Abstract
It has been demonstrated that nonintegrating lentiviral vectors (NILVs) are efficient in maintaining transgene expression in vitro and in vivo. Gene delivery by NILVs can significantly reduce nonspecific vector integration, which has been shown to cause malignant transformation in patients receiving gene therapy for X-linked severe combined immunodeficiency. Strong and sustained immune responses were observed after a single immunization with NILVs carrying viral antigens. However, there is no report to date that evaluates the efficacy of NILVs in inducing antigen-specific antitumor immunity. Using a well-characterized tumor model, we tested in vivo immunization with a self-inactivating lentiviral vector harboring a defective integrase. A high frequency of ovalbumin peptide (OVAp1)-specific CD8+ T cells and a substantial antibody response were detected in naive mice immunized with an NILV encoding an OVA transgene. Furthermore, this immunization method completely protected the mice against the growth of E.G7 tumor cells expressing the OVA antigen. Thus, this study provides evidence that immunization using NILVs can be a safe and promising approach for exploring cancer immunotherapy.
Introduction
Since the first report of the successful generation of a lentiviral vector derived from human immunodeficiency virus (HIV)-1 (Naldini et al., 1996), lentiviral vectors have become not only a research tool for the study of basic biology, but also a potent delivery vehicle for the development of novel gene therapeutics (Vigna and Naldini, 2000; Verma and Weitzman, 2005; Kohn, 2007). One attractive feature of lentiviral vectors, as compared with other retrovirus-based vectors, is their ability to transduce nondividing cells (Naldini et al., 1996). It had been demonstrated that lentiviral vectors can mediate the integration of transgenes into the genome of target cells, thereby maintaining long-term stable expression of transgenes in a wide range of cell types, including dendritic cells (DCs) (Dullaers and Thielemans, 2006; Breckpot et al., 2007). It is of great interest to genetically modify DCs for cancer immunotherapy because these cells are the most powerful antigen-presenting cells (APCs) and are directly involved in initiating, programming, and regulating tumor-specific immune responses (Steinman and Banchereau, 2007; Melief, 2008). Lentiviral vectors were found to be extremely efficient in transducing human and mouse DCs to express tumor antigens in vitro without affecting their antigen presentation function. In addition, immunization with these in vitro-transduced DCs could induce a sufficient antitumor immune response to tumor challenges in mouse models (Breckpot et al., 2007; He et al., 2007).
Given the labor-intensive nature of in vitro manipulation of DCs for immunization, there is growing interest in the direct administration of lentiviral vectors via subcutaneous or intravenous injection to target APCs, including DCs, to elicit encoded antigen-specific immunity (Vandendriessche et al., 2002; Esslinger et al., 2003; Palmowski et al., 2004; Kim et al., 2005; Dullaers et al., 2006; Rowe et al., 2006; Iglesias et al., 2007; Lopes et al., 2008). It was clear from these studies that DCs in spleens and/or draining lymph nodes could be targeted by vector injection, presumably because of the nature of lentiviral vectors as being capable of transducing nondividing and resting cell types. Induction of vigorous tumor-specific T cell immunity by direct lentiviral vector immunization was demonstrated with several mouse and human tumor antigens (He et al., 2007). He and colleagues carefully analyzed the various subsets of DCs after lentiviral vector-mediated immunization and found that skin-derived DCs with the surface phenotype of CD8−/loDEC205+ were the predominant APCs migrating to draining lymph nodes to prime the potent antigen-specific immune responses (He et al., 2006).
Safety remains one of the most outstanding concerns for the broad use of lentiviral vectors for stimulating immune responses. The occurrence of leukemia in X-linked severe combined immunodeficiency (X-SCID) children cured by retrovirus-mediated gene therapy (Hacein-Bey-Abina et al., 2003) necessitates the continuous effort to evolve and improve methods to address the potential low genotoxicity induced by the random integration of vector DNA into the target cell genomes (Nienhuis et al., 2006). Even though lentiviral vectors retain insertional activation, they have been shown to exhibit low genotoxicity in certain mouse models (Montini et al., 2006; Bokhoven et al., 2009), which makes them favorable for immunological applications. Transcriptional targeting (Kimura et al., 2007) or the development of novel lentiviral systems to limit the transduction to DCs (Yang et al., 2008) can ameliorate certain off-target effects and continuously improve the safety profile of the vector. Nonintegrating lentiviral vectors (NILVs) have been developed that further mitigate the risk of insertional mutagenesis by disabling the integrase function (Philpott and Thrasher, 2007). The resulting NILVs are defective in vector integration but retain the ability to genetically modify target cells to express transgenes. Transgene expression was achieved by the episomal double-stranded lentiviral DNA in either a linear form or a circular form with a single long terminal repeat (LTR), or in a circular form with two LTRs (Saenz et al., 2004; Vargas et al., 2004; Philpott and Thrasher, 2007). One HIV-1-based NILV with the replacement of the integrase RRK motif by AAH displayed sustainable transgene expression in nondividing cells in vitro and in vivo and its residual integration was measured to be only 1/1250- to 1/500-fold that of the integrating vector in a clone survival assay (Philippe et al., 2006). To date, effective transduction and expression afforded by NILVs have been observed in many postmitotic tissues, such as the brain, ocular tissue, muscle, and liver (Philippe et al., 2006; Yanez-Munoz et al., 2006; Apolonia et al., 2007; Bayer et al., 2008). Initial experiments involving a single dose injection of integrase-deficient lentiviral vector encoding the envelope protein of either HIV-1 (Negri et al., 2007) or West Nile virus (WNV) (Coutant et al., 2008) resulted in significant and prolonged immune responses specifically against the delivered antigen, although the studies did not characterize the target APCs, particularly the DCs and their immune stimulatory functions on NILV-mediated modification in vitro and in vivo. This kind of study is of fundamental importance for fully exploiting the potential of NILVs as effective carriers to deliver tumor vaccines.
In this paper, we evaluated the ability of an NILV to modify DCs to stimulate antigen-specific T cell responses in vitro and the efficacy of this vector system to mount tumor-specific immune responses. We generated a vesicular stomatitis virus glycoprotein (VSVG)-pseudotyped and HIV-1-based self-inactivating NILV harboring a deficient integrase with a single amino acid mutation from aspartic acid to valine at position 64 (D64V) and encoding an ovalbumin (OVA) antigen. We demonstrated that the direct administration of such a vector induced substantial and durable antigen-specific T cell and antibody responses. Moreover, a single immunization could provide protection against the growth of tumor cells bearing the OVA antigen. This work shows that immunization with NILV can be a promising and safe approach for the development of cancer vaccines.
Materials and Methods
Mice
C57BL/6 (denoted as B6) female mice were purchased from Charles River Laboratories (Wilmington, MA). The OT1 transgenic [C57BL/6-Tg(TcraTcrb)1100Mjb/J] and OT2 transgenic [C57BL/6-Tg(TcraTcrb)425Cbn/J] mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in an animal facility at the University of Southern California (Los Angeles, CA) in accordance with institute regulations.
Plasmid construction
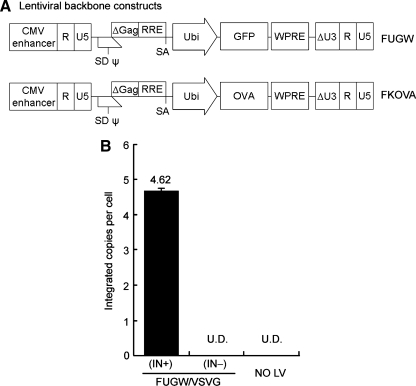
The packaging plasmid pCMVΔR(int-)8.2 is an integrase-defective construct that contains a single amino acid mutation from aspartic acid to valine at position 64 (D64V) of the class I catalytic domain of HIV-1 integrase (Leavitt et al., 1996). The lentiviral backbone plasmid FKOVA (Fig. 1A) was generated from the FUGW construct (Lois et al., 2002) by substituting the gene encoding green fluorescent protein (GFP) with the cDNA of chicken ovalbumin downstream of the human ubiquitin-C promoter. FUW, an empty lentiviral backbone, is a derivative of FUGW that lacks the GFP reporter gene. These lentiviral backbone plasmids (FUGW, FKOVA, and FUW) are self-inactivating (SIN) constructs with a deleted U3 region in their 3′ long terminal repeat (LTR). The packaging plasmids for making the integrating vector, pMDLg/pRRE and pRSV-REV, and the envelope expression plasmid pMD.G(VSV), have been described previously (Dull et al., 1998).
FIG. 1.
The D64V point mutation in HIV-1 integrase prohibits vector integration on transduction. (A) A schematic diagram of the lentiviral transfer vector encoding a green fluorescent protein (GFP) reporter gene (FUGW) or encoding ovalbumin antigen (FKOVA). R, U5, and ΔU3 are components of the long terminal repeat (LTR) and ΔU3 contains the self-inactivating deletion; SD, splicing donor; SA, splicing acceptor; ψ and Δgag, the encapsulation sequence; RRE, the Rev-responsive element; Ubi, human ubiquitin-C promoter; WPRE, woodchuck hepatitis virus posttransductional regulatory element. (B) HEK293T cells (1.5 × 105 cells were transduced with 1.5 ml of fresh viral supernatant of FUGW/VSVG(IN+) or FUGW/VSVG(IN–). Transduced cells were cultured for 2 weeks and genomic DNA was extracted for quantitative PCR analysis of the integration of the viral vector. Untransduced 293T cells were included as a control. The respective transduction titers for FUGW/VSVG(IN+) and FUGW/VSVG(IN–) were estimated to be 8 × 107 and 1.8 × 107 TU/ml, respectively. The p24 concentration of fresh viral supernatants of FUGW/VSVG(IN+) and FUGW/VSVG(IN–) was 260.9 and 179.1 ng/ml, respectively.
Lentiviral vector production
All lentiviral vectors described in this study were pseudotyped with the vesicular stomatitis virus glycoprotein (VSVG). The standard calcium phosphate precipitation procedure was used in the transient transfection of virus-producing cells for the making of lentiviral vectors. HEK293T cells seeded in a 6-cm culture dish (BD Biosciences, San Jose, CA) were transiently transfected with an appropriate combination of various plasmids. Integrating lentiviral vectors were generated by the transfection of lentiviral backbone plasmid (5 μg), packaging plasmids (pMDLg/pRRE, 2.5 μg; pRSV-REV, 2.5 μg), and the envelope plasmid pMD.G(VSV) (2.5 μg). Integration-deficient vectors were produced by replacing plasmids pMDLg/pRRE and pRSV-REV with integrase-mutated packaging plasmid pCMVΔR(int-)8.2 (10 μg). Two days after transfection, the viral supernatant was collected and filtered through a 0.45-μm pore size filter (Nalgene, Rochester, NY). Concentrated vectors for the in vivo studies were prepared by ultracentrifugation, using an Optima L-90 K preparative ultracentrifuge and an SW 28 rotor (Beckman Coulter, Fullerton, CA) at 25,000 rpm for 90 min. The pellets were resuspended in a proper volume of sterile phosphate-buffered saline (PBS).
Quantitative polymerase chain reaction analysis
HEK293T cells were spin-transduced with either FUGW/VSVG(IN–) or FUGW/VSVG(IN+) and cultured for 2 weeks. Nontransduced 293T cells were included as a negative control. Genomic DNA was extracted, using a DNeasy blood and tissue kit (Qiagen, Valencia, CA). Vector integration was assessed by the quantitative polymerase chain reaction (PCR) of 300 ng of genomic DNA with a primer pair spanning a short region within the 5′-LTR (MH531, 5′-TGTGTGCCCGTCTGTTGTGT-3′; MH532, 5′-GAGTCCTGCGTCGAGAGAGC-3′) (Butler et al., 2001). The quantitative PCR reagent (SYBR green PCR master mix) and instrument (7300 real-time PCR system) were from Applied Biosystems (Foster City, CA). The number of copies per cell was calculated by using the FUW plasmid to generate a standard curve and converting the 300 ng of genomic DNA to the equivalent number of 293T cells.
Lentiviral vector transduction in vitro
Total bone marrow cells were harvested from naive C57BL/6 mice and cultured for 6 days in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (from J558L cells; diluted 1:20) and interleukin (IL)-4 to obtain bone marrow-derived dendritic cells (BMDCs) (Yang and Baltimore, 2005). The resulting BMDCs were spin-transduced twice with fresh viral supernatant of either nonintegrating vector FUGW/VSVG(IN–) or integrating vector FUGW/VSVG(IN+) in a 24-well plate at 2500 rpm and 27°C for 90 min. The GFP+ cells were monitored by flow cytometric analysis from 2 to 21 days posttransduction. At each time point, BMDCs were collected from one well out of each transduction and analyzed for GFP+ cells, with nontransduced BMDCs included as a control. All BMDCs were maintained by changing the medium containing GM-CSF and IL-4 every 2 days. Similarly, DC2.4 cells were spin-transduced once and passaged to monitor GFP+ cells.
In vivo transduction of dendritic cells
FUGW/VSVG(IN–) (1.08 × 109 transduction units [TU]) or FUGW/VSVG(IN+) (4.8 × 109 TU) was concentrated in 200 μl of PBS by ultracentrifugation. The concentrated lentiviral vectors were subcutaneously injected into the right flanks of C57BL/6 mice. Three days later, cells harvested from the right inguinal lymph nodes close to the injection sites were stained with anti-mouse CD11c (BD Biosciences) to detect GFP+ DCs.
Stimulation of OT1 and OT2 transgenic T cells with vector-modified BMDCs
On day 6, BMDCs were spin-infected twice with fresh supernatant of either FKOVA/VSVG(IN–), FKOVA/VSVG(IN+), or FUW/VSVG(IN+). On day 9, nonadherent cells were collected and matured overnight in RPMI medium containing 10% fetal bovine serum (FBS), GM-CSF (from J558L cells; diluted 1:20), and lipopolysaccharide (LPS, 1 μg/ml). On day 10, nonadherent vector-treated BMDCs were collected and cocultured with OT1 or OT2 T cells harvested from OT1 or OT2 transgenic mice at the indicated ratio of BMDCs to responding T cells. Nontransduced BMDCs (day 9) were matured overnight and then pulsed with OVAp1 (OVA257–264, recognized by CD8+ OT1 T cell receptor [TCR]) or OVAp2 (OVA323–339, recognized by CD4+ OT2 TCR) to serve as positive controls. Three days later, culture supernatants were examined by enzyme-linked immunosorbent assay (ELISA) to measure interferon (IFN)-γ secretion. The proliferative responses of cocultured OT1 or OT2 cells were measured in an [3H]thymidine incorporation assay (Yang and Baltimore, 2005).
Enzyme-linked immunosorbent spot assay
T cells were harvested from spleens and cocultured with either OVAp1 or OVAp2 peptide (GeneScript, Piscataway, NJ) overnight. The next day, restimulated T cells were counted and transferred to a MultiScreen plate (Millipore, Billerica, MA) coated with anti-mouse IFN-γ or IL-2 antibodies (BD Biosciences). The plate was incubated at 37°C and 5% CO2 for 18–22 hr. On the third day, biotinylated anti-mouse IFN-γ or IL-2 antibodies (BD Biosciences) were added to the plate, followed by the addition of streptavidin–alkaline phosphatase (Millipore) and BCIP/NBTplus substrate (Chemicon International, Temecula, CA) to develop spots. Spot development was stopped by rinsing thoroughly with deionized water. The plate was air dried and read with a Zeiss ELISPOT reader (Carl Zeiss MicroImaging, Göttingen, Germany). The number of spot-forming counts (SFCs) per 106 cells was used to plot results.
In vivo immunization of naive mice
Fresh viral supernatant of FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) was concentrated in 50 μl of PBS by ultracentrifugation. The amount of viral particles present was quantified by p24 ELISA. The procedure was performed according to the protocol provided with the p24 capture ELISA kit from ImmunoDiagnostics (Woburn, MA). Concentrated lentiviral vectors were injected into the lower two footpads of C57BL/6 mice. The immunized mice were analyzed for immune responses 2 weeks postinjection.
Surface marker and intracellular staining
Mouse lymphocytes were collected and washed in PBS. The prepared cells were stained with anti-mouse CD16/32 to block Fc receptors. For tetramer staining, cells were stained with anti-mouse CD8–PE/Cy5, H-2Kb-SIINFEKL–PE tetramer (Beckman Coulter), and anti-mouse CD44–FITC. For intracellular staining, splenocytes were restimulated for 6 hr with OVAp1 (1 μg/ml) or OVAp2 (10 μg/ml), with GolgiPlug (BD Biosciences) to inhibit IFN-γ secretion. The cell surface markers were stained with anti-mouse CD8–FITC and anti-mouse CD4–PE/Cy5. Cells were then permeabilized and stained with anti-mouse IFN-γ–PE. Stained cells were analyzed by flow cytometry (FACSort; BD Biosciences). All monoclonal staining antibodies were from BD Biosciences or from BioLegend (San Diego, CA).
Tumor challenge study
Two weeks after immunization with the indicated dose of FKOVA/VSVG(IN–), FKOVA/VSVG(IN+), or FUW/VSVG(IN+), C57BL/6 mice were challenged with EL4 (C57BL/6, H-2b, thymoma) or EG.7 (EL4 cells stably expressing one copy of chicken OVA cDNA) tumor cells (5 × 106 cells per mouse) (Yang et al., 2008). Tumor growth was monitored with fine calipers and was calculated as the product of the two largest perpendicular diameters (mm2). On days 15 and 41 after tumor challenge, peripheral blood mononuclear cells (PBMCs) obtained by eye bleeding or splenocytes from the mice challenged with the EG.7 tumor cells were analyzed for the presence of H-2Kb-SIINFEKL–PE tetramer-positive CD8+ T cells.
Results
Efficient transduction mediated by nonintegrating lentiviral vectors in vitro and in vivo
To confirm the integration deficiency of our NILV system, we performed a quantitative PCR analysis of the genomic DNAs extracted from vector-modified cells. It was found that the integration of vector DNA was approximately 4.62 copies per cell in HEK293T cells transduced by integrating FUGW lentiviral vector pseudotyped with vesicular stomatitis virus glycoprotein [FUGW/VSVG(IN+)] (Fig. 1A), but was undetected in cells transduced with nonintegrating vector, FUGW/VSVG(IN–) (Fig. 1B).
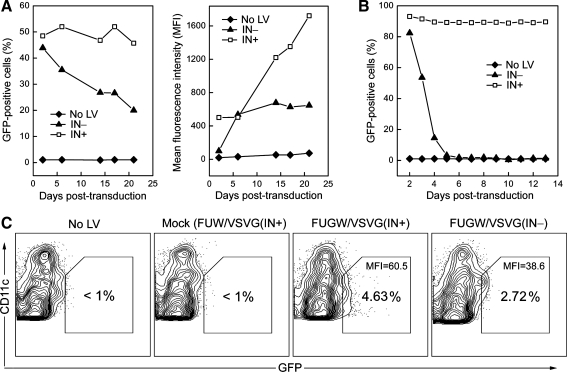
The frequency of the GFP+ cell population in bone marrow-derived dendritic cells (BMDCs) was maintained from >40% on day 2 to about 20% on day 21 posttransduction by FUGW/VSVG(IN–) (Fig. 2A, left), whereas a dramatic decrease in GFP expression, from 90 to 2% within 4 days posttransduction, was observed for the dendritic cell line (DC2.4) modified with the same nonintegrating vector (Fig. 2B). Notably, the mean fluorescence intensity (MFI) of the GFP expression from BMDCs transduced with FUGW/VSVG(IN–) was lower than that of the GFP provided by the integrating vector (Fig. 2A, right), but its expression level was stably sustained up to 3 weeks in this in vitro setting (Fig. 2A). As expected, stable GFP expression was kept in both BMDCs and DC2.4 cells transduced with the integrating vector (Fig. 2A and B). Nonintegrating vector particles were evaluated by p24 capture ELISA, and the p24 concentrations of vector supernatants containing integration-deficient and integration-competent FUGW/VSVG were determined to be about 179.1 and 260.9 ng/ml, respectively. The transduction titer for harvested unconcentrated vector supernatant against HEK293T cells was measured to be 1.8 × 107 TU/ml for FUGW/VSVG(IN–) and 8 × 107 TU/ml for FUGW/VSVG(IN+). These results suggest that efficient production of NILV enveloped by VSVG could be achieved by the transient transfection approach and that the resulting NILV could maintain transgene expression for a remarkably long duration in the less proliferative BMDCs.
FIG. 2.
Nonintegrating lentivector encoding a GFP reporter gene mediated efficient transduction of dendritic cells (DCs) in vitro and in vivo. (A) Bone marrow cells harvested from naive B6 mice were cultured in the presence of GM-CSF and IL-4 for 6 days to generate bone marrow-derived dendritic cells (BMDCs). The BMDCs then received two spin transductions in the following 2 days with the fresh supernatant of FUGW/VSVG(IN+) or FUGW/VSVG(IN–). GFP expression was monitored by flow cytometric analysis and results are shown as the percentage of GFP+ BMDCs (left) and its mean fluorescence intensity (MFI; right). (B) DC2.4 cells were transduced with the fresh supernatant of FUGW/VSVG(IN+) or FUGW/VSVG(IN–) and GFP expression was monitored. (C) Fresh supernatant of FUW/VSVG(IN+) (mock), FUGW/VSVG(IN+), or FUGW/VSVG(IN–) was concentrated in 200 μl of sterile PBS and subcutaneously injected into B6 mice. Three days later, the corresponding right inguinal lymph nodes, close to the injection sites, were collected and analyzed to measure the frequency and MFI of GFP+CD11c+ DCs.
To assess whether NILV could transduce DCs in vivo, we examined GFP+ DCs from the draining lymph nodes of mice injected with the concentrated vectors. On day 3 after subcutaneous delivery of FUGW/VSVG(IN–), about 2.72% of the total CD11c+ DCs isolated from the right inguinal lymph node of a treated mouse were GFP+, compared with about 4.63% GFP+ DCs from a mouse treated with FUGW/VSVG(IN+) (Fig. 2C). These GFP+ DCs were likely vector-modified dermal DCs that migrated from the initial injection sites. These data show that nonintegrating vector could efficiently transduce DCs in vivo and that the resulting DCs could migrate to the nearby lymph nodes.
Stimulation of CD8+ and CD4+ T cell responses in vitro
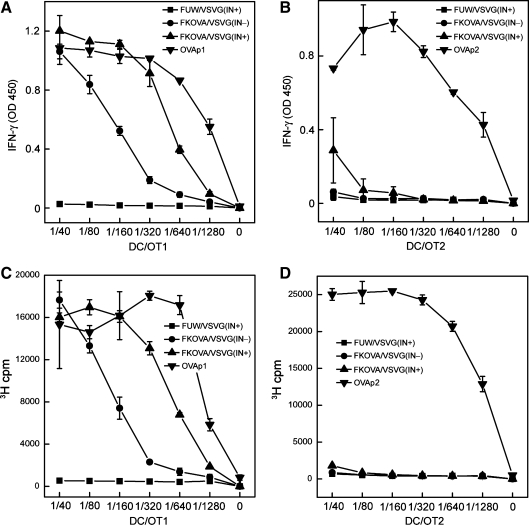
Taking advantage of the model antigen chicken ovalbumin (OVA), which contains two well-characterized epitopes, OVA257–264 (OVAp1) and OVA323–339 (OVAp2), which are recognized by the CD8+ OT1 T cell receptor (TCR) and the CD4+ OT2 TCR (Hogquist et al., 1994; Barnden et al., 1998), we tested the ability of OVA-encoding NILV (FKOVA; Fig. 1A) to stimulate antigen-specific CD8+ and CD4+ T cell responses through the genetic modification of DCs to express an antigen in vitro. On day 6 of culture, BMDCs were transduced with FKOVA/VSVG(IN–), FKOVA/VSVG(IN+), or FUW/VSVG(IN+)—the empty vector that served as a mock control; these modified DCs were designated FKOVA(IN–)/DCs, FKOVA(IN+)/DCs, and MOCK/DCs, respectively. Vector-modified BMDCs were then matured with lipopolysaccharide (LPS) and cocultured with OVA-specific OT1-transgenic CD8+ or OT2-transgenic CD4+ T cells for 3 days. In parallel, unmodified BMDCs were matured with LPS and pulsed with OVAp1 (designated OVAp1/DCs) or OVAp2 (designated OVAp2/DCs), as positive controls. Activation of OT1 and OT2 T cells was assessed in an IFN-γ secretion assay and a cell proliferation assay. FKOVA(IN–)/DCs, FKOVA(IN+)/DCs, and OVAp1/DCs all induced substantial IFN-γ production and proliferative activity in OT1 cells. On the basis of the responses of the OT1 T cells to various amounts of DCs, FKOVA(IN–)/DCs, FKOVA(IN+)/DCs, and OVAp1/DCs could be ranked from low to high in their capacity to stimulate OT1 cells (Fig. 3A and C). No marked activation was detected in OT2 cells cocultured with FKOVA(IN–)/DCs or FKOVA(IN+)/DCs, whereas vigorous IFN-γ secretion and proliferation responses were seen in OVAp2/DC-treated OT2 cells (Fig. 3B and D). It seems that the genetic modification of DCs by NILV results in a weaker MHC class II presentation for the stimulation of CD4+ T cells. Nevertheless, these in vitro coculture experiments clearly show that antigen-specific CD8+ T cell responses could be efficiently generated via the delivery of antigens to DCs by NILV.
FIG. 3.
Stimulation of OVA-specific CD8+ and CD4+ T cell responses in vitro by murine bone marrow-derived dendritic cells (BMDCs) modified by nonintegrating lentiviral vector encoding OVA antigen. (A–D) CD8+ and CD4+ T cells specific for OVA were collected from the spleens of OT1 and OT2 TCR transgenic mice and cocultured in vitro with FKOVA/VSVG(IN+)- or FKOVA/VSVG(IN–)-modified BMDCs for 3 days. FUW/VSVG(IN+)-transduced BMDCs served as the negative control. BMDCs pulsed with OVAp1 (SIINFEKL) or OVAp2 (ISQAVHAAHAEINEAGR), which are recognized by OT1 T cells and OT2 T cells, respectively, were included as positive controls. (A and B) OT1 and OT2 T cells were cocultured with various ratios of BMDCs to OT1 or OT2 T cells in vitro for 3 days. The culture supernatant was measured for the secretion of IFN-γ by ELISA. (C and D) An 3H incorporation assay was used to measure the proliferative activities of (A) treated OT1 T cells and (B) OT2 T cells.
Substantial induction of antigen-specific CD8+ T cell responses in vivo
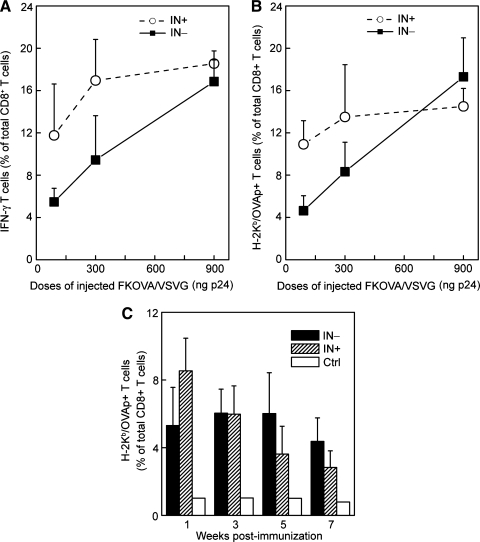
To investigate whether the direct administration of NILV could induce an antigen-specific CD8+ T cell response, we injected serial doses of FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) with amounts of p24 ranging from 90 to 900 ng via the footpad route. Two weeks after the immunizations, OVA-specific CD8+ T cell responses were examined by intracellular staining of IFN-γ secretion and tetramer staining of T cells harvested from the spleens of treated animals. After 6 hr of restimulation of the splenocytes with OVAp1 peptide, intracellular staining detected high frequencies of IFN-γ+ cell populations within the CD8+ T cells, which correlated proportionally with the amount of vectors administered (Fig. 4A). Consistent with these results, the tetramer staining assay also detected high populations of CD8+ T cells that were OVAp1 specific (Fig. 4B). As compared with the integrating vector FKOVA/VSVG(IN+), the lower doses (30 or 100 ng of p24) of FKOVA/VSVG(IN–) generated approximately half the number of antigen-specific T cells, but similar response levels were attained at the higher dose (900 ng of p24) (Fig. 4A and B). These results suggest that NILV can effectively deliver antigens to induce vigorous cellular immune responses in vivo.
FIG. 4.
Footpad injection of nonintegrating lentiviral vector encoding OVA antigen could generate a substantial antigen-specific CD8+ T cell response in wild-type B6 mice. (A and B) B6 mice received immunizations with various doses of FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) via footpad injections. The amount of viral particles injected was quantified by p24 capture ELISA. Two weeks later, T cells were harvested from the treated mice and analyzed by flow cytometry. Mice without immunization were included as a negative control. (A) T cells harvested from spleens were restimulated with OVAp1 for 6 hr and analyzed for IFN-γ secretion by intracellular cytokine staining. (B) The quantity of OVAp1-specific CD8+ T cells was estimated with H-2Kb-SIINFEKL–PE tetramer. (C) Kinetic study of immune responses in mice immunized with 300 ng of FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+). Peripheral blood was collected by retro-orbital breeding at the indicated time postimmunization and analyzed by tetramer staining.
Kinetic study of antigen-specific CD8+ T cell responses in vivo
We then investigated the kinetics of antigen-specific immune responses elicited by NILV. FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) (300 ng of p24) were injected into B6 mice via the footpad route. OVAp1-specific CD8+ T cells in the peripheral blood were monitored by tetramer staining every 2 weeks, starting at week 1 postimmunization. As for the mice immunized with FKOVA/VSVG(IN+), the percentage of OVAp1-specific CD8+ T cells gradually decreased from 8.54% at week 1 to 2.83% at week 7 (Fig. 4C). The frequency of OVAp1-specific CD8+ T cells from FKOVA/VSVG(IN+)-immunized mice was 5.31% at week 1, was stably maintained at 6.02% during week 3 to week 5, and decreased to 4.37% at week 7 (Fig. 4C). In comparing the kinetics of immune responses resulting from FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) immunization, FKOVA/VSVG(IN+) induced a stronger response at the initial stage, but FKOVA/VSVG(IN–) retained a higher frequency of OVAp1-specific CD8+ T cells at the later stage of immune responses (Fig. 4C). Therefore the kinetic study suggests that NILV can effectively mount long-term antigen-specific immune responses.
Comparison of nonintegrating and integrating lentiviral vectors for in vivo immunization
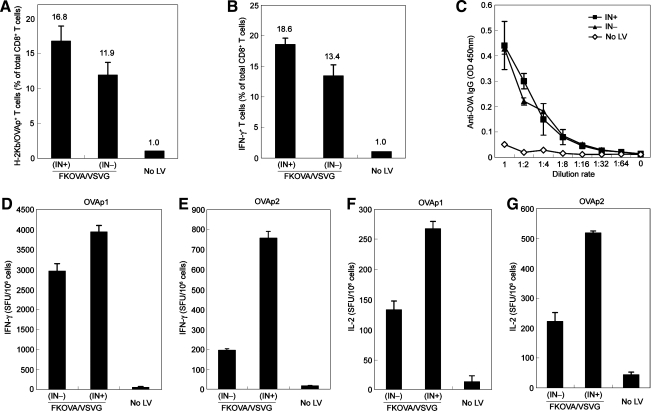
Having verified that NILV could maintain efficient transduction and induce strong CD8+ T cell responses in vitro and in vivo, we next compared the immunization potency of NILV with that of an ordinary integrating lentiviral vector. Wild-type mice were immunized with a single dose of FKOVA/VSVG(IN–) or INFKOVA/VSVG(IN+), using the same amount of viral particles (equivalent to 900 ng of p24), and analyzed 2 weeks later. Flow cytometric analysis of splenocytes by OVA–tetramer staining showed that the frequency of OVAp1-specific CD8+ T cells induced by immunization with FKOVA/VSVG(IN–) (∼11.9%) was slightly lower than that by immunization with the integrating FKOVA/VSVG(IN+) (∼16.8%) (Fig. 5A).
FIG. 5.
Nonintegrating lentiviral vector expressing OVA antigen was comparable to its integrating counterpart in inducing antigen-specific CTLs and humoral responses in vivo. (A–G) B6 mice were immunized with either FKOVA/VSVG(IN+) or FKOVA/VSVG(IN–) via footpad injections. The amount of viral particles used for each immunization was quantified to be 900 ng of p24. Mice without immunization (No LV) were included as a control. Mice were analyzed 2 weeks postimmunization. (A) T cells were collected from spleens and examined for the presence of OVAp1-specific CD8+ T cells by tetramer staining. (B) Spleen cells from (A) were restimulated with OVAp1 for 6 hr and IFN-γ secretion was evaluated by intracellular staining. (C) IgG serum specific for OVA was assessed by ELISA. (D–G) On restimulation with OVAp1 or OVAp2, spleen cells from (A) were analyzed by ELISPOT to measure IFN-γ or IL-2 spot-forming cells.
Enzyme-linked immunosorbent spot (ELISPOT) assay and intracellular staining analysis of splenocytes restimulated with OVAp1 indicated that significant numbers of IFN-γ-secreting CD8+ T cells specific for OVAp1 were induced by single-dose immunization with FKOVA/VSVG(IN–), although the numbers were a little less than those elicited by immunization with FKOVA/VSVG(IN+) (Fig. 5B and D). On restimulation with OVAp1 or OVAp2, spleen cells from FKOVA/VSVG(IN–)-immunized mice produced approximately half the IL-2 spot-forming counts (SFCs) per million cells as compared with spleen cells harvested from FKOVA/VSVG(IN+)-immunized mice (Fig. 5F and G). However, on restimulation with OVAp2, splenocytes from FKOVA/VSVG(IN–)-immunized mice were much less potent in secreting IFN-γ than those from FKOVA/VSVG(IN+)-immunized mice (Fig. 5E), suggesting that NILV might be less competent in the activation of CD4+ helper T type 1 (Th1) cells in vivo, which was consistent with the results of the in vitro stimulation experiment, in which OT2 T cells were cocultured with antigen-modified DCs.
Sera were also collected to assess the production of OVA-specific IgG after vectored immunization. The original sera were diluted 1000 times and then a series of finer dilutions were made. As compared with integrating vector [FKOVA/VSVG(IN+)], immunization with nonintegrating FKOVA/VSVG mounted a similar level of OVA-specific serum IgG (Fig. 5C), suggesting that NILV was as potent as integrating vector in inducing B cell responses to secrete antigen-specific antibodies. Taken together, these results strongly support the concept of using NILV as an effective vector system for inducing antigen-specific cellular and humoral responses.
Antitumor protection sustained by a durable CD8+ T cell response in vivo
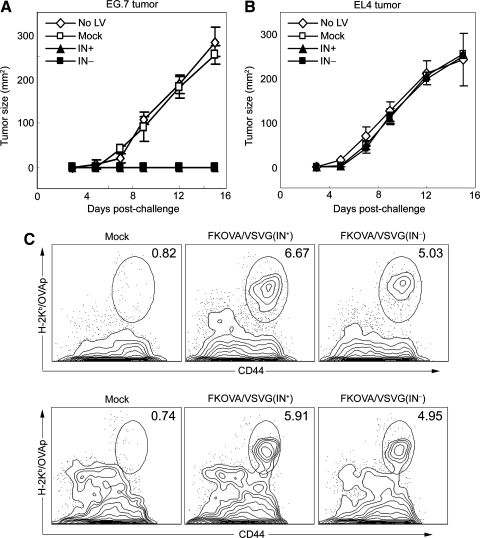
We employed the EG.7 tumor cell line expressing OVA as a tumor model to examine the efficacy of an in vivo vaccination delivered by NILV to protect against tumor growth. To this end, we administered a single dose of FKOVA/VSVG(IN–), FKOVA/VSVG(IN+), or FUW/VSVG(IN+) (as a mock control) into wild-type mice by footpad injections, each with an amount equivalent to 900 ng of p24. Two weeks later, each mouse was challenged with 5 × 106 EG.7 tumor cells and tumor growth was monitored thereafter. As a control, mice receiving the same immunization protocol were challenged with EL4 tumor cells, which lack the expression of OVA antigen. Whereas the unimmunized or mock lentiviral vector-immunized mice displayed rapid growth of both EG.7 and EL4 tumors, mice immunized with FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+) permitted the growth only of the control EL4 tumors, and not the EG.7 tumors (Fig. 6A and B). Thus, this result indicates that immunization with NILV could elicit efficacious protection against an EG.7 tumor challenge in an antigen-specific manner. We further traced the existence of OVAp1-specific CD8+ T cells in the mice after tumor challenge. Mice that were immunized with FKOVA/VSVG(IN–), FKOVA/VSVG(IN+), or FUW/VSVG(IN+) and later challenged with EG.7 cells were killed on day 15 after tumor injection. T cells harvested from the spleens were analyzed by OVA–tetramer staining, resulting in ~6.67 and ~5.03% OVAp1-specific CD8+ T cells, relative to the total amount of CD8+ T cells from mice immunized with FKOVA/VSVG(IN–) or FKOVA/VSVG(IN+), respectively (Fig. 6C). The analysis of peripheral blood monocytes (PBMCs) obtained by retro-orbital bleeding showed similar results on day 41 after tumor challenge (Fig. 6D), suggesting that long-term and effective antitumor protection could be sustained by durable antigen-specific CD8+ T cells generated by immunization with nonintegrating lentiviral vectors.
FIG. 6.
Protection against tumor growth and durable OVAp1-specific CD8+ T cells were observed after a single administration of nonintegrating lentiviral vector encoding OVA antigen. (A and B) B6 mice were immunized with mock vector FUW/VSVG(IN+) (Mock), FKOVA/VSVG(IN+), or FKOVA/VSVG(IN–) via footpad injections. The amount of viral particles used for each immunization was quantified to be 900 ng of p24. Mice without immunization were included as a control. (A and B) Two weeks after immunization, each mouse was implanted with either 5 × 106 EG.7 tumor cells, which express OVA antigen, or EL4 tumor cells, which lack OVA antigen. Tumor progression was monitored with fine calipers and is represented as the product of the two largest perpendicular diameters (mm2). Each group consisted of four mice. (C) On day 15 after tumor challenge, spleen cells were harvested from the treated mice from (A) and analyzed by tetramer staining. (D) On day 41 after tumor challenge, peripheral blood mononuclear cells (PBMCs) obtained by eye bleeding of treated mice from (A) were also analyzed by tetramer staining.
Discussion
One safety concern raised by the use of retrovirus-based gene delivery vectors is the insertional mutagenesis associated with vector genome integration (Nienhuis et al., 2006; Bohne and Cathomen, 2008). This has been demonstrated in animal models (Li et al., 2002; Seggewiss et al., 2006) and has been seen in human clinical investigations, in which patients with X-SCID treated by autologous transplantations of retrovirus-modified hematopoietic stem cells developed leukemia (Hacein-Bey-Abina et al., 2003). Although HIV-based lentiviral vectors have integration properties different from those of the gammaretrovirus used for the X-SCID trial (Schroder et al., 2002; Mitchell et al., 2004; Montini et al., 2006), insertional mutagenesis remains one of the most important safety considerations preventing the movement of lentiviral vectors to clinical usage. Studies of the biology of lentiviral vectors have shown that mutations at the catalytic domains of HIV-1 integrase and/or the integrase attachment sites could prevent lentiviral integration (Philpott and Thrasher, 2007). The resulting NILVs could retain their ability to effectively transduce dividing and nondividing cells while providing long-term transgene expression in a variety of postmitotic tissues (Philippe et al., 2006; Yanez-Munoz et al., 2006; Apolonia et al., 2007). NILV transgene expression could be further enhanced by a large U3 deletion of the long terminal repeat (LTR) region; this deletion probably removes the cis-acting elements in U3 that might be involved in negatively regulating transgene expression (Bayer et al., 2008).
The goal of this study was to evaluate the potential of in vivo immunization by NILV for inducing antigen-specific antitumor immune responses. We produced a self-inactivating NILV harboring a defective integrase (D64V mutation) and enveloped by VSVG. The D64V mutation has been shown to effectively disable integration of the viral genome (Leavitt et al., 1996; Nightingale et al., 2006). We also failed to detect vector integration in HEK293T cells transduced with integration-deficient FUGW/VSVG(IN–) by quantitative PCR analysis, a sharp contrast to the significant integration found in cells transduced with its conventional integrating counterpart [FUGW/VSVG(IN+)]. DCs are the gatekeeper cell type for stimulating adaptive immune responses and are therefore the most important cell type to target for the purpose of a vaccine (Banchereau and Steinman, 1998; Banchereau and Palucka, 2005; Steinman and Banchereau, 2007; Melief, 2008). We thus investigated the ability of NILV to mediate transgene expression in DCs. As a result of rapid dilution of the episomal forms of vector DNA throughout cell division, a nonintegrating phenotype, evidenced by transient GFP expression, was observed in a DC line (DC.2.4) transduced with FUGW/VSVG(IN–). On the other hand, NILV-mediated GFP expression was sustained in primary DCs in vitro for as long as the culture could be conducted (up to 20 days). This is consistent with previous reports that durable gene expression could be obtained in resting or less proliferative cells treated with integration-deficient vectors (Philippe et al., 2006; Yanez-Munoz et al., 2006; Apolonia et al., 2007), suggesting that transduction by NILV could be sufficient for targeted DCs to induce antigen-specific immunity. GFP-expressing DCs were identified by flow cytometric analysis of lymphocytes harvested from lymph nodes close to the site where concentrated NILV was injected, indicating that direct subcutaneous injection of nonintegrating vectors could target DCs in vivo to express transgenes.
In an effort to ensure that antigens delivered by NILV to DCs could be appropriately processed and presented to T cells, we tested the capacity of NILV [FKOVA/VSVG(IN–)] to genetically modify BMDCs to express the OVA antigen for the stimulation of antigen-specific CD8+ and CD4+ T cell responses. Results show that the nonintegrating vector was comparable to its integrating counterpart in inducing the CD8+ OT1 T cell response, but was inefficient in generating a measurable CD4+ OT2 T cell response. One possible explanation for the lack of CD4+ T cell stimulation is that the nonintegrating vector had a lower level of OVA expression (Bayer et al., 2008) and that this prevented sufficient secretion of OVA by the DCs for subsequent cross-presentation to the MHC class II pathway. The strategy of linking OVA with either a transferrin receptor or an invariant chain reportedly facilitates MHC class II presentation and therefore could be used to partially overcome the limitation of NILV in activating CD4+ T cells (Rowe et al., 2006). Experiments are underway to test this idea.
The experiment involving direct immunization with NILV showed that a high population of epitope-specific CD8+ T cells could be detected by tetramer staining after a single injection of FKOVA/VSVG(IN–) through the footpad of a naive mouse. These T cells were able to make the inflammatory cytokine IFN-γ on peptide restimulation. Remarkably, one immunization with a modest dose of nonintegrating vector (671.6 ng of p24) could yield a substantial population (∼14%) of antigen-responsive CD8+ T cells (Fig. 4A and B). This finding suggests that NILV is effective as a vaccine vector in mounting strong antigen-specific immune responses, which is in line with the results of a previous study on anti-HIV immune responses elicited by a nonintegrating vector carrying a codon-optimized HIV gp120 sequence (Negri et al., 2007). Furthermore, the response appeared to be dose dependent, as higher populations of CD8+ T cells could be generated after administration of larger doses of the vector. Injection of 2686.5 ng of p24 of vector yielded as much as 27% OVAp1-specific CD8+ T cells measured by tetramer staining with the dominant epitope (data not shown). A comparative study of in vivo immunization demonstrated that the nonintegrating vector produced a slightly lower percentage of antigen-specific, IFN-γ-secreting CD8+ T cells than the integrating vector. Compared with integrating vector, splenocytes from NILV-immunized mice also produced measurable, but less IL-2 and IFN-γ on stimulation with a CD4-specific epitope peptide, consistent with the inefficiency of FKOVA/VSVG(IN–) in activating OT2 CD4+ T cells in vitro. Interestingly, a similar level of antigen-specific IgG response was observed for these two sets of vectors, suggesting that NILV was able to stimulate and engage enough CD4+ T cells to orchestrate an effective B cell response.
We further observed that the nonintegrating vector was as effective as the integrating vector in mounting an antitumor immune response in a tumor challenge model, suggesting that this vector format could elicit an antitumor immune response that was prolonged and antigen specific. The kinetic study revealed that OVAp1-specific CD8+ T cells in the peripheral blood could be maintained up to 7 weeks after immunization with NILV. While this manuscript was under review, a study by Collins and coworkers was published, describing similar findings using NILV to induce in vivo antigen presentation up to 30 days and eliciting persistent CD8+ T cell and antibody responses, using OVA as a model antigen (Karwacz et al., 2009).
In summary, we have presented in this study an evaluation of the potential of nonintegrating lentiviral vectors as a vaccine carrier for cancer immunotherapy. Simultaneous cellular and humoral immune responses were generated by NILV. One round of immunization was able to elicit a high level of antigen-specific CD8+ T cell responses that was sufficient to prevent tumor growth in a model tumor antigen system. Considering the lower level of transgene expression afforded by NILV (Bayer et al., 2008), we were surprised by the high degree of vectored immune response, confirming the results of a previous study of anti-HIV immunity generated by NILV-associated immunization (Negri et al., 2007). This extraordinary efficiency, combined with its inherent safety feature, makes NILV a promising and intriguing vector system for vaccine delivery. However, more studies are needed in order to fully gauge the utility of this vector system for vaccine applications. For example, a thorough comparison of the quality (avidity, polyfunctionality, killing efficiency, memory, etc.) of immune responses mounted by NILV with that of the integrating lentiviral vector and other immunization methods will be important for exploring the effectiveness of this vectored vaccine. We have already seen that NILV is less potent by certain measures in stimulating a CD4+ T cell response. A further investigation of the cell types targeted by NILV would advance our understanding of the biology of this vector for immunization purposes. We have shown that DCs can be targeted, but we cannot rule out other antigen-presenting cells, such as macrophages, that can be modified to express antigens as well. We certainly need to evaluate more self-tumor antigens to assess the potential of NILV for the delivery of cancer vaccines.
Acknowledgments
The authors are grateful to Steven Froelich and Lili Yang for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Apolonia L. Waddington S.N. Fernandes C. Ward N.J. Bouma G. Blundell M.P. Thrasher A.J. Collins M.K. Philpott N.J. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 2007;15:1947–1954. doi: 10.1038/sj.mt.6300281. [DOI] [PubMed] [Google Scholar]
- Banchereau J. Palucka A.K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- Banchereau J. Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Barnden M.J. Allison J. Heath W.R. Carbone F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bayer M. Kantor B. Cockrell A. Ma H. Zeithaml B. Li X. McCown T. Kafri T. A large U3 deletion causes increased in vivo expression from a nonintegrating lentiviral vector. Mol. Ther. 2008;16:1968–1976. doi: 10.1038/mt.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne J. Cathomen T. Genotoxicity in gene therapy: An account of vector integration and designer nucleases. Curr. Opin. Mol. Ther. 2008;10:214–223. [PubMed] [Google Scholar]
- Bokhoven M. Stephen S.L. Knight S. Gevers E.F. Robinson I.C. Takeuchi Y. Collins M.K. Insertional gene activation by lentiviral and gammaretroviral vectors. J. Virol. 2009;83:283–294. doi: 10.1128/JVI.01865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K. Aerts J.L. Thielemans K. Lentiviral vectors for cancer immunotherapy: Transforming infectious particles into therapeutics. Gene Ther. 2007;14:847–862. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- Butler S.L. Hansen M.S. Bushman F.D. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- Coutant F. Frenkiel M.P. Despres P. Charneau P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS ONE. 2008;3:e3973. doi: 10.1371/journal.pone.0003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T. Zufferey R. Kelly M. Mandel R.J. Nguyen M. Trono D. Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M. Thielemans K. From pathogen to medicine: HIV-1-derived lentiviral vectors as vehicles for dendritic cell based cancer immunotherapy. J. Gene Med. 2006;8:3–17. doi: 10.1002/jgm.846. [DOI] [PubMed] [Google Scholar]
- Dullaers M. Van Meirvenne S. Heirman C. Straetman L. Bonehill A. Aerts J.L. Thielemans K. Breckpot K. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13:630–640. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- Esslinger C. Chapatte L. Finke D. Miconnet I. Guillaume P. Levy F. MacDonald H.R. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8+ T cell responses. J. Clin. Invest. 2003;111:1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M. McCormack M.P. Wulffraat N. Leboulch P. Lim A. Osborne C.S. Pawliuk R. Morillon E. Sorensen R. Forster A. Fraser P. Cohen J.I. De Saint Basile G. Alexander I. Wintergerst U. Frebourg T. Aurias A. Stoppa-Lyonnet D. Romana S. Radford-Weiss I. Gross F. Valensi F. Delabesse E. MacIntyre E. Sigaux F. Soulier J. Leiva L.E. Wissler M. Prinz C. Rabbitts T.H. Le Deist F. Fischer A. Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- He Y. Zhang J. Donahue C. Falo L.D., Jr. Skin-derived dendritic cells induce potent CD8+ T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. Munn D. Falo L.D., Jr. Recombinant lentivector as a genetic immunization vehicle for antitumor immunity. Expert Rev. Vaccines. 2007;6:913–924. doi: 10.1586/14760584.6.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A. Jameson S.C. Heath W.R. Howard J.L. Bevan M.J. Carbone F.R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Iglesias M.C. Mollier K. Beignon A.S. Souque P. Adotevi O. Lemonnier F. Charneau P. Lentiviral vectors encoding HIV-1 polyepitopes induce broad CTL responses in vivo. Mol. Ther. 2007;15:1203–1210. doi: 10.1038/sj.mt.6300135. [DOI] [PubMed] [Google Scholar]
- Karwacz K. Mukherjee S. Apolonia L. Blundell M.P. Bouma G. Escors D. Collins M.K. Thrasher A.J. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J. Virol. 2009;83:3094–3103. doi: 10.1128/JVI.02519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H. Majumder N. Lin H. Watkins S. Falo L.D., Jr. You Z. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum. Gene Ther. 2005;16:1255–1266. doi: 10.1089/hum.2005.16.1255. [DOI] [PubMed] [Google Scholar]
- Kimura T. Koya R.C. Anselmi L. Sternini C. Wang H.J. Comin-Anduix B. Prins R.M. Faure-Kumar E. Rozengurt N. Cui Y. Kasahara N. Stripecke R. Lentiviral vectors with CMV or MHCII promoters administered in vivo: Immune reactivity versus persistence of expression. Mol. Ther. 2007;15:1390–1399. doi: 10.1038/sj.mt.6300180. [DOI] [PubMed] [Google Scholar]
- Kohn D.B. Lentiviral vectors ready for prime-time. Nat. Biotechnol. 2007;25:65–66. doi: 10.1038/nbt0107-65. [DOI] [PubMed] [Google Scholar]
- Leavitt A.D. Robles G. Alesandro N. Varmus H.E. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J. Virol. 1996;70:721–728. doi: 10.1128/jvi.70.2.721-728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Dullmann J. Schiedlmeier B. Schmidt M. Von Kalle C. Meyer J. Forster M. Stocking C. Wahlers A. Frank O. Ostertag W. Kuhlcke K. Eckert H.G. Fehse B. Baum C. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- Lois C. Hong E.J. Pease S. Brown E.J. Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lopes L. Dewannieux M. Gileadi U. Bailey R. Ikeda Y. Whittaker C. Collin M.P. Cerundolo V. Tomihari M. Ariizumi K. Collins M.K. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 2008;82:86–95. doi: 10.1128/JVI.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief C.J. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Mitchell R.S. Beitzel B.F. Schroder A.R. Shinn P. Chen H. Berry C.C. Ecker J.R. Bushman F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M. Sanvito F. Ponzoni M. Bartholomae C. Sergi Sergi L. Benedicenti F. Ambrosi A. Di Serio C. Doglioni C. Von Kalle C. Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gallay P. Ory D. Mulligan R. Gage F.H. Verma I.M. Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Negri D.R. Michelini Z. Baroncelli S. Spada M. Vendetti S. Buffa V. Bona R. Leone P. Klotman M.E. Cara A. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 2007;15:1716–1723. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- Nienhuis A.W. Dunbar C.E. Sorrentino B.P. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Nightingale S.J. Hollis R.P. Pepper K.A. Petersen D. Yu X.J. Yang C. Bahner I. Kohn D.B. Transient gene expression by nonintegrating lentiviral vectors. Mol. Ther. 2006;13:1121–1132. doi: 10.1016/j.ymthe.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Palmowski M.J. Lopes L. Ikeda Y. Salio M. Cerundolo V. Collins M.K. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J. Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- Philippe S. Sarkis C. Barkats M. Mammeri H. Ladroue C. Petit C. Mallet J. Serguera C. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17684–17689. doi: 10.1073/pnas.0606197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott N.J. Thrasher A.J. Use of nonintegrating lentiviral vectors for gene therapy. Hum. Gene Ther. 2007;18:483–489. doi: 10.1089/hum.2007.013. [DOI] [PubMed] [Google Scholar]
- Rowe H.M. Lopes L. Ikeda Y. Bailey R. Barde I. Zenke M. Chain B.M. Collins M.K. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 2006;13:310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Saenz D.T. Loewen N. Peretz M. Whitwam T. Barraza R. Howell K.G. Holmes J.M. Good M. Poeschla E.M. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: Analysis with class I integrase mutants. J. Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder A.R. Shinn P. Chen H. Berry C. Ecker J.R. Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Seggewiss R. Pittaluga S. Adler R.L. Guenaga F.J. Ferguson C. Pilz I.H. Ryu B. Sorrentino B.P. Young W.S., III Donahue R.E. Von Kalle C. Nienhuis A.W. Dunbar C.E. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M. Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Vandendriessche T. Thorrez L. Naldini L. Follenzi A. Moons L. Berneman Z. Collen D. Chuah M.K. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100:813–822. doi: 10.1182/blood.v100.3.813. [DOI] [PubMed] [Google Scholar]
- Vargas J., Jr. Gusella G.L. Najfeld V. Klotman M.E. Cara A. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum. Gene Ther. 2004;15:361–372. doi: 10.1089/104303404322959515. [DOI] [PubMed] [Google Scholar]
- Verma I.M. Weitzman M.D. Gene therapy: Twenty-first century medicine. Annu. Rev. Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Vigna E. Naldini L. Lentiviral vectors: Excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med. 2000;2:308–316. doi: 10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yanez-Munoz R.J. Balaggan K.S. Macneil A. Howe S.J. Schmidt M. Smith A.J. Buch P. Maclaren R.E. Anderson P.N. Barker S.E. Duran Y. Bartholomae C. Von Kalle C. Heckenlively J.R. Kinnon C. Ali R.R. Thrasher A.J. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yang L. Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Yang H. Rideout K. Cho T. Joo K.I. Ziegler L. Elliot A. Walls A. Yu D. Baltimore D. Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]