Abstract
NVP-BEZ235 is a dual PI3K/mTOR inhibitor currently in phase I clinical trials. We profiled this compound against a panel of breast tumor cell lines to identify the patient populations that would benefit from such treatment. In this setting, NVP-BEZ235 selectively induced cell death in cell lines presenting either HER2 amplification and/or PIK3CA mutation, but not in cell lines with PTEN loss of function or KRAS mutations, for which resistance could be attributed, in part to ERK pathway activity. An in depth analysis of death markers revealed that the cell death observed upon NVP-BEZ235 treatment could be recapitulated with other PI3K inhibitors and that this event is linked to active PARP cleavage indicative of an apoptotic process. Moreover, the effect seemed to be partly independent of the caspase-9 executioner and mitochondrial activated caspases, suggesting an alternate route for apoptosis induction by PI3K inhibitors. Overall, this study will provide guidance for patient stratification for forthcoming breast cancer phase II trials for NVP-BEZ235.
Keywords: kinase inhibitor, small molecule, PI3K, breat cancer, mTOR
The PI3K pathway has long been known as an important growth and survival pathway of the cell, and pathway activation is frequently found in human cancers. Such activation can be the result of a variety of genetic and epigenetic abnormalities (1). A number of activating mutations conferring oncogenic potential has been found in the PIK3CA gene itself, which encodes the PI3K catalytic subunit p110α (2, 3). The gene encoding PTEN is often deleted, mutated, or silenced in tumors, which results in PI3K pathway activation (4). Another mechanism of constitutively activating PI3K is by pathway alterations upstream of the kinase. In HER2 amplified breast cancer, ErbB3, which is responsible for PI3K recruitment and activation has been shown to strongly potentiate HER2 oncogenic potential (5, 6). The K-Ras oncoprotein, which is mutated and activated in 20% of all human tumors, also possesses the ability to interact and recruit PI3K to the plasma membrane. It was recently reported that mice coexpressing oncogenic mutant K-Ras (V12D) and a mutant form of PI3Kα unable to interact with K-Ras in lieu of the endogenous PI3Kα protein, would not develop lung tumors (7).
Targeted therapies against specific components of this pathway are expected to be efficacious as single agents or in combination in a variety of human cancers. Hence, the identification and characterization of PI3K pathway inhibitors with good drug-like properties were eagerly awaited to initiate clinical trials (8). NVP-BEZ235 is a dual PI3K/mTOR inhibitor that has proven its ability to significantly reduce the tumor growth of a number of human xenograft models (9–11), as well as of a murine PIK3CA driven lung tumor model (12). However, it is still unclear if compounds with a dual inhibitory profile would be equally efficacious in cancers with distinct genetic lesions in the PIK3CA, PTEN, HER2, or KRAS genes.
To predict the response of breast tumors with distinct genetic alterations toward NVP-BEZ235 in man, breast preclinical models bearing distinct genetic abnormalities that could lead to PI3K activation were used to profile NVP-BEZ235. The data show that the anti-proliferative activity of NVP-BEZ235 in vitro cannot be used as predictor of response. However, sensitive lines could be distinguished from the insensitive ones by the ability of the compound to induce cell death through an apoptotic mechanism. Responders were harboring either PIK3CA mutations or HER2 amplification or both. In depth characterization of the apoptotic responses induced by NVP-BEZ235 suggested activation of the extrinsic pathway through caspase-2. Interestingly, the presence of KRAS but also PTEN mutations did not trigger sensitivity to BEZ235. In both cases, ERK pathway activation seemed to be responsible for the lack of response, by bypassing the canonical RPS6 activation through mTORC1. The data presented here suggest tailored proof-of-principle for NVP-BEZ235 in breast cancer presenting either HER2 amplification or PIK3CA amplification, or both. Furthermore, this study provides an experimental basis for the examination of other cell lines representative of different indications for phase II studies in man.
Results
NVP-BEZ235 Induces Apoptosis in a Subset of Breast Cancer Cell Lines.
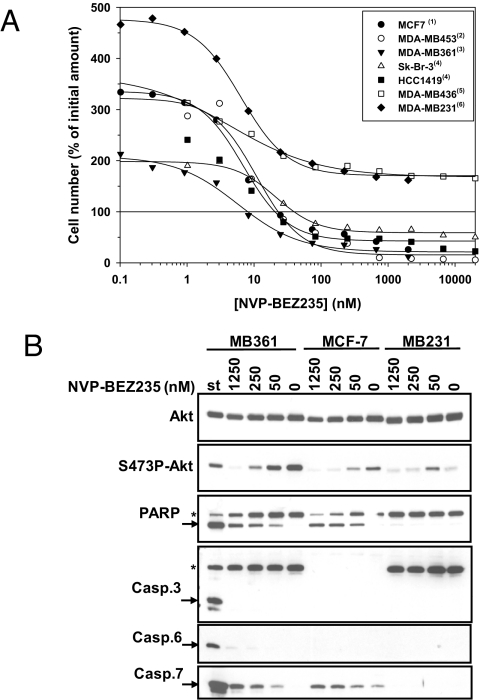
The anti-proliferative activity of NVP-BEZ235 was tested in a panel of 18 breast cell lines (Table S1). The calculated GI50 values were all in the low nanomolar range, underlining that NVP-BEZ235-induced growth inhibition in 2-D settings is not amenable for stratification prediction. However, a careful examination of the anti-proliferative curves (Fig. S1A) revealed that the cell lines could be divided into two categories: group A (e.g., MDA-MB453, MDA-MB361, Sk-Br-3, HCC1419, and MCF7) where NVP-BEZ235 treatment caused cell death as revealed by the reduction of cell number below the initial amount (below 0% difference vs. control) present at drug treatment start allowing calculation of lethal dose 50 (LD50) values; group B (e.g., MDA-MB231 and MDA-MB468), where the drug only led to proliferation inhibition (Fig. 1A and Table S1). All group A cell lines are characterized by the presence of either a PIK3CA (significantly associated with cell death, P = 0.038, Fisher exact test) activating mutation alone (n = 2 out of 2), the amplification of the HER2 gene in the presence of a PIK3CA activating mutation (n = 4 out of 4) or the amplification of the HER2 gene alone (n = 4 out of 4, significantly associated with cell death, P = 0.05, Fisher exact test). With the exception of BT-549, all of the PTEN deleted, mutated or silenced cell lines (n = 5 out of 6) and the K-Ras/B-Raf mutated cell line MDA-MB231 fall into group B (significantly associated with no cell death for PTEN, P = 0.013, Fisher exact test). Concomitant to the expected dose-dependent inhibition of S473P-Akt levels, NVP-BEZ235 induced efficient PARP cleavage in MDA-MB361 and MCF-7, but not in MDA-MB231 cells (Fig. 1B), at the lowest concentration tested (50 nM), roughly corresponding to the determined LD50 values. MCF-7 cells do not express caspase-3, and its cleaved form was not detected in MDA-MB361 cells. Similarly, caspase-6 seemed to be weakly activated in presence of the compound in both cell lines. However, a strong increase in cleaved caspase-7 was observed in both models. A 72-h time-course analysis with NVP-BEZ235 revealed that efficient PARP and caspase-7 cleavages could be observed as early as 24 h of treatment, in both MCF7 and MDA-MB361 cell lines (Fig. S1B). Furthermore, cotreatment with the pan-caspase inhibitor Z-VAD resulted in a complete blockade of PARP cleavage (Fig. S1C). Altogether, these data show that NVP-BEZ235 rapidly induces apoptosis in the group-A, but not in the group-B cell lines tested. NVP-BEZ235 induced apoptosis is most likely mediated by caspase-7 activation.
Fig. 1.
NVP-BEZ235 induces cell death through induction of apoptosis in a subset of breast cancer cell lines. (A) The mentioned cell lines were incubated with increasing concentrations of NVP-BEZ235 for a period of 72 h. Then, the cells were fixed and the effect on viability was recorded and plotted as a percentage of the number of cells present at the treatment start (100% straight line). All points beyond this line are indicative of cell death. (B) MDA-MB361, MCF-7 and MDA-MB231 cells were incubated either for 48 h with the indicated amount (in nM) of NVP-BEZ235 or for 3 h with 1 μM of staurosporine (st). The corresponding cell extracts were then analyzed by Western blot for the indicated markers. Legend: *, uncleaved form; arrow, cleaved form; (1), PIK3CA mut.; (2), HER2 amp./PIK3CA-H1047R; (3), HER2 amp./PIK3CA-E545K; (4), HER2 amp; (5), PTEN alterations; (6), KRAS mut. This legend applies to all of the Figures.
NVP-BEZ235 Induces Apoptosis More Potently than Other Modulators of the PI3K Pathway.
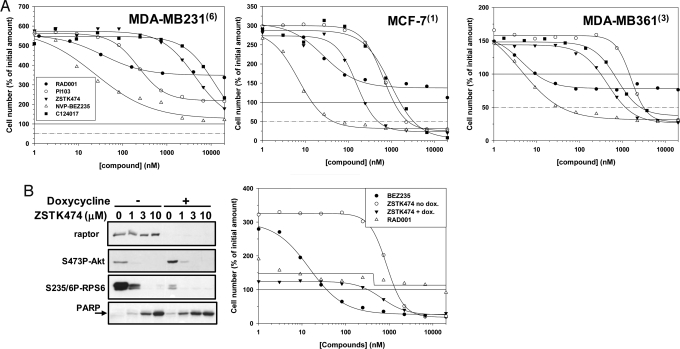
To better understand and refine the inhibitory profile responsible for the apoptosis induction, the activity of mTORC1 (RAD001), Akt (C124017), and PI3K (PI103 and ZSTK474) inhibitors (Table S2) on cell proliferation and survival were compared in the same cellular models. None of the compounds were able to induce cell death in the NVP-BEZ235 insensitive lines MDA-MB231 (Fig. 2A) and MDA-MB436 (Fig. S2A). When compared to NVP-BEZ235, PI103, ZSTK474, and C124017 were found to have reduced anti-proliferative activities as indicated by the 2 log right shift observed on the fit curves. However, although less potently than NVP-BEZ235, the three compounds were able to induce cell death in MCF-7 (Fig. 2A, LD50 of 2,446, 490, and 3,267 nM, respectively), MDA-MB361 (Fig. 2A, LD50 of 3,132, 1,180, and 2,647 nM, respectively), MDA-MB453 (Fig. S2A, LD50 of 575 and 1,547, respectively, not determined for C124017), HCC1419, EFM192A, and MDA-MB175-VII cells (Fig. S2A, LD50 for ZSTK474 only of 3,788, 3,667, and 3,517 nM, respectively).
Fig. 2.
Antiproliferative and cell death induction activities of PI3K, Akt, and mTORC1 inhibitors in breast cancer cell lines. (A) MDA-MB231 (Left), MCF-7 (Center), and MDA-MB361 (Right) cells were incubated with increasing concentrations of either RAD001, PI-103, ZSTK474, NVP-BEZ235, or C124017 for a period of 72 h. Cells were then fixed and effect on viability plotted as described. The dashed line represents the concentration for which the compound is able to kill 50% of the cells (LD50 values). (B) Raptor protein knock-down was induced in the HCC1954(3) cell line by the addition of doxycycline (200 ng/mL) to the growth media. Seventy-two hours later, the cells were analyzed for the effects on proliferation (Right) and signaling events (Left), either in absence or presence of the indicated amounts of the PI3K inhibitor ZSTK474.
In contrast, the mTORC1 allosteric inhibitor RAD001 was found to profoundly inhibit cell proliferation but had no or limited effect on cell survival (Fig. 2A and Fig. S2A, LD50 > 20 μM for all cell lines). Doxycycline-induced expression of an shRNA directed against raptor, the essential mTORC1 component resulted in efficient knock-down of raptor protein in the NVP-BEZ235 sensitive cell line HCC1954. This led, as expected, in an increase of S473P-Akt levels, a decrease of S235/6-RPS6 levels, and to a modest increase in PARP cleavage (Fig. 2D Left, compare 0 lines in presence or absence of doxycycline) and to a strong (circa 3-fold) reduction in cell proliferation (325 to 120%, Fig. 2D Right). Moreover, raptor knock-down had a minor effect on ZSTK474 induced cell death, as indicated by the slight increase in PARP cleavage and to the minor fit curve and LD50 value (from 2,341 to 1,369 nM) shift observed when the cells were exposed to doxycycle. Similar results were obtained upon raptor knockdown in MDA-MB361 cells (Fig. S2B). Overall, these data suggest that PI3K and Akt inhibition are key components of cell death induction by NVP-BEZ235, and that mTORC2 more than mTORC1 is likely involved in this phenomenon.
NVP-BEZ235 and PI3K Inhibitor Induced Apoptosis Is in Part Caspase-9 Independent.
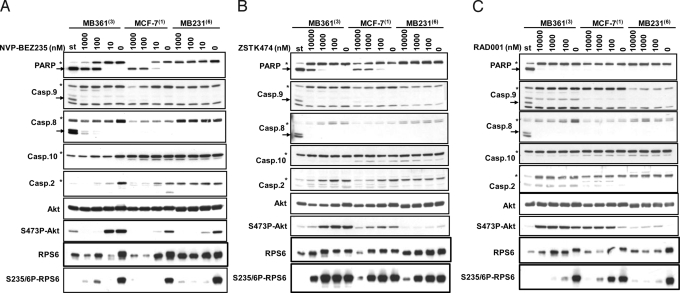
In MCF-7 and MDA-MB361 cells, at concentrations of NVP-NVP-BEZ235 resulting in pathway modulation and PARP cleavage (100 nM and above), caspase-9, -8, and -10 were not cleaved (Fig. 3A). However, NVP-BEZ235 provoked in a dose-dependent manner an almost complete disappearance of caspase-2, and appearance of its cleaved form (Fig. S3A). A similar phenomenon was observed in HCC1419, EFM192A, MDA-MB175-VII, Sk-Br-3 (Fig. S3B), and MDA-MD453 (Fig. S3C) group-A cell lines, but not in the MDA-MB231 (Fig. 3A) and MDA-MB436 (Fig. S3C) group-B cell lines. Similar effects were observed, starting at higher concentrations (1,000 nM and above), for the PI3K inhibitors ZSTK474 (Fig. 3B and Fig. S3C) and PI-103 (Fig. S3 C and D), but not for the mTORC1 inhibitor RAD001 (Fig. 3C and Fig. S3C). As seen for the parental cells, NVP-BEZ235 (LD50 of 133 and 37 nM, respectively), ZSTK474 (LD50 of 677 and 1,293 nM, respectively), PI-103(LD50 of 3,763 and 2,680 nM, respectively) and CS124017 (LD50 of 6,799 and 4,572 nM, respectively) were able to induce cell death (Fig. S4B) in MCF-7- and MDA-MB361-Bcl2 over-expressing cell lines (Fig. S4A) with similar potency. Moreover, in these two chimeric cell lines, NVP-BEZ235 efficiently reduced S473P-Akt levels as well as it induced PARP and caspase-2 cleavage, in a dose-dependent manner (Fig. S4C). Overall, these data suggests that NVP-BEZ235 and other PI3K inhibitors could induce apoptosis by a mechanism that seems to be independent of caspase-9 activation. Rather, the extrinsic caspase-2 seems to be the executioner caspase activated upon PI3K pathway inhibition.
Fig. 3.
Apoptosis induction by the PI3K inhibitors involves the executioner caspase. Caspase-2. (A–C). MDA-MB361, MCF-7, and MDA-MB231 cells were incubated either for 48 h with the indicated amount (in nM) of NVP-BEZ235 (A), ZSTK474 (B), or RAD001 (C) for 48 or 3 h with 1 μM of staurosporine (st). The corresponding cell extracts were then analyzed by Western blot for the indicated markers.
Effects of NVP-BEZ235 in HER2 Amplified Cells Are Distinct from HER2 Inhibitors.
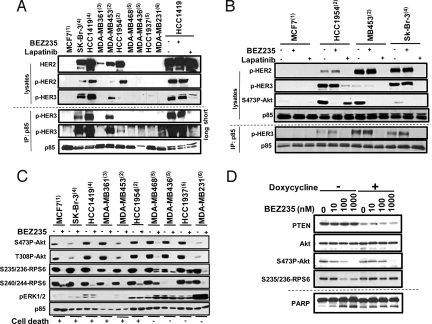
HER2 amplified breast cancer cells rely on HER3 for PI3K recruitment and activation. Indeed, in all high HER2 expressers (Sk-Br-3, HCC1419, MB453, HCC1954), p85 immunoprecipitation showed recruitment of PI3K to HER3. This was not the case for the PIK3CA mutant only MCF7 cell line, for the PTEN null MDA-MB468, MDA-MB436, and HCC1937 cell lines nor for the K-RAS mutant MDA-MB231 cell line and neither for the low HER2 expresser MDA-MB361 (Fig. 4A). Treatment of HER2 amplified cells with NVP-BEZ235 did not reduce PI3K recruitment to HER3, while treatment with the HER1/2 inhibitor lapatinib completely disrupted this interaction (Fig. 4B and Fig. S5A). While NVP-BEZ235 treatment led to efficient Akt inhibition in all models tested, lapatinib could only efficiently inhibit Akt activity if PI3K was recruited strongly to HER3. These data suggest that NVP-BEZ235 efficiently inhibits the PI3K pathway in breast cancer cell lines containing either PIK3CA mutations, HER2 amplification or both. In contrast, the response to lapatinib is dependent on the extent of PI3K recruitment to HER2/HER3 heterodimers and this is highly correlated to HER3 phosphorylation levels.
Fig. 4.
NVP-BEZ235 activities in HER2 amplified and PTEN deficient breast cancer lines. (A) Extracts from the indicated cell lines were pretreated for 1 h (HCC1419 only) or not (all others) with either NVP-BEZ235 or lapatinib (1 μM) and analyzed either directly (lysates) or indirectly after immune-precipitation with anti-p85 immobilized beads (IP: p85) for their HER2, pHER2, and pHER3 levels. (B) Same as in A, but all of the cell lines were treated for 1 h with either NVP-BEZ235 or lapatinib (1 μM). (C) The indicated cell lines were pretreated with 1 μM of NVP-BEZ235 for 1 h, and the corresponding cell extracts analyzed for the indicated proteins. (D) PTEN knock-down was induced (+) or not (−) in the HCC1954 cell line stably expressing inducible shRNA hairpins against PTEN by addition of doxycycline (200 ng/mL) to the culture media. Seventy-two hours later, NVP-BEZ235 (1 μM) was added to the media for either 1 or 24 h. Then, the corresponding cell extracts were analyzed for PTEN, Akt and activated Akt (1-h time points), and cleaved PARP levels (72-h time points).
PTEN Null Breast Cancer Cells Are Insensitive to NVP-BEZ235 Treatment by Providing ERK Signaling to RPS6.
To better understand the mechanisms by which K-RAS mutant and PTEN null cells remain insensitive to NVP-BEZ235 treatment, a careful examination of both PI3K (Akt) and mTORC1 (RPS6) downstream effectors was performed in this panel of breast cancer lines. As expected, we observed an efficient abrogation of both S473- and T308-Akt phospho-levels upon NVP-BEZ235 treatment in all cell lines (Fig. 4C). However, a persistent S235/236P-RPS6 signal was detected in the insensitive lines. Moreover, detectable signals could still be monitored even upon 24 h of drug exposure (Fig. S5B). All of the PTEN null lines tested also presented higher activated ERK levels (pERK). To check whether the loss of PTEN expression could be responsible for the enhancement of active ERK and RPS6 levels, the HCC1954 cell line was used to generate pools allowing inducible expression of small hairpins directed against the endogenous human protein (13). Upon doxycycline treatment, efficient reduction in PTEN expression was observed (Fig. 4D). Cotreatment with NVP-BEZ235 resulted in an almost complete protection of pRPS6 levels, even at the higher dose tested (1 μM), for which complete inhibition of S473P-Akt is monitored. Moreover, PTEN reduction also caused protection from NVP-BEZ235 induced PARP cleavage. To check whether the residual pRPS6 levels in the insensitive cell lines was the result of ERK pathway activation, MDA-MB468 and MDA-MB436 were treated with a MEK inhibitor either alone or in presence of NVP-BEZ235 (Fig. S5B). Indeed, cotreatment resulted in a complete inhibition of S235/6-RPS6 levels, in both cell models. Altogether, these data suggest that the lack of PTEN in breast cancer lines might result in redundant phosphorylation of the mTORC1 downstream effecter RPS6 by the ERK pathway.
Efficient Cell Death Induction Translates in Strong Antitumor Activity in Vivo.
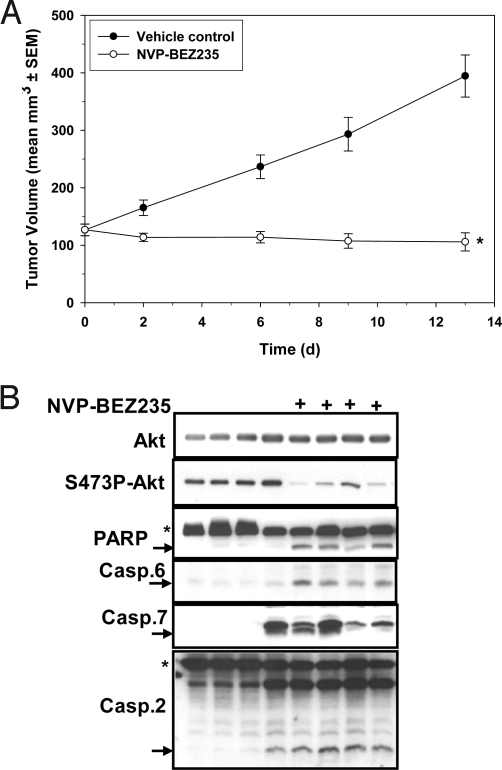
MDA-MB361, MCF-7, and MDA-MB453 cells were injected orthotopically into immunodeficient mice, and efficacy studies were started when the tumors reached the log linear growth phase. For all three models, NVP-BEZ235 was able to induce tumor shrinkage (MDA-MB361, Fig. 5A, T/C = −16.5%; MCF-7, Fig. S6A, T/C = −21%; MDA-MB453, T/C = −15%, Fig. S6B). The anti-VEGFR2 antibody DC101 (14) was almost completely inactive against MDA-MB361 tumors (T/C >80%, Fig. S6C) ruling out the possibility that NVP-BEZ235 induced antitumor effect is dominantly due to its anti-angiogenic properties (15). Analysis of MDA-MB361 tumor extracts from mice treated or not with NVP-BEZ235, revealed that the compound was able to efficiently reduce S473P-Akt signaling, and to induce PARP, caspase-7, and caspase-2 cleavage (Fig. 5B and Fig. S6D). These data suggest that in these breast models, NVP-BEZ235 treatment leads to tumor regression, through an active apoptosis induction process.
Fig. 5.
NVP-BEZ235 has antitumor activity against MDA-MB361, tumors, and induces apoptosis in vivo. Female Harlan nude mice bearing orthotopic MDA-MB361 tumors were treated p.o., once per day, either with 45 mg/kg of NVP-BEZ235 or with the vehicle control. Tumor volumes were recorded during the treatment period and T/C values were calculated. (B) One hour after the last dose, MDA-MB361 tumors from the efficacy study (A) were excised and tumor extracts analyzed by Western blotting for the level of expression of the indicated proteins.
Discussion
The observation that constitutive PI3K pathway activation is commonly observed in tumors has triggered the development of several PI3K pathway inhibitors. NVP-BEZ235 is a dual PI3K/mTOR inhibitor, which was previously shown to display strong antiproliferative and antitumoral activity (9). The scope of this study was to identify those cancers that would maximally respond to NVP-BEZ235 treatment. As described for example for MEK inhibitors, simple antiproliferative assays are sufficient to discriminate between sensitive and insensitive subsets in vitro (16, 17). In contrast, for NVP-BEZ235, all tested cell lines responded similarly in the conditions used, precluding any stratification. In contrast, the GI50s recently reported for the mTORC1 allosteric inhibitor RAD001 range between 0.55 to 4125 nM (18, 19), suggesting that mTORC2 and PI3K inhibition are likely also involved in the antiproliferative activity of NVP-BEZ235. Consistent with this, the catalytic mTOR inhibitor PP242 was found to cause a more profound proliferation blockade than rapamycin (20).
NVP-BEZ235 treatment results in differential effects on cell viability in a panel of breast cancer cell lines. HER2 and PIK3CA status were positively correlated with the cell death induction property and antitumor activity of NVP-BEZ235, as reported with allosteric Akt inhibitor (21). Here, we show that NVP-BEZ235 efficiently shuts down the PI3K pathway in HER2 amplified cells, and does not cause the disruption of the upstream HER2/HER3 complex. Hence, PI3K inhibitors are expected to display synergistic activities with anti-HER2/HER3 agents in HER2 amplified tumors. Data emphasizing this were recently disclosed (22, 23). In the panel of breast cancer cells that we used, PIK3CA mutations were associated with the luminal lineage and there they often co-occurred with HER2 amplification, whereas most of the PTEN mutations tend to occur in the basal-like lineage. It was recently reported that basal-like breast cancer lines have an activated RAS-like transcriptional profile independent of their K-Ras/B-Raf mutation status (24). We have shown here that at least in three PTEN loss-of-function lines, ERK was higher phosphorylated in comparison to luminal cells. One possible conclusion would be that PTEN loss of function can result in simultaneous PI3K and Ras pathway activation. However, to date the mechanism how this might happen is not understood and requires further investigation. PTEN′s lipid phosphatase function is very well described and associated with its tumor suppressor function, but other activities have been reported and those might also play a role for its tumor suppressing function. For instance, PTEN also exists as a nuclear protein (25) and is involved in chromosomal stability and DNA-repair, notably through interaction with the centromere-specific binding protein C (CENP-C) (26). In this context, the absence of PTEN might have consequences in blocking p53-dependent apoptotic phenomena, in genomically unstable tumor cells. Moreover, other evidences exist that PTEN might positively and directly regulate apoptosis, when localized either in the cytosol or in the nucleus (27, 28). Further studies would be needed to delineate the PI3K independent prosurvival molecules involved in the insensitive cell lines.
We have shown that the cell killing induced by NVP-BEZ235 is principally mediated by an active apoptotic process that seems to preferentially require the activation of the initiator caspase-2, whereas the intrinsic mitochondrial and caspase-9 route seems to be dispensable. The regulation of caspase-2 is still not completely understood. A short (Casp-2S) and a long form (Casp-2L) coexist in cells, due to the activity of two different upstream promoters and alternative splicing (29). Functional assays have identified Casp-2L and CASP-2S as positive and negative regulator of apoptosis, respectively (30, 31). The precise mechanism for caspase-2 (Casp-2L) activation is still a matter of debate (32), but seems to require, at least in response to genotoxic stress, the interaction with the PIDD and RAID proteins, in a complex called the PIDDosome (33–35). Interestingly, caspase-2 has been shown to be responsible for mediating the apoptotic response upon gamma-irradiation in a mitochondrial- and p53-independent manner, and this effect required the activity of the class IV PI3Ks ATM and ATR (36). A similar scenario might apply in the sensitive breast cancer cell lines but the interplay between PI3K/mTOR and caspase-2 remains to be determined.
In conclusion, this study has allowed the identification of breast cancer with HER2 and/or PIK3CA mutations as the diseases for which NVP-BEZ235 treatment might have the most favorable prognosis. This provides a strong rationale for phase II studies using NVP-BEZ235 alone or in combination with the anti-HER2 therapy Trastuzumab (Herceptin). Breast cancer patients with mutated PTEN should be avoided in NVP-BEZ235 single therapy trials, and might also benefit from a combination with a MEK inhibitor. A retrospective analysis of genetic biomarkers and clinical efficacy in patients enrolled in phase I with the various PI3K inhibitors is eagerly awaited as this will certainly provide a good basis to confirm or infirm the predictability of the approach described in this study.
Materials and Methods
Chemical Compounds, Biologics, and Preparation.
NVP-BEZ235 (Novartis), RAD001 (Novartis), Staurosporine (Alexis Biochemicals), ZSTK474 (Alexis Biochemicals), PI-103 (Axon Medchem), and the Akt inhibitor VIII (Merck Calbiochem) and Z-VAD (Bachem), and Doxycyclin (Sigma) were all prepared as a 10 mM stock solution, except Z-VAD (200 mM) in 100% DMSO and Doxycyclin (1 mg/mL) in water. Working solutions were prepared freshly by dilution in 100% DMSO prior addition to the cell media (see Antiproliferation and Cell Death Assays). For in vivo studies, NVP-BEZ235 (tosylate salt) was formulated in NMP/PEG300 (10/90, V/V). Solutions (5 mg/mL) were prepared fresh each day of dosing as follow: the powder was dissolved in NMP, upon sonication; then the remaining volume of PEG300 was added. The application volume was 10 mL/kg.
Cell Lines and Cell Culture.
The cell lines were purchased from ATCC except of PC3M (J. Fiedler, MD Anderson Cancer Center), U87MG, and LN229 (A. Merlo, University of Basel), BT474, Sk-Br-3, T47D, MDA-MB453, and AU565 (N. Hynes, Friedrich Miescher Institute), GTL-16 was from (Comoglio and Christina-Stella, Institute for Cancer Research), and were cultured at 37 °C in a 5% CO2 and 80% relative humidity atmosphere incubator, in DMEM high glucose (Gibco) complemented with 10% FBS, 2 mM glutamine, 1% penicillin-streptomycine, and 1% sodium-pyruvate. The MCF-7- and MDA-MB361-HA-Bcl2 cell lines were generated by transfecting the parental cell line with pcDNA3.1-HA-Bcl2 expression vector (details on the plasmid are available on request). Pools were selected and enriched by addition of 0.5 mg/mL Geneticin (Gibco) in the culture medium. HCC1954 expressing doxycycline inducible shRNA hairpins (sequences are available on request) targeting raptor or PTEN were established using the pLKO-Tet-ON lentiviral system as described in ref. 37.
Antiproliferation and Cell Death Assays.
Antiproliferative assays were as described in ref. 9. To determine the cell death activities, a similar approach was used with the exception that the cells were seeded at a 10,000–16,000 cells per well density. The resulting values were expressed as a percentage of the original absorbance (100%), at the time of incubation with NVP-BEZ235. The fitted curves were then used to determine the concentration of the compound necessary to reduce the cell number by 50% or LD50.
Biochemical Characterization of Apoptotic Markers.
Two million cells were seeded in a 10-cm dish. Eighteen hours later the medium was discarded and replaced with 10 mL of fresh medium containing the test items (by diluting the DMSO working solutions by 1,000-fold, for a final and constant DMSO concentration of 0.1%), at the concentration indicated in the figures (otherwise at a concentration of 1 μM for the PI3K inhibitors, and 200 μM for Z-VAD). Unless specified in the legend of the figures, the incubation period was 48 h, except for staurosporine (3 h). Then, the cells were washed twice, lysed and processed by Western blot analysis as described in ref. 9. Immunoprecipitation procedures and antibodies are described in SI Materials and Methods.
Establishment of Xenograft Tumors, Efficacy Studies, Tumor Lysates, and Analytics.
Establishment of tumors, group randomization tumor, and body weight recording during efficacy studies were described elsewhere (9). All experimental procedures (approved by the Kantonales Veterinäramt Basel-Stadt under license no. 1769 and 1979) strictly adhered to the Eidgenössisches Tierschutzgesetz and the Eidgenössische Tierschutzverordnung.
Supplementary Material
Acknowledgments.
We thank Marc Hattenberger, Fabian Stauffer, Juliane Vaxelaire, Malika, Kazic, Gregory Marszalek, Vincent Romanet and Matthieu Klopfenstein for excellent technical assistance; Dr. David Kwiatkowski (Brigham and Women's Hospital, Boston, MA) for the TSC1/2 null MEFs; Dr. Tobias Schmelzle (Novartis Institutes for BioMedical Research Oncology, Basel, Switzerland) for the pLKO-Tet-ON-raptor construct; and Dr Frank Stegmeier (Novartis Institutes for BioMedical Research Oncology, Cambridge, MA) for the pLKO-Tet-ON-PTEN construct.
Footnotes
The authors declare a conflict of interest. S.M.B., C.S., C.F., S. Wang, C.G.-E. and S.-M.M. are employees of Novartis Pharma. S. Wee is an employee of Bristol-Myers Squibb. H.L. is an employee of Basilea Pharmaceuticals
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905152106/DCSupplemental.
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiles BL. Phosphatase and tensin homologue deleted on chromosome 10: Extending its PTENtacles. Int J Biochem Cell Biol. 2009;41:757–761. doi: 10.1016/j.biocel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alimandi M, et al. Cooperative signaling of erbb3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 6.Lee-Hoeflich ST, et al. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, et al. Binding of Ras to phosphoinositide 3-kinase p110 is required for Ras-driven tumorigenesis in Mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K//Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 9.Maira SM, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 10.Serra V, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 11.Cao P, Maira SM, Garcia-Echeverria C, Hedley DW. Activity of a novel, dual PI3-kinase/mTor inhibitor NVP-BEZ235 against primary human pancreatic cancers grown as orthotopic xenografts. Br J Cancer. 2009;100:1267–1276. doi: 10.1038/sj.bjc.6604995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant KRas G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee S, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4289–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- 14.Prewett M, et al. Antivascular endothelial growth factor receptor (Fetal Liver Kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 15.Schnell CR, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: Implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 16.Solit DB, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratilas CA, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res. 2008;68:9375–9383. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane HA, et al. mTOR Inhibitor RAD001 (Everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 19.Breuleux M, et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8:742–753. doi: 10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PloS Biology. 2009;7:371–383. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.She QB, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Junttila T, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Yao E, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combination of GDC-0941 PI3K inhibitor, Trastuzumab, and Pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- 24.Hoeflich KP, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 25.Trotman LC, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Gil A, et al. Nuclear localization of PTEN by a ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 2006;17:4002–4013. doi: 10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 29.Logette E, et al. The human caspase-2 gene: Alternative promoters, pre-mRNA splicing and AUG usage direct isoform-specific expression. Oncogene. 2001;22:935–946. doi: 10.1038/sj.onc.1206172. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 31.Droin N, et al. Modulation of apoptosis by procaspase-2 short isoform: Selective inhibition of chromatin condensation, apoptotic body formation, and phosphatidylserine externalization. Oncogene. 2001;20:260–269. doi: 10.1038/sj.onc.1204066. [DOI] [PubMed] [Google Scholar]
- 32.Krumschnabel G, Sohm B, Bock F, Manzl C, Villunger A. The enigma of caspase-2: The laymen's view. Cell Death Differ. 2008;16:195–207. doi: 10.1038/cdd.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 34.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappa-B activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Park HH, et al. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell. 2007;128:533–546. doi: 10.1016/j.cell.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidi S, et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and Caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiederschain D, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.