual flt4, nf1a, nf1b, or nf1a + nf1b MO-treated embryos.
Additional confirmation of the role of nf1a/nf1b in vascular development derives from studies using a genetic background sensitized to vascular insult. Previous studies used MOs directed against flt4, the zebrafish VEGF receptor-3 orthologue, to investigate genetic interactions during zebrafish artery development (37). Additionally, flt4 morphant zebrafish embryos display variable defects in segmental artery formation reminiscent of those identified in our nf1a/nf1b morphants. Endothelial-GFP expressing zebrafish embryos were injected with flt4 MO alone and in combination with a MO directed against nf1a, nf1b, or a combination of both. At low MO doses, 85% of flt4/nf1a, 24% of flt4/nf1b, and 36% of flt4/nf1a + nf1b compound morphants displayed abnormal vascular shunts at 48 hpf compared with only 3–8% of individual flt4, nf1a, or nf1b morphants (Fig. 5C, Fig. S7B, and Movie S6). This defect was not apparent in controls (Movie S7). The shunts occur between the dorsal aorta and the dorsal longitudinal anastomotic vessel with retrograde flow through segmental arteries back into the dorsal aorta or through intersegmental veins into the posterior cardinal vein. In some cases, there were interruptions of the dorsal aorta.
Vascular Patterning Defects in Mouse Embryos Lacking Nf1.
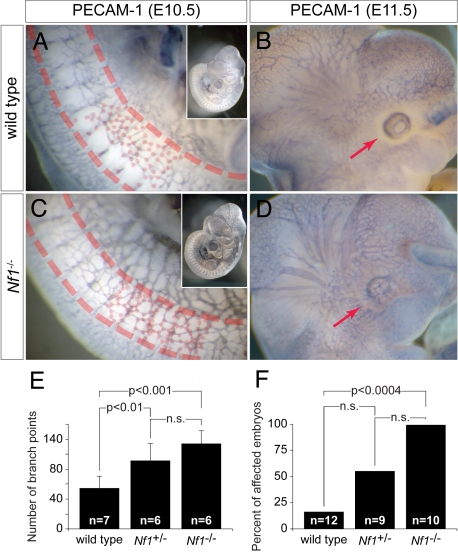
Although cardiac defects have been reported in mouse embryos lacking Nf1, a phenotype that has been attributed to a role for neurofibromin in endothelium (11), abnormalities in vascular patterning have not been previously identified. Nf1−/− mice succumb during midgestation and exhibit significant peripheral hemorrhage that has been hypothesized to be secondary to the intracardiac defects, although similar hemorrhage is not a common feature of mouse embryos with congenital heart disease. In light of our observation of peripheral vascular patterning defects in nf1 morphant zebrafish embryos, we reevaluated murine Nf1 knockouts by whole-mount platelet/endothelial cell adhesion molecule-1 (PECAM-1) staining to visualize endothelium at E10.5–E11.5 time points before the development of overt cardiac failure or significant endocardial cushion defects. Although no overt differences were appreciated in E11.5 yolk sacs (Fig. S8), we identified vascular abnormalities in embryos, including an increase in overall vascularity and a failure of the primitive vascular plexuses in the somitic region and head to remodel as seen in wild-type embryos (Fig. 6). These findings suggest that peripheral hemorrhage commonly noted on Nf1−/− mouse embryos may be related to an intrinsic vascular defect similar to that identified in MO knockdown zebrafish.
Fig. 6.
Nf1−/− mouse embryos display defects in vascular patterning. (A and C) Whole-mount PECAM-1 staining of E10.5 wild-type (A) and Nf1−/− (C) mouse embryos reveals abnormal vascular patterning in Nf1−/− embryos, with an increased number of vessels and branching (dots at branch points) particularly evident in the somites (between dashed lines). Low-magnification insets show an overall increase in vascular staining in Nf1−/− embryos. (E) Quantification of vessel branch points over four somites immediately rostral to the anterior limb buds at E10.5 shows a significant increase in Nf1+/− and Nf1−/− embryos compared with wild type (±SD). (B and D) Similar staining of stage-matched littermates at E11.5 reveals loss of the normal avascular zone around the developing eye (D) in Nf1−/− embryos compared with wild type (B). (F) Quantification of abnormal eye vasculature shows a significant increase in the number of affected Nf1−/− embryos compared with wild types (P < 0.004).
Discussion
We report the identification and initial characterization of the zebrafish orthologues of the human neurofibromatosis gene NF1. The two zebrafish nf1 genes likely arose from a genome duplication event and are highly related to one another in structure, sequence, and expression pattern through early development. MO-mediated knockdown of either gene alone results in developmental defects involving cardiac and neural crest structures that are even more prominent when both genes are knocked down in concert, suggesting partial functional redundancy. Interestingly, the entire spectrum of cardiovascular defects we have identified, including pericardial effusions and functional valve abnormalities, segmental vessel defects, and aberrant arteriovenous shunts, are greater with knockdown of the nf1a orthologue compared with nf1b, suggesting that nf1a may play a more prominent role in cardiovascular development.
Nf1−/− mice die during midgestation as a result of severe cardiac failure, and display gross cardiovascular and neural crest defects. Closer examination of these Nf1-defcient mice reveals hyperproliferative endocardial cushions, the precursors of the cardiac valves, which have been shown to result from a cell-autonomous role for Nf1 in endothelial cells (11). Here, we show that zebrafish embryos also display cardiovascular and neural abnormalities following transient knockdown of the orthologous nf1 genes. These defects resemble those seen in mouse models, including the presence of pericardial effusions, thinned myocardium, abnormal cardiac valves, and an increase in Schwann-glial derivatives.
Importantly, the generation of a new vertebrate model of NF1 allowed us to identify a previously unrecognized role for neurofibromin in vascular patterning during early zebrafish and murine development. The ability to distinguish a primary vascular defect from a phenotype resulting secondary to cardiac failure is possible in zebrafish because early vascular development does not require an intact circulation, and adequate oxygenation is achieved via passive diffusion (38). In mice, this distinction is much more difficult to define, emphasizing one of the advantages of developing a zebrafish model of NF1. It is worth noting that the degree of peripheral hemorrhage noted in Nf1 knockout embryos is unusual for mouse models of congenital heart disease and is not seen in embryos with double-outlet right ventricle, truncus arteriosus, or atrioventricular canal defects despite pericardial effusions indicating heart failure (39). We hypothesize that a peripheral vascular defect produced by endothelial dysfunction in Nf1-deficient mouse embryos accounts for the observed degree of hemorrhage. Though the complexity and severity of vascular patterning defects in mouse and zebrafish embryos lacking neurofibromin are distinct, we suggest that they are highly likely to be related, and there is precedent for similar differences in the vascular manifestation of genetic mutations in fish and mice (40, 41).
Vascular patterning defects represent a well-recognized component of the pleiotropic spectrum of NF1 disease phenotypes in affected individuals (3). NF1 patients often exhibit a characteristic vascular lesion known as moyamoya, a name that derives from its appearance as a puff of smoke on computed tomography scans of the head due to abnormal small-vessel patterning in the brain (42, 43). Other vascular defects, including hypertension and renal artery stenosis, have also been documented (3). Neurofibromin has been shown to modulate the activity of Ras proto-oncogenes through its GAP-related domain (GRD), and multiple lines of evidence support a role for Ras signaling in normal vascular patterning and development. For example, mouse embryos deficient for p120 GAP activity display vascular defects such as abnormalities in endothelial cell organization (44). In addition, mutations in RASA1, the gene encoding p120 GAP, are associated with vascular anomalies in affected individuals (45, 46). Experiments in chicken and mouse endothelial tissues have identified a role for H-Ras in angiogenesis and vascular permeability (47). Studies in zebrafish also support a role for Ras signaling in vascular development; MO knockdown of k-ras or overexpression of a dominant negative mutant of k-ras both result in defective vascular development (48). These data, taken together with our own, suggest a necessity for tight regulation of Ras signaling in normal vascular development. The flt4/(nf1a, nf1b, nf1a + nf1b) vascular shunting phenotype represents an inappropriate arteriovenous malformation, which is also present in the embryos with cystic expansion of the dorsal vein. Other vascular patterning defects observed in the nf1a/nf1b morphants may represent distinct functions of neurofibromin in the vasculature, or may be related by common underlying mechanisms. The genetic interaction between nf1a/nf1b and flt4 that we show suggests that these molecules may function in a common molecular pathway, although alternative interpretations cannot be ruled out.
In an effort to determine the degree to which neurofibromin function can be ascribed to its activity as a Ras-GAP, we have previously generated a mouse model in which the isolated neurofibromin GRD is expressed in a tissue-restricted manner upon Cre-mediated activation (20). These studies have shown that reconstitution of the neurofibromin GRD in endothelial cells of Nf1−/− mice is sufficient to rescue cardiac development and midgestational lethality. The resulting mice, however, are abnormal and succumb in the early postnatal period. Analysis of these animals reveals massive overgrowth of peripheral nervous tissues that mimic those of the neural crest-specific Nf1-deleted mice. This finding strongly suggests that additional domains outside the GRD are important for neural crest growth and homeostasis. Our development of a zebrafish model of NF1 will be particularly useful for examining the potential activities of these domains through in vivo structure-function analyses. Enhancer and suppressor screens may identify signaling pathways, in addition to the Ras/MAPK and mTOR pathways, that impact disease progression. In addition, high-throughput small-molecule screens will allow for the rapid identification of compounds with the potential for modifying NF1 disease phenotypes. The generation of stable mutant lines for nf1a and nf1b serve as a necessary prerequisite to pursue these exciting possibilities.
Materials and Methods
Morpholino Injections.
Morpholino oligonucleotides (Gene Tools) corresponding to nf1a, nf1b, flt4, and associated controls were dissolved in water and supplemented with 0.1% vol/vol phenol red. One-cell zebrafish embryos were injected with ≈1 nL of the appropriate MO solution(s). MO sequences were as follows: nf1a ATG MO 5′-GGCTTGTGCGCCGCCATGCTCAGGG, nf1a ATG 5MP MO (nf1a ATG 5-mispair control MO) 5′-GGCTTCTGCCCCGGCATGGTCACGC, nf1b ATG MO 5′-CCGCTCACGCCGATAGTGATGAAGA, nf1b ATG 5MP MO 5′-CCCCTCAGGCCCATAGTCATCAAGA, nf1a SB MO 5′-GTCCAAGTAGTGTTTTCCTTACCTG, nf1a SB 5MP MO 5′-GTCCAACTACTCTTTTGCTTAGCTG, nf1b SB MO 5′-CTCAGTATTTATCTGCACCTGGTGG, nf1b SB 5MP MO 5′-CTGAGTATATATGTGCAGCTGCTGG, flt4 MO (37), and standard control MO 5′-CCTCTTACCTCAGTTACAATTTATA.
Additional information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Jie He (University of Pennsylvania CDB Zebrafish Core) and Nicole Antonucci for assistance with animal husbandry, Andrea Stout (University of Pennsylvania CDB/CVI Microscopy Core) for assistance with microscopy, and Michael Pack and Mary Mullins for helpful discussions and reagents. This work was supported by National Institutes of Health Grants R01-HL062974 (to J.A.E.) and K08-HL075179 (to F.A.I.) and Department of Defense Grant NF050175 (to J.A.E. and A.T.L.). A.P. was supported by a fellowship from the Sarnoff Cardiovascular Research Foundation, and J.-S.L. was supported by a Young Investigator Award from the Children's Tumor Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901932106/DCSupplemental.
References
- 1.Ferner RE, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol. 2002;17(8):562–570. doi: 10.1177/088307380201700804. discussion: 571–562, 646–551. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM, et al. Cardiovascular disease in neurofibromatosis 1: Report of the NF1 Cardiovascular Task Force. Genet Med. 2002;4(3):105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cichowski K, Jacks T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell. 2001;104(4):593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 5.Guo HF, The I, Hannan F, Bernards A, Zhong Y. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276(5313):795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- 6.The I, et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276(5313):791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 7.Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5(2):95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- 8.Hegedus B, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Gutmann DH. Mutations in the GAP-related domain impair the ability of neurofibromin to associate with microtubules. Brain Res. 1997;759(1):149–152. doi: 10.1016/s0006-8993(97)00328-4. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh Y, Roberts AM, Volta M, Sheng M, Roberts RG. Bipartite interaction between neurofibromatosis type I protein (neurofibromin) and syndecan transmembrane heparan sulfate proteoglycans. J Neurosci. 2001;21(11):3764–3770. doi: 10.1523/JNEUROSCI.21-11-03764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitler AD, et al. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33(1):75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brannan CI, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8(9):1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 13.Jacks T, et al. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7(3):353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 14.Cichowski K, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286(5447):2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 15.Vogel KS, et al. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286(5447):2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 17.Costa RM, et al. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27(4):399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296(5569):920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismat FA, Xu J, Lu MM, Epstein JA. The neurofibromin GAP-related domain rescues endothelial but not neural crest development in Nf1 mice. J Clin Invest. 2006;116(9):2378–2384. doi: 10.1172/JCI28341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph NM, et al. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13(2):129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13(2):105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng H, et al. Induction of abnormal proliferation by nonmyelinating Schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13(2):117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Silva AJ, et al. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15(3):281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 25.Costa RM, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Velasco-Miguel S, Vass WC, Parada LF, DeClue JE. Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res. 2002;62(15):4507–4513. [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le L, Parada L. Tumor microenvironment and neurofibromatosis type I: Connecting the GAPs. Oncogene. 2007;26(32):4609–4616. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Side LE, et al. Mutations of the NF1 gene in children with juvenile myelomonocytic leukemia without clinical evidence of neurofibromatosis, type 1. Blood. 1998;92(1):267–272. [PubMed] [Google Scholar]
- 30.Le DT, et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004;103(11):4243–4250. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 31.Gitler A, et al. Tie2-Cre-induced inactivation of a conditional mutant Nf1 allele in mouse results in a myeloproliferative disorder that models juvenile myelomonocytic leukemia. Pediatr Res. 2004;55(4):581–584. doi: 10.1203/01.PDR.0000113462.98851.2E. [DOI] [PubMed] [Google Scholar]
- 32.Yang FC, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135(3):437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amores A, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282(5394):1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 34.Covassin L, et al. Global analysis of hematopoietic and vascular endothelial gene expression by tissue specific microarray profiling in zebrafish. Dev Biol. 2006;299(2):551–562. doi: 10.1016/j.ydbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robu ME, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3(5):e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130(21):5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 37.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA. 2006;103(17):6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel AM, Weinstein BM. Studying vascular development in the zebrafish. Trends Cardiovasc Med. 2000;10(8):352–360. doi: 10.1016/s1050-1738(01)00068-8. [DOI] [PubMed] [Google Scholar]
- 39.Gruber PJ, Epstein JA. Development gone awry: Congenital heart disease. Circ Res. 2004;94(3):273–283. doi: 10.1161/01.RES.0000116144.43797.3B. [DOI] [PubMed] [Google Scholar]
- 40.Gitler A. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7(1):107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Torresvazquez J. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Norton KK, Xu J, Gutmann DH. Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis. 1995;2(1):13–21. doi: 10.1006/nbdi.1995.0002. [DOI] [PubMed] [Google Scholar]
- 43.Cairns AG, North KN. Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatr. 2008;79(10):1165–1170. doi: 10.1136/jnnp.2007.136457. [DOI] [PubMed] [Google Scholar]
- 44.Henkemeyer M, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377(6551):695–701. doi: 10.1038/377695a0. [DOI] [PubMed] [Google Scholar]
- 45.Eerola I, et al. Capillary malformation–arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73(6):1240–1249. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revencu N, et al. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29(7):959–965. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- 47.Serban D, Leng J, Cheresh D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008;102(11):1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Zhu S, Gong Z, Low BC. K-ras/PI3K-Akt signaling is essential for zebrafish hematopoiesis and angiogenesis. PLoS ONE. 2008;3(8):e2850. doi: 10.1371/journal.pone.0002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.