Abstract
The Ca2+-binding proteins (CBPs) parvalbumin, calbindin, and calretinin are phenotypic markers of terminally differentiated neurons in the adult brain. Although subtle phylogenetic variations in the neuronal distribution of these CBPs may occur, morphologically and functionally diverse subclasses of interneurons harbor these proteins in olfactory and corticolimbic areas. Secretagogin (scgn) is a recently cloned CBP from pancreatic β and neuroendocrine cells. We hypothesized that scgn is expressed in the mammalian brain. We find that scgn is a marker of neuroblasts commuting in the rostral migratory stream. Terminally differentiated neurons in the olfactory bulb retain scgn expression, with scgn being present in periglomerular cells and granular layer interneurons. In the corticolimbic system, scgn identifies granule cells distributed along the dentate gyrus, indusium griseum, and anterior hippocampal continuation emphasizing the shared developmental origins, and cytoarchitectural and functional similarities of these neurons. We also uncover unexpected phylogenetic differences in scgn expression, since this CBP is restricted to primate cholinergic basal forebrain neurons. Overall, we characterize scgn as a neuron-specific CBP whose distribution identifies neuronal subtypes and hierarchical organizing principles in the mammalian brain.
Keywords: cortex, development, interneuron, neurogenesis, stem cell
The ability to release neurotransmitters at chemical synapses, to integrate the activity of diverse synaptic inputs and trigger molecular mechanisms underlying neuronal adaptation, as well as to maintain excitability in neurons rely on the refined spatial and temporal control of momentary changes in cytosolic [Ca2+] (1, 2). Ca2+-binding proteins (CBPs) represent a means to effectively regulate intracellular Ca2+ dynamics (3). Members of the EF-hand family of CBPs invariably contain a 3-D motif to bind Ca2+ at its physiological cytosolic concentrations (4). Some ancestral representatives of this protein family, such as calmodulin, are ubiquitously expressed with a high degree of evolutionary conservation and are involved in the control of fundamental cellular functions ranging from the cell cycle, cell motility and axon polarization to synaptic signaling (3). In contrast, the parvalbumin (PV) and calbindin subfamilies of CBPs, the latter including the vitamin D-dependent 28 kDa isoform of calbindin (CB) and calretinin (CR), exhibit restricted tissue-specific expression patterns in vertebrates (5, 6). During the past decades, PV, CB, and CR received significant attention because of their exquisite developmentally regulated cell type-specific expression in the mammalian nervous system (6–8).
CBPs show a unique association with newly generated neurons in the adult brain (9, 10). Neural progenitors that are born in the subependymal zone and migrate in the rostral migratory stream (RMS) to differentiate into interneurons in the olfactory bulb (OB) commonly express CR at their neuroblast stage with select subpopulations becoming CB+ upon their arrival to their final position in the OB. Similarly, neurons generated in the subgranular zone of the dentate gyrus (DG) express CBPs, CR transiently and CB permanently, during terminal differentiation into granule cells and integration into adult neuronal networks (10, 11). Transient or permanent CBP expression in new neurons of the adult brain can be of functional significance as CBPs can effectively modulate intracellular Ca2+ signaling to achieve optimal temporal control of cell motility, neurite outgrowth, and synaptogenesis (3).
Although their quantitative distribution can vary greatly, the laminar organization and cellular identity of PV, CB, and CR expression in the mammalian corticolimbic system remain phylogenetically preserved (3). These CBPs define largely nonoverlapping and morphologically distinct subpopulations of GABAergic interneurons in rodent, primate, and human cortices (5, 6, 12) with the exception of CB, which, although at low levels, is also expressed by cortical (layer 2/3 and 4) and hippcampal (CA1–CA3) pyramidal cells and DG granule cells (GCs) (5, 12).
Secretagogin (scgn) is a recently discovered EF-hand CBP cloned from β cells of the pancreatic islands of Langerhans and endocrine cells of the gastrointestinal tract (13, 14). Scgn harbors six putative EF hand motifs (13) and has been functionally implicated as a Ca2+ sensor in insulin synthesis and secretion (13). However, the precise expression pattern of this CBP, the neurochemical identity of scgn+ nerve cells in neurogenic niches and in the corticolimbic system of the adult mammalian brain and its subcellular targeting in neurons remain unexplored. Here, we report that scgn characterizes precursor cells committed to the RMS and terminally differentiated periglomerular and granular layer interneurons in the OB. We find that scgn is a selective marker for GCs in the DG and the anterior hippocampal continuation (AHC). We also uncover striking phylogenetic differences of scgn expression in the RMS and in basal forebrain cholinergic neurons projecting to the hippocampal formation. Cumulatively, our results classify scgn as a fourth major cell type-specific CBP in the mammalian corticolimbic system and reveal an unexpected neurochemical signature unifying GCs in the DG, AHC, and indusium griseum (IG).
Results and Discussion
Expression Profiling in Adult Brain.
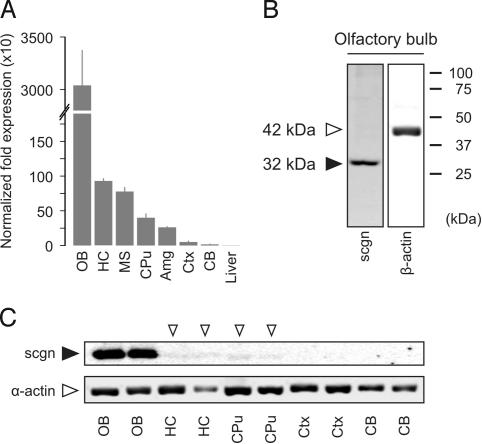
Although scgn mRNA expression has been shown in cortical areas of the human brain (15), subtle regional variations of scgn expression in rodent brain remain unknown. We assessed scgn mRNA levels in OB, telencephalic areas and cerebellum of the adult mouse by real-time quantitative PCR (qPCR) analysis. We establish that scgn mRNA expression is robust in the OB, moderate in the hippocampus (HC), medial septum (MS), and striatum (CPu), and low in amygdaloid nuclei, neocortex, and cerebellum (Fig. 1A and Fig. S1 A and B).
Fig. 1.
scgn is expressed in the OB and corticolimbic territories of the adult brain. (A) scgn mRNA expression profiling of microdissected mouse brain tissues as measured by qPCR relative to gapdh. (B) An anti-scgn antibody (HPA006641) selectively recognizes a single protein band corresponding to scgn's calculated molecular weight. (C) Regional differences in protein expression in adult mouse brain as determined by Western analysis. Open arrowheads indicate scant protein expression levels. Abbreviations are listed in Table S1.
Within the framework of the Human Protein Atlas program (16), we have generated antibodies to >3,000 proteins, including a powerful polyclonal antibody recognizing a phylogenetically conserved scgn epitope (Fig. S2 A and B) and tested these on rat and mouse nervous tissues (17). Here, we confirmed that this antibody selectively recognizes scgn in Western blot applications (Fig. 1B and Fig. S2C), and it visualizes a scgn expression pattern in primate and rodent brain and peripheral tissues that corresponds to previously established scgn mRNA expression profiles (13) (Fig. S1B and Fig. S2 D–G). To further explore whether scgn expression is prominently associated to the corticolimbic system at the protein level, we detected scgn protein in OB, HC, and CPu (including pallidal territories) upon loading whole cell lysates (20 μg) on denaturing SDS-PAGE (Fig. 1C). Overall, these data demonstrate regional specificity of scgn expression in the mammalian brain with the OB and corticolimbic system exhibiting pronounced mRNA and protein concentrations of this CBP (Figs. S1B and S3 and Tables S1 and S2).
The RMS and OB.
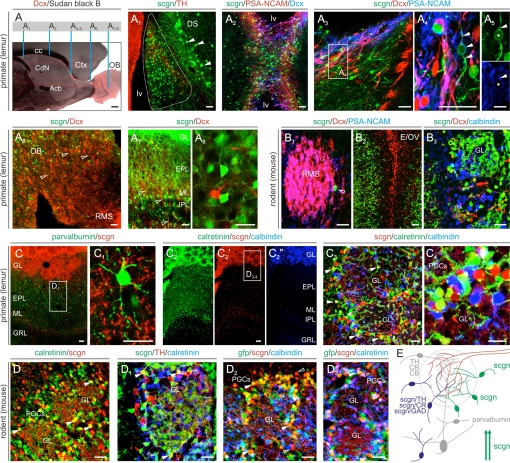
Neurogenesis occurs throughout life in the subependymal zone with cells traveling in the RMS (Fig. 2A) to populate the OB where neuroblasts differentiate into periglomerular interneurons (9). However, the rate at which neurons are generated, the distribution of migrating cells along the RMS, and the molecular control of their phenotypic differentiation are substantially different between primates and rodents (18). We find small (6–8 μm) scgn+ cells in the MS region of the subventricular zone of the gray mouse lemur brain (Microcebus murinus, Primates; Fig. 2A1) that is otherwise known as the birthplace of CR+ OB interneurons in rodents (9). scgn identifies doublecortin (Dcx)−, but PSA-NCAM+ neuroblasts throughout their chain migration in the RMS (Fig. 2 A2–A5). Scgn+ neuroblasts, like Dcx+ ones, penetrate the primate OB and contribute cells to the granular, plexiform, and periglomerular layers (Fig. 2 A6–A8). A fundamental difference between primate and rodent brain is that no scgn+ neuroblast is seen in the mouse RMS (Fig. 2 B1 and B2). Instead, scgn selectively marks postmigratory granular and periglomerular OB neurons (Fig. 2 B2 and B3).
Fig. 2.
scgn in the RMS and OB. (A) Neurons are continuously committed to the OB through the RMS. Vertical bars indicate the location of panels A1–A8. In primate brain, scgn labels migrating neuroblasts at their birth places (A1), en route in the RMS (A2–A5), upon invading the OB (A6), and at their final positions (A7 and A8). Arrowheads in panel A1 identify scgn+ AHC neurons. Arrowheads in panels A4 and A5 denote doublecortin (Dcx)−/scgn+/PSA-NCAM+ processes. In contrast, scgn is absent from rostrally migrating neuroblasts in mouse brain (B1–B3). (C–C4) In the primate OB, scgn+ neurons are present in the granular (GRL), internal/external plexiform (I/EPL), and glomerular (GL) layers and are distinct from PV+ or CB+ PGCs. CR+/scgn+ cells are marked by solid arrowheads. (D–D3) In the mouse OB, scgn often coexists with CR and GAD67 (as identified by gfp expression), rarely with tyrosine hydroxylase (TH, solid arrowheads) but not CB in PGCs. (E) Overview of the neurochemical diversity of PGCs. scgn+ PGCs belong to GAD+/TH+ and CR+ PGCs (blue) or to a separate subclass of PGCs (green). Arrows indicate migrating scgn+ neuroblasts. Open arrowheads invariably point to a lack of colocalization of histochemical markers. Open boxes denote the positions of insets. Abbreviations are referred to in Table S1. [Scale bars, 22 μm (A1–B3, C1, and C3–D3), 60 μm (C and C2), and 100 μm (A).]
The laminar distribution of neurochemically diverse GABAergic interneurons in the OB underpins olfactory processing (19). Glutamic acid decarboxylase (GAD)/GABA, tyrosine hydroxylase (TH), CB, and CR are phenotypic markers of periglomerular cells (PGCs) (20). In the primate and rodent OB, scgn+ neurons populate the glomerular, internal plexiform/mitral, and periglomerular layers (Fig. 2 C–D and Fig. S4). We observe that scgn+ cells are invariably distinct from PV+ short axon cells (Fig. 2D1), CB+ Blanes cells, or CB+ PGCs (Fig. 2 C2 and D2) in both species. Instead, scgn may coexist with CR, TH, and GAD in PGCs (Fig. 2 C3–D2). Ramifying dendrites of scgn+ PGCs extend into the glomeruli and receive both inhibitory and excitatory inputs (Fig. S4 C and D).
Overall, we identify scgn as a specific marker of primate RMS neuroblasts. In contrast, we demonstrate that scgn is invariably expressed in interneurons undergoing terminal differentiation in the OB of both primates and rodents. These findings reinforce that species-specific spatial and temporal differences exist in the onset of neurochemical specification of newly born OB interneurons in the adult mammalian brain (18). Only approximately 40% of PGCs have been neurochemically accounted for and subdivided into (i) GAD/GABA+, (ii) CB+, and (iii) CR+ subclasses (20). While a subset of scgn+ PGCs belongs to GAD/GABA+ and CR+ subclasses, the remainder of scgn+ PGCs appears to make up a separate subclass (Fig. 2E). This finding may have important functional ramifications, as continued identification of PGCs will help elaborating the OB wiring diagram and refining the structural backbone of local neuronal subnetworks modulating olfactory perception.
Hippocampal Formation.
The extended hippocampal formation is comprised of the interlocking gyri of the Ammon's horn (CA1–3 subfields) and DG, and a continuum of nuclei engulfing the corpus callosum (cc): The faciola cinereum (FC), IG, AHC, and ventral tenia tecta (vTT) (Figs. S3 and S5). The neurochemical and cytoarchitectural identity of IG and AHC has been a subject of long debate with conflicting views linking these areas to either the OB or DG (21, 22).
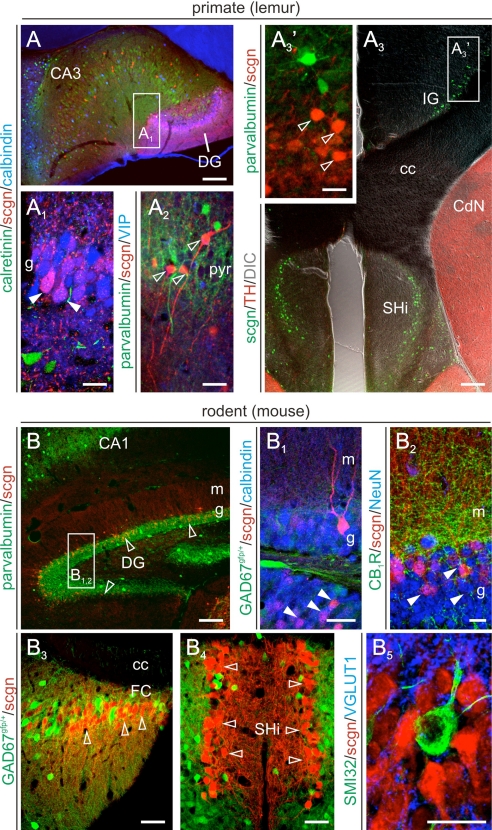
Scgn labels GCs at varying intensities in all layers of the primate DG (Fig. 3 A and A1). In addition, scgn+ nonpyramidal cells with morphologies reminiscent of bistratified cells (12) were found in CA3 stratum pyramidale (Fig. 3A2), while immunoreactive cells with variable morphologies were infrequently encountered in stratum oriens. Although scgn also labeled GCs in the mouse, we find that mostly mature GCs in superficial DG laminae receiving dense innervation in the inner molecular layer contain this CBP (Fig. 3 B–B2). Notably, scgn+ GC-like neurons form a continuum along the FC (Fig. 3B2), IG (Figs. 3A3 and 4 A–B1), AHC (Fig. 3 A3 and B3–B5), but not vTT in both species.
Fig. 3.
Scgn identifies GCs in the hippocampal formation. (A) In the primate hippocampus, scgn labels CB+ dentate GCs (A1) and nonpyramidal cells in CA1–3 subfields (A2). In the AHC/SHi and IG of monkeys, scgn expression is restricted to GCs but is absent in GABAergic, e.g., PV+, interneurons. In rodent brain, scgn marks GCs mapped onto the superficial layers of the DG (B–B2). In areas developmentally related to the DG, such as the FC (B3), AHC/SHi (B4 and B5) scgn reveals a continuum of GCs. Solid and open arrowheads mark double- and single-labeled structures, respectively. Abbreviations are referred to in Table S1. [Scale bars, 25 μm (A1–A3′ and B1–B5) and 100 μm (A, A3, and B).]
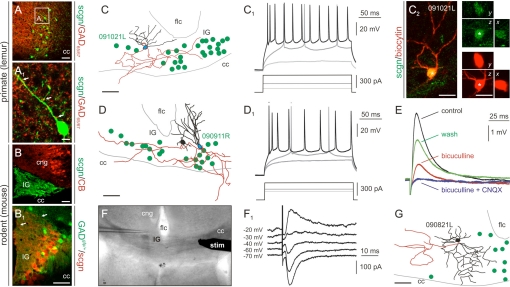
Fig. 4.
Cellular characteristics of scgn+ neurons in IG. (A and A1) scgn+ cells receive inhibitory inputs (arrows, A1) and are non-GABAergic (B and B1) with apical dendrites targeted to the subpial surface (arrows, B1). The dendritic morphology (C and D) and action potential waveform (C1 and D1) of scgn+ neurons resembles those of dentate GCs. Data are from biocytin-filled cells unequivocally harboring scgn (C2). (E) scgn+ GCs receive both inhibitory (bicuculline-sensitive) and excitatory (CNQX-sensitive) local synaptic inputs. (F and F1) cc stimulation evokes IPSCs in GCs. (G) scgn+ cells are intermingled with morphologically diverse scgn− interneurons in this region. Abbreviations are referred to in Table S1. [Scale bars, 10 μm (A1) and 25 μm (all others).]
The characteristic GC-like morphology of scgn+ neurons in IG (Fig. 4 A–B1), the segregated subcellular targeting of their excitatory (Fig. 3B5) and inhibitory (Fig. 4A1) synaptic inputs, and their lack of CB expression (Fig. 4B) prompted us to determine the cytoarchitecture, discharge properties, and afferentation of these cells. We show by correlated patch-clamp electrophysiology and morphological reconstruction that scgn+ IG cells have GC-like dendritic complexities (Fig. 4 C, C2, and D), and their action potential pattern in response to somatic depolarization (Fig. 4 C1 and D1) together with passive membrane properties (Vrest = −74.3 ± 0.5 mV; Ri = 210 ± 48 MΩ) also resemble those of dentate GCs (23). Ipsilateral extracellular stimulation (200–500 μm from the soma) triggered both inhibitory and excitatory inputs (Fig. 4E), while cc stimulation resulted in monosynaptic PSCs that reversed close to the calculated value for EGABA (Fig. 4 F and F1). We also show that the IG contains various subclasses of interneurons (Fig. 4 B1 and G) with a sudden increase in neuronal heterogeneity at the anatomical boundaries of this nucleus (Fig. S6).
Similarities in the ontogeny, lamination pattern, cellular physiology, and origins of synaptic afferents (22) argue for a close relationship between the IG/AHC and DG. Our findings support this concept by recognizing scgn as a unifying GC marker in these territories and by demonstrating fundamental common features in their intrinsic electrical properties. The fact that GCs in IG but not in DG are CB− may suggest that the CBP composition of GCs reflects regional differences in the developmental program and turnover of these cells in adult brain.
Phylogenetic Differences in scgn Expression by Cholinergic Neurons.
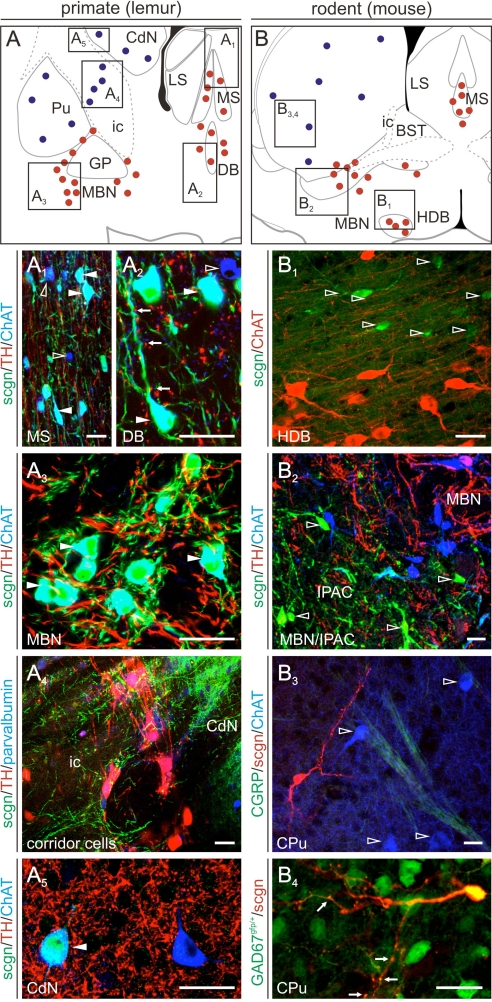
Cholinergic basal forebrain neurons primarily innervate principal cells in both CA1–3 subfields and DG (6) to drive hippocampal network oscillations (24). Although cholinergic projection neurons in rodents appear largely homogenous, striking phylogenetic differences concerning the expression of nitric oxide synthase, neurotransmitter receptors, and CBPs exist between primates and rodents (25). Therefore, we studied scgn expression in lemur and mouse cholinergic neurons including both projection cells and interneurons (Fig. 5 A and B). We determined that scgn coexists in the vast majority of choline acetyltransferase (ChAT)+ projection neurons of the primate MS (Fig. 5A1), diagonal band complex (Fig. 5A2), and magnocellular basal nucleus (Fig. 5A3 and Fig. S7) (26). As an exception to this rule, ChAT−/scgn+ neurons were found scattered in the substantia innominata of the lemur brain (Fig. S7A3). In contrast, scgn exclusively identified a spatially segregated noncholinergic cell population in mouse basal forebrain territories (Fig. 5 B1 and B2). Next, we assessed scgn expression in cholinergic interneurons of both species and found that scgn immunoreactivity decorates a heterogeneous neuron population in the nuclei putamen (Pu) and caudatus (CdN) and connecting cell columns in the capsula interna (Fig. 5A4) in primates with approximately 50% of cholinergic interneurons being scgn+ in Pu (Fig. 5A5), but not CdN (≈10%) or ventral pallidum (VP) (Fig. S7). In contrast, scgn is absent from cholinergic interneurons of the mouse CPu (Fig. 5B3). Instead, scgn+ cells are GABAergic with elaborate, spiny dendritic tufts in this (Fig. 5B4) and also in pallidal areas (Fig. S7). These findings are significant as the presence of scgn in primate but not rodent cholinergic neurons may add a facet of understanding to explain vast interspecies differences in the sensitivity of these cells to noxious stimuli through differential control of intracellular Ca2+ signaling.
Fig. 5.
Phylogenetic differences in scgn expression of cholinergic basal forebrain neurons. (A–A5) Cholinergic projection (A1–A3) and interneurons (A4 and A5) in the primate basal forebrain frequently contain scgn. Arrows (A2) identify putative TH-positive afferents to cholinergic neurons. (B–B4) In contrast, neither cholinergic projection neurons (B1 and B2) nor striatal cholinergic interneurons (B3 and B4) express scgn in mouse forebrain. Note that scgn is, however, present in spiny (arrows) striatal GABAergic interneurons in mouse (B4). Solid and open arrowheads point to double and single labeled structures, respectively. Open rectangles in (A and B) identify the general position of particular images. Red and blue circles mark cholinergic projection and interneurons, respectively. (Scale bars, 25 μm.)
Conclusions
scgn is a neuronal CBP whose cell-type specificity is distinct from those of other known CBPs. Besides that scgn uniquely labels chain-migrating RMS neuroblasts in primate brain, its OB distribution allows us to propose that scgn+ cells otherwise lacking known classification markers of PGCs represent a distinct periglomerular neuron subclass. Scgn is also expressed by newly born dentate GCs and is enriched in GC dendrites targeted onto the subpial surface in the IG. Since scgn is expressed in (entero-)endocrine cells and in neuroendocrine cells at the periphery (Fig. S2) and in the brain (Fig. S3), respectively, and has been implicated in the control of hormone secretion, we propose that scgn may regulate the release of diverse factors from neuroblasts and dendrites of differentiated PGCs and GCs that are required for new neurons to survive or to develop. Our hypothesis may also help establishing a functional link between the expression of basic fibroblast growth factor and neurotrophin-3 being restricted to the IG and FC in adult brain (27) and the activity-dependent release of these essential substances into the liquor space.
Materials and Methods
Expression Profiling.
qPCRs were performed as described in ref. 28 with custom designed primers (Fig. S1A3). RNA isolated from tissues microdissected from adult C57BL/6N mouse brains (n = 2) were subjected to scgn expression analysis after validating RNA integrity (Fig. S1A). Results from qPCR experiments were subsequently compared to scgn mRNA distribution as determined by in situ hybridization (Fig. S1B).
Tissues and Histochemistry.
Young adult C57Bl6/N (n = 4) and heterozygous GAD67-GFP (Δneo) mice (GAD67gfp/+; n = 6) (29), and gray mouse lemurs born in a laboratory breeding colony (n = 5; Brunoy, France) (30) were maintained, transcardially perfused, and had their brains processed as described in ref. 26. Free-floating coronal and sagittal cryostat sections (40 μm) from animals of both sexes were used for histochemistry. Experiments in rodents and primates confirmed to the 86/609/EEC directive and were approved by the Home Office and local French authorities (#962773), respectively.
We generated rabbit anti-scgn antibodies by designing protein epitope signature tags (PrESTs) of 100–150 aa (17) based on the human protein sequence by using bioinformatic tools (Fig. S2A). PrESTs were recombinantly produced in Escherichia coli and used to immunize rabbits. Mono-specific polyclonal antibodies were generated by immunoaffinity purification of ensuing antisera. Multiple immunofluorescence histochemistry with cocktails of primary antibodies (Table S3) was performed according to published protocols (31). Sudan black-B counterstaining of primate tissues was applied to quench tissue autofluorescence (26).
Imaging.
Single x-y plane or orthogonal z image stacks were captured by laser-scanning microscopy (510META; Zeiss). Colocalization was defined as immunosignals being preset without physical signal separation in <1.0-μm optical slices (31). Reconstruction of identified neurons was performed as described in ref. 32.
Biochemistry.
Protein samples were analyzed under denaturing conditions. Western blot analysis was undertaken by using rabbit anti-scgn (1:2,000) and mouse anti-β-actin (1:4,000) primary antibodies (17). Immunoprecipitation was performed in cell lysates prepared from adult mouse OB (Fig. S2C). Blots were scanned on a Lycor Odyssey-IR imager and quantified by using ImageJ1.32j.
Electrophysiology.
Patch clamp recordings, differential interference contrast (DIC) imaging and extracellular stimulation with a bipolar electrode positioned either proximally or in the cc (0.2-ms pulse duration) in coronal slices (300-μm) prepared from postnatal 14- to 19-day-old mouse brains were performed as described in ref. 32. Presence of inhibitory and excitatory synaptic inputs on GCs was in IG were tested by applying bicuculline (5 μM) and CNQX (10 μM), respectively, in the superfusate.
Supplementary Material
Acknowledgments.
This work was supported by the Scottish Universities Life Science Alliance, Alzheimer's Association, Alzheimer's Research Trust (ART) UK, European Molecular Biology Organization Young Investigator Program, National Institutes of Health grant DA023214, Swedish Medical Research Council, European Commission (HEALTH-F2–2007-201159), and Knut and Alice Wallenberg Foundation. J.M. is recipient of a postdoctoral fellowship from ART UK.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912484106/DCSupplemental.
References
- 1.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnashev N, Rozov A. Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy. Cell Calcium. 2005;37:489–495. doi: 10.1016/j.ceca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Andressen C, Blumcke I, Celio MR. Calcium-binding proteins: Selective markers of nerve cells. Cell Tissue Res. 1993;271:181–208. doi: 10.1007/BF00318606. [DOI] [PubMed] [Google Scholar]
- 4.Heizmann CW. Intracellular calcium-binding proteins: Structure and possible functions. J Cardiovasc Pharmacol. 1986;(Suppl 8):S7–S12. [PubMed] [Google Scholar]
- 5.Celio MR. Calbindin-D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 6.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka K, et al. Chemically-defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neuroscience Research. 1995;23:73–88. [PubMed] [Google Scholar]
- 8.Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience. 1993;54:461–476. doi: 10.1016/0306-4522(93)90266-i. [DOI] [PubMed] [Google Scholar]
- 9.Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–174. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- 12.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner L, et al. Cloning and expression of secretagogin, a novel neuroendocrine- and pancreatic islet of Langerhans-specific Ca2+-binding protein. J Biol Chem. 2000;275:24740–24751. doi: 10.1074/jbc.M001974200. [DOI] [PubMed] [Google Scholar]
- 14.Birkenkamp-Demtroder K, et al. Secretagogin is a novel marker for neuroendocrine differentiation. Neuroendocrinology. 2005;82:121–138. doi: 10.1159/000091207. [DOI] [PubMed] [Google Scholar]
- 15.Gartner W, et al. Cerebral expression and serum detectability of secretagogin, a recently cloned EF-hand Ca(2+)-binding protein. Cereb Cortex. 2001;11:1161–1169. doi: 10.1093/cercor/11.12.1161. [DOI] [PubMed] [Google Scholar]
- 16.Uhlen M, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Mulder J, et al. Tissue profiling of the mammalian central nervous system using human antibody-based proteomics. Mol Cell Proteomics. 2009;8:1612–1622. doi: 10.1074/mcp.M800539-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaka T, et al. An aspect of the organization of the GABAergic system in the rat main olfactory bulb: Laminar distribution of immunohistochemically defined subpopulations of GABAergic neurons. Brain Res. 1987;411:373–378. doi: 10.1016/0006-8993(87)91090-0. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka K, Kosaka T. Synaptic organization of the glomerulus in the main olfactory bulb: Compartments of the glomerulus and heterogeneity of the periglomerular cells. Anat Sci Int. 2005;80:80–90. doi: 10.1111/j.1447-073x.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 21.Wyss JM, Sripanidkulchai K. The indusium griseum and anterior hippocampal continuation in the rat. J Comp Neurol. 1983;219:251–272. doi: 10.1002/cne.902190302. [DOI] [PubMed] [Google Scholar]
- 22.Adamek GD, Shipley MT, Sanders MS. The indusium griseum in the mouse: Architecture, Timm's histochemistry and some afferent connections. Brain Res Bull. 1984;12:657–668. doi: 10.1016/0361-9230(84)90147-3. [DOI] [PubMed] [Google Scholar]
- 23.Mody I, Kohr G, Otis TS, Staley KJ. The electrophysiology of dentate gyrus granule cells in whole-cell recordings. Epilepsy Res Suppl. 1992;7:159–168. [PubMed] [Google Scholar]
- 24.Leranth C, Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog Brain Res. 2007;163:63–84. doi: 10.1016/S0079-6123(07)63004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartig W, et al. Functional recovery of cholinergic basal forebrain neurons under disease conditions: Old problems, new solutions? Rev Neurosci. 2002;13:95–165. doi: 10.1515/revneuro.2002.13.2.95. [DOI] [PubMed] [Google Scholar]
- 26.Harkany T, et al. Redistribution of CB1 cannabinoid receptors during evolution of cholinergic basal forebrain territories and their cortical projection areas: A comparison between the gray mouse lemur (Microcebus murinus, primates) and rat. Neuroscience. 2005;135:595–609. doi: 10.1016/j.neuroscience.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Emoto N, et al. Basic fibroblast growth factor (FGF) in the central nervous system: Identification of specific loci of basic FGF expression in the rat brain. Growth Factors. 1989;2:21–29. doi: 10.3109/08977198909069078. [DOI] [PubMed] [Google Scholar]
- 28.Castelo-Branco G, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 30.Perret M, Aujard F. Regulation by photoperiod of seasonal changes in body weight and reproductive function in the lesser mouse lemur (Microcebus murinus): Differential responses by sex. Int J Primatol. 2001;22:5–24. [Google Scholar]
- 31.Martin-Ibanez R, et al. Vesicular glutamate transporter 3 (VGLUT3) identifies spatially segregated excitatory terminals in the rat substantia nigra. Eur J Neurosci. 2006;23:1063–1070. doi: 10.1111/j.1460-9568.2006.04601.x. [DOI] [PubMed] [Google Scholar]
- 32.Zilberter M, et al. Input specificity and dependence of spike timing-dependent plasticity on preceding postsynaptic activity at unitary connections between neocortical layer 2/3 pyramidal cells. Cereb Cortex. 2009;19:2308–2320. doi: 10.1093/cercor/bhn247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.