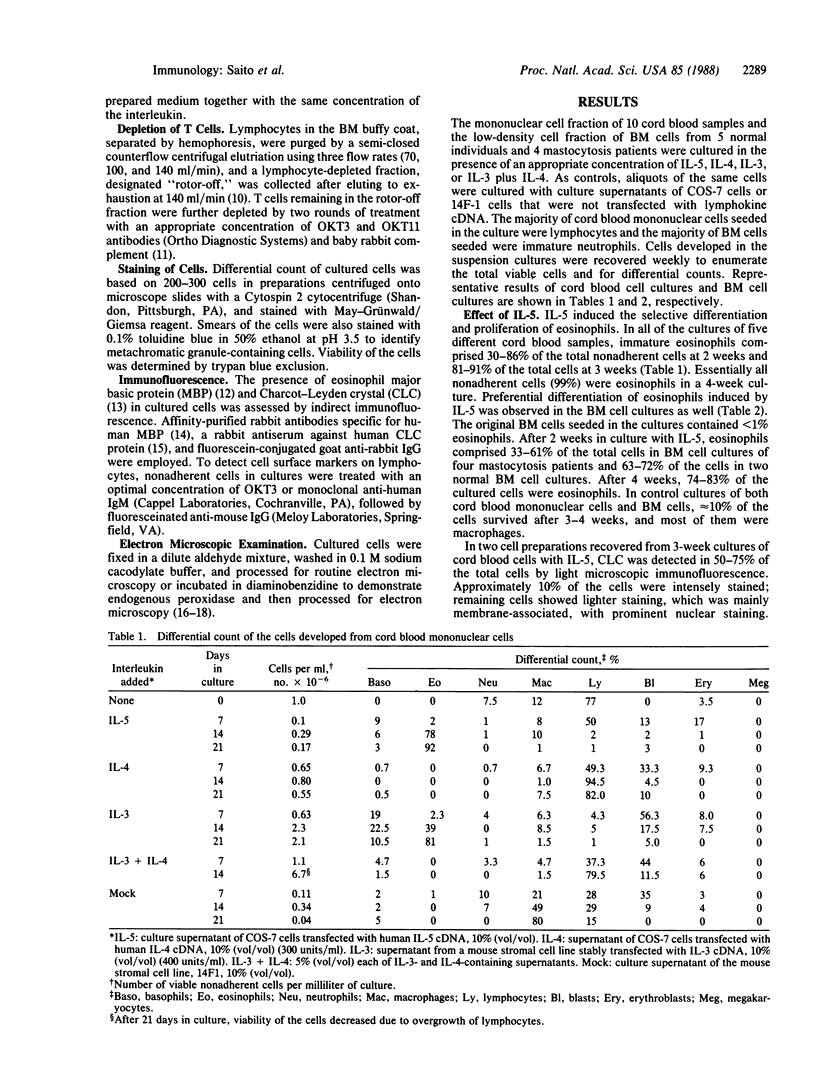
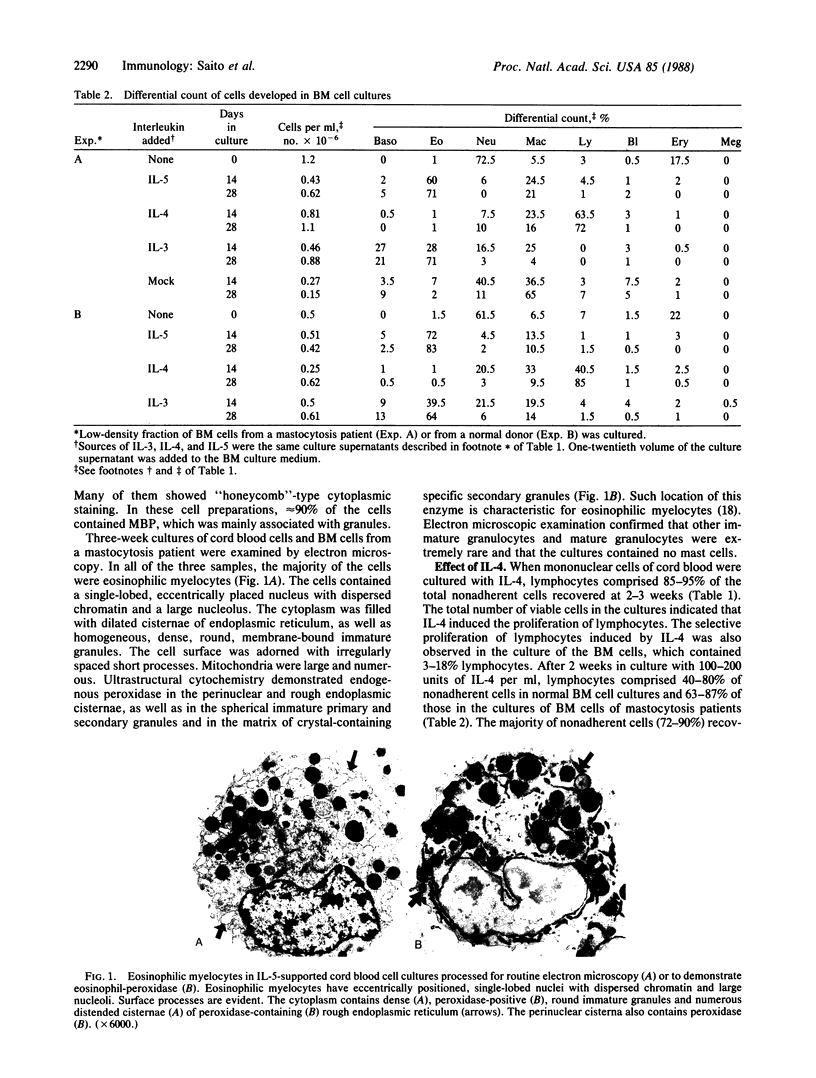
Abstract
Effects of recombinant human interleukins on hematopoiesis were explored by using suspension cultures of mononuclear cells of human umbilical-cord blood and bone marrow. The results showed that interleukin 5 induced the selective differentiation and proliferation of eosinophils. After 3 weeks in culture with interleukin 5, essentially all nonadherent cells in both bone marrow and cord blood cell cultures became eosinophilic myelocytes. Culture of the same cells with interleukin 4 resulted in the selective growth of OKT3+ lymphocytes. However, OKT3+ cells did not develop if the bone marrow cells were depleted of OKT3+/OKT11+ cells prior to the culture, indicating that interleukin 4 induced the proliferation of a subpopulation of resting T cells present in cord blood and bone marrow cell preparations. In suspension cultures of bone marrow cells and cord blood cells grown in the presence of interleukin 3, basophilic, eosinophilic, and neutrophilic myelocytes and macrophages developed within 2 weeks. By 3 weeks, however, the majority of nonadherent cells became eosinophilic myelocytes. In contrast to mouse bone marrow cell cultures, neither interleukin 3 nor a combination of interleukins 3 and 4 induced the differentiation of mast cells in human bone marrow or cord blood cell cultures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. J., Kephart G. M., Habermann T. M., Greipp P. R., Gleich G. J. Localization of eosinophil granule major basic protein in human basophils. J Exp Med. 1983 Sep 1;158(3):946–961. doi: 10.1084/jem.158.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman S. J., Weil G. J., Gleich G. J. Formation of Charcot-Leyden crystals by human basophils. J Exp Med. 1982 Jun 1;155(6):1597–1609. doi: 10.1084/jem.155.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Dvorak A. M., Klebanoff S. J., Henderson W. R., Monahan R. A., Pyne K., Galli S. J. Vesicular uptake of eosinophil peroxidase by guinea pig basophils and by cloned mouse mast cells and granule-containing lymphoid cells. Am J Pathol. 1985 Mar;118(3):425–438. [PMC free article] [PubMed] [Google Scholar]
- Filley W. V., Ackerman S. J., Gleich G. J. An immunofluorescent method for specific staining of eosinophil granule major basic protein. J Immunol Methods. 1981;47(2):227–238. doi: 10.1016/0022-1759(81)90123-x. [DOI] [PubMed] [Google Scholar]
- Gao I. K., Noga S. J., Wagner J. E., Cremo C. A., Davis J., Donnenberg A. D. Implementation of a semiclosed large scale counterflow centrifugal elutriation system. J Clin Apher. 1987;3(3):154–160. doi: 10.1002/jca.2920030305. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Park L. S., Morrissey P. J., Sassenfeld H., Price V., Urdal D. L., Widmer M. B. Regulation of murine T cell proliferation by B cell stimulatory factor-1. J Immunol. 1987 Aug 15;139(4):1148–1153. [PubMed] [Google Scholar]
- Hess A. D., Donnenberg A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. V. Analysis of responding T lymphocyte subpopulations in primary MLR with monoclonal antibodies. J Immunol. 1983 Feb;130(2):717–721. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ishizaka T., Dvorak A. M., Conrad D. H., Niebyl J. R., Marquette J. P., Ishizaka K. Morphologic and immunologic characterization of human basophils developed in cultures of cord blood mononuclear cells. J Immunol. 1985 Jan;134(1):532–540. [PubMed] [Google Scholar]
- Leiferman K. M., Gleich G. J., Kephart G. M., Haugen H. S., Hisamatsu K., Proud D., Lichtenstein L. M., Ackerman S. J. Differences between basophils and mast cells: failure to detect Charcot-Leyden crystal protein (lysophospholipase) and eosinophil granule major basic protein in human mast cells. J Immunol. 1986 Feb 1;136(3):852–855. [PubMed] [Google Scholar]
- Ogawa M., Nakahata T., Leary A. G., Sterk A. R., Ishizaka K., Ishizaka T. Suspension culture of human mast cells/basophils from umbilical cord blood mononuclear cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4494–4498. doi: 10.1073/pnas.80.14.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Saito H., Okajima F., Molski T. F., Sha'afi R. I., Ui M., Ishizaka T. Effects of ADP-ribosylation of GTP-binding protein by pertussis toxin on immunoglobulin E-dependent and -independent histamine release from mast cells and basophils. J Immunol. 1987 Jun 1;138(11):3927–3934. [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Tadokoro K., Stadler B. M., De Weck A. L. Factor-dependent in vitro growth of human normal bone marrow-derived basophil-like cells. J Exp Med. 1983 Sep 1;158(3):857–871. doi: 10.1084/jem.158.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D., Tamir M., Toledo J., Oren T. Differentiation stage and lineage-specific inhibitor from the stroma of mouse bone marrow that restricts lymphoma cell growth. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4547–4551. doi: 10.1073/pnas.83.12.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]