Abstract
Platelet-derived growth factor (PDGF) signaling is essential for processes involving cell motility and differentiation during embryonic development in a wide variety of organisms including the mouse, frog, zebrafish, and sea urchin. In early Xenopus laevis embryos, PDGF-AA provides guidance cues for the migration of anterior mesendoderm cells as they move across a fibronectin-rich extracellular matrix. The long form of PDGF-A includes a positively charged carboxyl-terminal retention motif that can interact with the extracellular matrix and heparan sulfate proteoglycans (HSPGs). In this study we demonstrate that PDGF-AA binds directly to fibronectin and that this association is greatly enhanced by heparin. The PDGF-AA-fibronectin binding occurs across a broad range of pHs (5.5–9), which is significant because the PDGF-guided migration of Xenopus mesendoderm cells occurs under basic extracellular conditions (pH 8.4). We further demonstrate that endogenous HSPG's are required for the PDGF-AA-guided mesendoderm movement, suggesting an in vivo role for HSPGs in mediating the interaction between PDGF-AA and fibronectin.
Keywords: embryo, embryonic development, mesendoderm, mesoderm
Embryonic development requires an extensive series of coordinated cell movements that are necessary to bring cells to the correct location at the proper time to build the organism. To move in a directed way, a cell must be able to detect and move in response to extracellular signals, extend leading edge protrusions such as lamellipodia, generate traction and force, and balance attachment and detachment to neighboring cells and the extracellular matrix (ECM). Two factors that have been shown independently to contribute to these processes are fibronectin (1) and platelet-derived growth factor (PDGF) (2).
Fibronectin is a major component of the ECM in vertebrates and is critical for cell migration in a variety of developing embryos. For example, fibronectin null mouse embryos die early in gestation and develop with defects that suggest a failure of mesoderm migration and adhesion (3). Fibronectin is also essential for mesendoderm cell migration in early amphibian embryos. The first period of cell movement in all embryos is called gastrulation, which acts to organize the basic body plan through a highly choreographed series of cell reorganizations. In Xenopus, a network of fibronectin fibrils appears just before gastrulation (4–6) and provides the substratum for migration of the anterior mesendoderm cells, which later contribute to the musculature of the face and head, and are among the first cells to move during gastrulation. In some amphibians, injection of antibodies to fibronectin or αvβ1 integrin, which acts as a fibronectin receptor, or synthetic peptides including the amino acid sequence corresponding to the cell binding domain of fibronectin (Arg-Gly-Asp), into the blastocoel cavity just before the onset of gastrulation inhibits this cell migration and in some cases blocks gastrulation entirely (4, 7, 8).
Anterior mesendoderm migration is directed by guidance cues provided by PDGF signaling (9). The PDGF receptor-α (PDGFR-α) is expressed by the migrating mesendoderm cells whereas the ligand, PDGF-A, is expressed by ectoderm cells that provide both the surface and ECM for this migration (10). Blocking PDGF signaling by disruption of the receptor or ligand causes the direction of migration of these cells to be randomized, although the cells are still able to move (9).
PDGF-A is found in two forms, a long form that includes a positively charged carboxyl-terminal retention motif and a short form that lacks it (9, 11). This motif enables PDGF-A to associate with the ECM and without it, PDGF-A diffuses away from the site of its secretion (9, 11). The motif has further been shown to interact with heparan sulfate proteoglycans (11–13), but its association with fibronectin is not known.
Recently vascular endothelial growth factor (VEGF), a member of the PDGF/VEGF family of growth factors, was shown to bind directly to fibronectin (14–17). This binding was significantly enhanced by heparin (14, 15, 18), which acts by modifying the structure of fibronectin, potentially revealing previously masked VEGF binding sites that remain available to bind VEGF even after heparin is removed (14). We demonstrate that PDGF also directly binds to fibronectin, and that this interaction depends on pretreatment of the fibronectin with heparin. Using an established embryo assay, we show that heparan sulfate is required for the directed migration of anterior mesendoderm cells and that it is specifically necessary during the deposition of the ECM. These data suggest a role for a heparan sulfate proteoglycan in the coordination of PDGF-AA-fibronectin interactions during gastrulation.
Results
Heparin Enhances PDGF-AA-Fibronectin Binding.
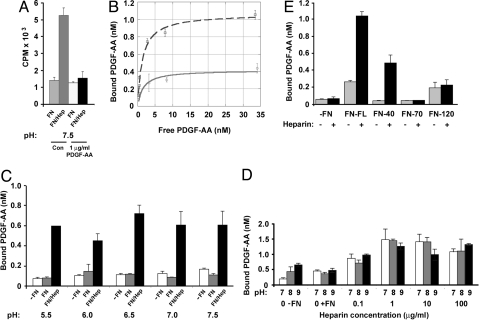
To examine the interaction between PDGF-AA and fibronectin, 125I-PDGF-AA-binding assays were conducted. Briefly, fibronectin was adsorbed onto polystrene surfaces at a concentration sufficient to form a monolayer, treated with and without heparin, and then incubated with 125I-PDGF-AA. Bound 125I-PDGF-AA was extracted and quantified. In the absence of heparin treatment there was minimal specific binding of PDGF-AA to fibronectin (Fig. 1A), with measured levels similar to wells lacking fibronectin (Fig. 1C). Pretreatment of the fibronectin with heparin, however, significantly increased PDGF-AA-fibronectin binding (Fig. 1A, 1 μg/mL heparin and Fig. 1C, 100 μg/mL heparin). The binding of 125I-PDGF-AA to heparin-treated fibronectin was effectively competed by excess unlabeled PDGF, indicating the specific nature of this interaction (Fig. 1A).
Fig. 1.
PDGF-AA binding to fibronectin is increased by pretreatment of the fibronectin with heparin. (A) Binding of 125I-PDGF-AA, in the presence or absence of excess (1 μg/mL) PDGF-AA, to 40 nM fibronectin was tested at pH 7.5, without (FN) or with (FN/Hep) pretreatment of the fibronectin with 1 μg/mL heparin. Control (Con) wells included 125I-PDGF-AA in the absence of excess growth factor. (B) Adsorbed fibronectin (40 nM) without (solid line) or with 1 μg/mL heparin pretreatment (dashed line), was incubated with 0.35 nM to 34.5 nM 125I-PDGF-AA and the amount bound determined (y axis). Note PDGF-AA effectively self competes and its binding to fibronectin alone reaches a maximum of 0.42 nM, but heparin pretreatment increased this to 1.07 nM. (C) PDGF-AA binding to fibronectin at pH 5.5–7.5 was examined without or with pretreatment of the fibronectin with 100 μg/mL heparin. Wells contained no fibronectin (-FN; white bars), fibronectin (FN; gray bars), or pretreated fibronectin (FN/Hep; black bars). (D) Binding of 125I-PDGF-AA to fibronectin was examined over an increasing concentration of heparin 0.1–100 μg/mL at pH 7 (white bars), 8 (gray bars), or 9 (black bars). (E) PDGF-AA binding to three fibronectin fragments, 40 kDa, 70 kDa, and 120 kDa, was examined without (−) or with (+) 1 μg/mL heparin pretreatment. Full-length fibronectin (FN-FL) and all fibronectin fragments were used at equimolar concentrations (40 nM). No fibronectin (−FN). Data are representative of at least two independent experiments.
Analysis of PDGF-AA binding to fibronectin (in the presence or absence of heparin pretreatment) over a range of concentrations showed classic saturation kinetics with an increase in maximum binding after heparin pretreatment (Fig. 1B). The Kd values for PDGF-AA binding to fibronectin without and with heparin pretreatment were 1.51 ± 0.36 nm and 1.35 ± 0.14 nm, respectively. These data indicate that heparin pretreatment led to the exposure or creation of nascent PDGF binding sites on fibronectin. This is similar to that observed previously for VEGF-A binding to fibronectin (14, 18). PDGF and VEGF are members of the same protein structural family, and previous studies have noted that VEGF-A binding to fibronectin is enhanced at acidic pH. The pH control of VEGF-fibronectin interactions potentially reflects important extracellular regulatory mechanisms as alterations in pH are know to be associated with tissue injury, disease state, and development (19, 20). Thus, we investigated whether PDGF-AA binding to fibronectin was modulated by pH. Analysis of PDGF-AA binding to fibronectin at a range of pHs between 5.5 and 9 showed no significant alterations (Fig. 1 C and D). Importantly, there was no reduction of PDGF-AA binding potential at basic pH. This is significant because the PDGF-guided migration of mesendoderm cells across a fibronectin-rich ECM occurs under basic extracellular conditions (pH 8.4) during gastrulation in Xenopus laevis (21–23). Hence, PDGF-AA interactions with fibronectin are likely to occur within the developing Xenopus embryo.
To further evaluate the heparin dependence of PDGF-AA-fibronectin binding, a more extensive dose–response analysis was conducted. The minimum heparin concentration necessary to increase PDGF-AA-fibronectin binding was 1 μg/mL (Fig. 1D). Lower heparin concentrations did not produce a measurable effect, whereas, higher amounts of heparin (10–100 μg/mL) did not further enhance binding (Fig. 1D). These data suggest that PDGF-AA can bind to fibronectin only after the binding sites on the fibronectin have been modified with heparin (14, 18).
PDGF-AA Binds to the Carboxyl Terminus of Fibronectin.
To localize the PDGF-AA binding site(s) on fibronectin, three fibronectin fragments were examined: FN-40, FN-70, and FN-120, corresponding to the C-terminal 40-kDa, N-terminal 70-kDa, and central 120-kDa fibronectin regions, respectively.125I-PDGF-AA binding to these fragments was conducted as described for full-length fibronectin (FN-FL). Briefly, fibronectin fragments adsorbed onto polystyrene plates were incubated with 125I-PDGF-AA, and bound 125I-PDGF-AA was measured. Only FN-40 bound PDGF-AA and similar to full-length fibronectin, binding required pretreatment with heparin (Fig. 1E). These results are consistent with those observed with VEGF-A, which also bound to the C-terminal 40-kDa domain in a heparin-dependent manner (18). Taken together, these data indicate specific PDGF-AA-fibronectin binding in vitro.
Heparinase III Randomizes Directed Mesendoderm Migration.
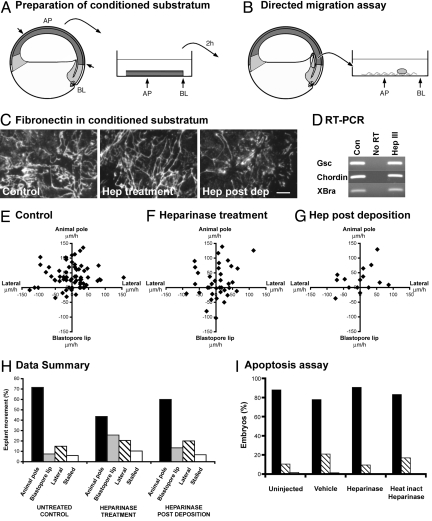
Next we investigated whether heparin mediated PDGF-AA-fibronectin interactions play a role in a well-established, in vivo system where PDGF-AA guides directional cell movement (24, 25). During early development of Xenopus embryos, cells that will ultimately form the musculature of the face and head (anterior mesendoderm cells) undergo a period of directed migration. These cells (gray cells in Fig. 2A) move into the embryo (involution) at the blastopore lip (BL; Fig. 2A), and then migrate toward the animal pole (AP; Fig. 2A). This directed movement can be recapitulated ex vivo by transferring the native cell migration substrate, a fibronectin-rich ECM, to a tissue culture dish (Fig. 2A), and placing an explant of migratory mesendoderm cells on that matrix midway between the sites equivalent to the BL and AP in the embryo (Fig. 2B) (24, 25). The mesendoderm explant will migrate in its normal direction that is, toward the position of the AP. Our previous work showed that PDGF signaling provides guidance cues for this directed movement, probably due to the retention of PDGF-AA in the substratum over which the PDGFR-α expressing mesendoderm cells migrate (9, 10).
Fig. 2.
Heparinase III treatment disrupts the directed migration of embryonic mesendoderm cells. (A and B) Ex vivo assay for directed cell movement of head mesendoderm cells. (A) Preparation of conditioned substrata. Schematic of a sagital section of a stage 10 Xenopus laevis embryo. The cell sheet (blastocoel roof) that supports the migration of mesendoderm cells in vivo is removed (short arrows indicate dissection) and placed matrix-side down on a tissue culture dish. The position and orientation of the tissue is marked on the dish. After 2 h, the tissue is removed leaving the deposited ECM. (B) Directed Migration Assay. An explant of anterior mesendoderm (circle) is dissected at stage 10.5 and placed on the ECM at the mid point between the blastocoel lip (BL) and animal pole (AP) marks. The explant position is recorded immediately and 1 h later. (C) Following the migration assay, the conditioned substrata are subjected to immunocytochemistry for fibronectin. (D) RT-PCR analysis of anterior mesendoderm explants. (E–G) The position of each mesendoderm explant after 1 h is plotted with all starting positions superimposed on the origin. Positive y values indicate movement toward the animal pole, the normal direction of migration. (E) Control, (F) 0.1 U/mL heparinase III included throughout the experiment. (G) ECM treated with 0.1 U/mL heparinase III for 45 min immediately following its deposition. (H) Bar graph of data in E–G. Direction of movement: animal pole (black bars), blastopore lip (gray bars), lateral (moved <15 μm on the y axis but >15 μm on the x axis; hatched bars), stalled (moved less that 15 μm on both axes; white bars). (I) Graph indicating the absence (black bars) or presence (hatched bars) of apoptotic cells in the blastocoel cavity following microinjection of vehicle, heparinase III, or heat-inactivated heparinase III. Dead embryos (white bars). All experiments were repeated at least three times. [Scale bar, 10 μm (E; applies to frames C–E.)]
To examine whether endogenous heparan sulfate proteoglycans (HSPGs) are required for the PDGF-AA-guided mesendoderm movement, heparinase III, which degrades heparan sulfate chains, was included in the ex vivo directed migration assay. Briefly, the native substratum for anterior mesendoderm migration was transferred to a tissue culture dish in the absence or presence of 0.1 U/mL heparinase III. In addition, substrata deposited in the absence of heparinase III, were treated immediately with 0.1 U/mL heparinase III for 45 min. Anterior mesendoderm explants (circled tissue in Fig. 2B) were dissected at stage 10.5 (the stage at which directed migration is normally occurring in vivo) and placed individually on substrata at the midpoint between the BL and AP marks. The position of each explant was recorded immediately, and then again 1 h later, and the direction and distance moved was assessed (see below). Time-lapse micrography was also used to monitor explants. Under all ECM treatment conditions, explants extended cytoplasmic protrusions such as lamellipodia around their entire circumference (Fig. S1). Movement forward was immediately preceded by a collapse of these protrusions at the lagging edge of the explant. Within 5 min of this collapse, cytoplasmic protrusions were again extended around the explants circumference.
To confirm correct deposition of each substratum, they were subjected to immunocytochemistry with an antibody to fibronectin immediately following the migration assay (Fig. 2C). Similar networks of fibronectin fibrils were deposited in the presence or absence of heparinase III treatment (Fig. 2C). Data obtained from substrata that lacked this network were discarded.
Microinjection of heparinase III into the blastocoel cavity during the blastula stages was shown previously to inhibit mesoderm formation (26). To confirm that heparinase III treatment during the gastrula stages did not have a similar affect, RT-PCR was performed on anterior mesendoderm explants cultured in the absence or presence of heparinase III. Expression of Xbrachyury (Xbra; pan mesoderm marker), chordin (dorsal mesoderm marker), and goosecoid (gsc; anterior mesoderm marker) were similar in untreated and heparinase III treated explants, suggesting that this tissue retained its mesoderm identity under these conditions (Fig. 2D).
As expected, in the directed migration assay the majority of control explants moved in their normal direction, toward the animal pole (72%). The remaining control explants either moved in the wrong direction, that is laterally (15%) or away from the animal pole (7%), or were stalled (6%) (Fig. 2 E and H and Table S1). In contrast, heparinase III treatment throughout the experiment randomized the direction of explant movement (P = 0.004), with only 44% having moved toward the animal pole. Of the remaining explants, 26% moved away from the animal pole, 20% moved laterally, and 10% stalled (Fig. 2 F and H and Table S1). Interestingly, when the ECM was treated with heparinase III after it had been transferred to the tissue culture dish, most explants moved in the normal, animal direction (60%; Fig. 2 G and H and Table S1), which was statistically similar to control explants (P = 0.37). The randomization of directed mesendoderm migration on matrices deposited in the presence of heparinase III, but not when the substratum was treated after deposition, is consistent with our in vitro data, suggesting that heparan sulfate stably modifies fibronectin to reveal binding sites for PDGF-AA.
To investigate whether a loss of heparan sulfate blocks PDGF signaling, the ability of heparinase III to induce apoptosis in migrating anterior mesendoderm cells was examined (Fig. 2I). Previous work showed that inhibition of PDGF signaling in vivo caused mesendoderm cells to die by apoptosis (10, 27). Microinjection of heparinase III into the blastocoel cavity at stage 6.5, the stage at which the most severe defects were seen in embryos in previous studies (ref. 26 and Fig. S2), did not cause a significant difference in the number of dying cells compared to controls (P > 0.2 in comparisons between all treatments).
Discussion
The data presented in this paper show that PDGF-AA can bind directly to fibronectin. This interaction is significantly enhanced by heparin and suggests that increased PDGF-AA binding sites on fibronectin are revealed by heparin treatment. Importantly, we found that removal of heparan sulfate from the native ECM causes disordered migration of mesendoderm cells in an ex vivo assay. Disordered migration only occurred, however, when the heparan sulfate was removed during deposition of the ECM. These findings indicate that the ability of PDGF-AA to interact with the fibronectin-rich ECM and guide cell migration during gastrulation may be controlled by heparan sulfate-mediated alterations in fibronectin that expose PDGF-AA binding sites. Consistent with this model, removal of heparan sulfate in vivo did not cause mesendoderm apoptosis suggesting that PDGF-AA signaling was not disrupted, but rather its localization disrupted. These data are also consistent with our previous findings that showed overexpression of the long form of PDGF-AA, which associates with the ECM via its carboxyl retention motif, disrupted the normal guidance cues and causes randomization of the direction of mesendoderm cell migration, whereas overexpression of the short form of PDGF-AA, which diffuses from its site of secretion, did not (9).
Heparinase III treatment of intact early Xenopus embryos, by microinjection into the blastocoel cavity, has been shown to disrupt mesoderm cell movements and the embryos develop with gastrulation defects (26). Similarly, incubation of presumptive dorsal axial mesoderm explants in heparinase III inhibits convergent extension movements of the tissue (28). In both cases, however, these treatments also inhibited the induction of the mesoderm (26, 28), which occurs before the onset of the cell reorganizations of gastrulation and thus, any direct effect on cell motility was difficult to distinguish. It is thought that this inhibition of mesoderm induction is due to a disruption of fibroblast growth factor (FGF) signaling (26, 28), which requires HSPGs (29–31). It seems unlikely that a disruption of mesoderm formation accounted for the disordered migration of the mesendoderm explants observed in our experiments because the tissues were heparinase III treated during the gastrula stages. There is a role for FGF signaling in the maintenance of the mesoderm at these stages (32), but RT-PCR analysis indicated that expression of mesoderm markers was retained by the explants (Fig. 2D), as was their ability to migrate on the ECM (Fig. 2C), a characteristic behavior of gastrula staged mesendoderm cells (33, 34).
Injection of heparinase III into the blastocoel cavity of Xenopus embryos at stage 10, the onset of gastrulation, alters the fibronectin fibrils on the roof of the blastocoel cavity (35), which are necessary for the guidance of migrating mesendoderm cells by conditioned substratum (36). In addition, fibronectin fibrillogenesis is adversely affected in cells with deficient heparan sulfate synthesis (37). However, in our assays, no differences could be seen between the matrices deposited in the presence or absence of heparinase III (Fig. 2C). This apparent inconsistency may be due to a localized, high concentration of injected heparinase compared with that found in solution. For example, gaps in the blastocoel roof ECM have also been reported in embryos microinjected with dominant negative Syndecan-1 or -2 mRNA (38). The gaps appeared at the injection site, where the concentration of mRNA was highest, but did not fully overlap with the field of diffusion of the mRNA (see figure 1D in ref. 38).
FGF signaling during the blastula stages is also thought to aid in the establishment of the guidance cues for the migrating mesendoderm cells in the embryo (36), which have been shown to be present by the beginning of gastrulation (24, 36). Our experiments began at stage 10+ after the guidance cues are laid down (36). In addition, experiments in which the native ECM was treated with heparinase III after it had been deposited on the culture dish did not disrupt the directed migration of mesendoderm cells (36).
Fibronectin is a modular protein that has a high degree of conformational flexibility that allows it to transition between globular and more extended forms (39–41). The transition from a globular to a more extended form can be mediated by factors including treatment with heparin/heparan sulfate (14). The extended conformation that is generated by heparin/heparan sulfate treatment is stable and does not require continued treatment for maintenance (14, 18). It is likely therefore, that in the ex vivo migration assay, once the native ECM had been deposited onto the culture dish in the presence of endogenous heparan sulfate, the fibronectin had already assumed an extended form where growth factor binding sites were revealed (14). When heparinase III was present during matrix deposition, however, it would have digested heparan sulfate and likely prevented the formation of matrix containing the extended form of fibronectin. The fact that heparinase III treatment after matrix deposition did not disrupt the irection of mesendoderm migration ex vivo (36), further suggests that PDGF-AA interacts directly with fibronectin in this assay rather than via an HSPG, since in this instance, heparinase III would have degraded HSPGs causing the release of HS-bound PDGF-AA from the HSPGs (43).
Taken together, these data support a model in which PDGF-A secreted from the ectodermal cells at the gastrula stages binds to HSPG-modified fibronectin in the blastocoel roof ECM to guide the directed migration of mesendoderm cells during Xenopus gastrulation. The dynamics of the PDGF-AA interaction with fibronectin will be important to consider as the kinetics of binding and release from the matrix may ultimately be responsible for controlling cell activation. The native HSPGs involved in this process are not known and require further investigation, however, several HSPGs are expressed in Xenopus embryos in tissue specific patterns during gastrulation including Syndecans-1 and -2 [expressed in the ectoderm (44)] and Syndecan-4, Glypican-4, and Biglycan [expressed in the ectoderm and mesoderm (44–47)]. Although the role(s) of these HSPGs in mesendoderm migration have not been investigated, they are involved in a wide range of processes that involve cell rearrangements including embryonic development, wound healing, angiogenesis, neuronal cell migration and cancer cell invasion through their interactions with ECM molecules, growth factors, cytokines, and chemokines (38, 48–51). For example, as discussed above, knock-down of either Syndecan-1 or -2 disrupts the blastocoel roof ECM and results in gaps in fibril deposition (38). In addition, Syndecan-4 and Glypican-4 are both essential for convergent extension, another important form of cell movement during gastrulation. Syndecan-4 also plays a role in the directional migration of neural crest cells (45, 46, 52). In these cases, Syndecan-4 and Glypican-4 act by regulating the planar cell polarity (PCP) pathway, which is involved in polarized matrix deposition during convergent extension (53). Compellingly, these studies show that embryos develop with anterior defects when either Syndecan-4 or Glypican-4 is knocked down, indicating that mesendoderm cell migration is also likely to be affected, and making them promising candidates for future studies.
It is possible that the specific structural composition of the heparan sulfate chains provides an additional element of control as we have previously noted that heparin/heparan sulfate-mediated modulation of fibronectin depends on the length and sulfation pattern of the polysaccharide (14). Thus, localized control of heparan sulfate biosynthesis through the regulation of the array of enzymes involved might be a critical component to this process. In any case, the present study provides strong evidence that heparan sulfate and HSPGs may function to modulate directed cell movements by controlling PDGF-AA deposition within the fibronectin-rich ECM of the blastocoel roof.
Materials and Methods
PDGF-AA Binding Assays.
125I-PDGF-AA was prepared and binding to human plasma fibronectin (40 nM; Chemicon International, Inc.) measured as described for 125I-VEGF (54). 125I-PDGF-AA (100 ng/mL; 50 μL per well) in binding buffer (25 mM Hepes and 0.15 M NaCl, pH 5.5–9.0) was incubated with fibronectin coated surfaces for 1 h at 4 °C and bound PDGF-AA was extracted with buffer (50 μL per well) containing 25 mM Hepes and 5M NaCl, pH 7.5, for 30 min at room temperature. The amount of extracted 125I-PDGF-AA was quantified using a Cobra AutoGamma 5005 counter (Packard Instruments).
Embryos, Substratum Conditioning, and Directed Migration Assay.
Embryos were fertilized in vitro, chemically dejellied in 2% cysteine, pH 7.8, and cultured in 10% Marc's Modified Ringer's [MMR (55)] at temperatures between 14 °C and 23 °C. They were staged according to Nieuwkoop and Faber (56).
The ex vivo assay used to examine directed mesendoderm migration was performed as described (9, 36) and summarized in the legend for Fig. 2 A and B. Transfer of the native ECM for cell migration was carried out in the presence or absence of 0.1 U/mL heparinase III (IBEX) at 14 °C for 2 h. A subset of the substrata transferred without heparinase III, were also heparinase III treated for 45 min. The position of plated mesendoderm explants was recorded using a COOLPIX 990 digital camera (Nikon) mounted on a SV6-GFP Microscope (Carl Zeiss Inc.). Time-lapse micrography of mesendoderm explants was performed using OpenLab software (Improvision) and an Orca Digital CCD camera (Hamamatsu Photonics) mounted on an Axiovert LM35 (Carl Zeiss Inc.). The distance and direction traveled by mesendoderm explants were assessed using Adobe Photoshop (Adobe). The data were analyzed using a X2 test with Bonferroni correction for increased stringency.
Immunocytochemistry was used to examine conditioned substrata for an evenly deposited fibrillar fibronectin matrix. Data obtained using conditioned substrata without evenly deposited fibronectin were discarded.
RT-PCR.
Anterior mesendoderm explants were dissected at stage 10.5 and incubated in the presence or absence of 0.1 U/mL heparinase III for 1 h (equivalent to the enzyme exposure in directed migration assays). Ten explants were pooled and immediately frozen on dry ice. RNA was extracted using the RNeasy Kit (Qiagen), and 200–400 ng RNA was used to generate cDNA using the SuperScript III First Strand Synthesis Kit (Invitrogen). The cDNA was subject to RT-PCR using GoTaq Green Master Mix (Promega) and PCR primers for gsc (57), chordin (58), and Xbra (57, 59).
Apoptosis Assay.
At the 2–4-cell stage, 750 pg mRNA encoding β-galactosidase containing a nuclear localization signal [β-gal (60)] was microinjected into the prospective dorsoanterior mesoderm. mRNA was synthesized using the mMessage mMachine Kit [Applied Biosystems/Ambion (10)]. At stage 6.5, 25 nL vehicle, heparinase III, or heat-inactivated heparinase III (final concentrations of 1.8 × 10−5 units) was microinjected into the blastocoel cavity. At stage 10.5 the embryos were fixed and processed for β-gal staining (10). Embryos were bisected and scored for the presence of apoptotic cells in the blastocoel cavity (27). The data were analyzed using a X2 test with Bonferroni correction for increased stringency.
Supplementary Material
Acknowledgments.
We thank members of the Boston University School of Medicine DIG and especially Isabel Dominguez for critical comments on this work, Castera Bresilla for his technical help, Marina Malikova for teaching E.M.S. the ex vivo migration assay, and Rudi Winklbauer for the gift of the fibronectin antibody. Many thanks go to Mike Levin and his laboratory for supplying embryos when our colony was producing poor quality eggs. Statistical analyses were performed in collaboration with H. Cabral, Boston University School of Public Health, Boston, Massachusetts. M.A.N. has served as a consultant for Momenta Pharmaceuticals, Inc. This work was supported in part by National Institutes of Health Grants CA87375 (to K.S.) and HL056200 (to M.A.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902510106/DCSupplemental.
References
- 1.Czirok A, Zamir EA, Filla MB, Little CD, Rongish BJ. Extracellular matrix macroassembly dynamics in early vertebrate embryos. Curr Top Dev Biol. 2006;73:237–258. doi: 10.1016/S0070-2153(05)73008-8. [DOI] [PubMed] [Google Scholar]
- 2.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 4.Boucaut J-C, et al. Evidence for the role of fibronectin in amphibian gastrulation. J Embryol Exp Morph. 1985;89:211–227. [PubMed] [Google Scholar]
- 5.Nakatsuji N, Johnson KE. Comparative study of extracellular fibrils on the ectodermal layer in gastrulae of five amphibian species. J Cell Sci. 1983;59:61–70. doi: 10.1242/jcs.59.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Lee G, Hynes RO, Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984;36:729–740. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- 7.Darribère T, et al. In vivo analyses of integrin β1 subunit function in fibronectin matrix assembly. J Cell Biol. 1990;110:1813–1823. doi: 10.1083/jcb.110.5.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: Position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–240. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- 10.Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- 11.Raines EW, Bowen-Pope DF, Ross R. In: Peptide Growth Factors and Their Receptors. Sporn MB, Roberts AB, editors. Vol 1. New York: Springer; 1991. pp. 173–262. [Google Scholar]
- 12.Betsholtz C. Biology of platelet-derived growth factors in development. Birth Defects Res C Embryo Today. 2003;69:272–285. doi: 10.1002/bdrc.10030. [DOI] [PubMed] [Google Scholar]
- 13.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 14.Mitsi M, Hong Z, Costello CE, Nugent MA. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–10328. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- 15.Goerges AL, Nugent MA. pH regulates vascular endothelial growth factor binding to fibronectin: A mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307–2315. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 16.Wijelath ES, et al. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25–31. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- 17.Wijelath ES, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: Enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsi M, Forsten-Williams K, Gopalakrishnan M, Nugent MA. A catalytic role of heparin within the extracellular matrix. J Biol Chem. 2008;283:34796–34807. doi: 10.1074/jbc.M806692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 20.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumor pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 21.Turin L, Warner AE. Intracellular pH in early Xenopus embryos: Its effect on current flow between blastomeres. J Physiol. 1980;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller RE, Danilchik M, Gimlich R, Shih J. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol. 1985;89(Suppl):185–209. [PubMed] [Google Scholar]
- 23.Gillespie JI. The distribution of small ions during the early development of Xenopus laevis and Ambystoma mexicanum embryos. J Physiol. 1983;344:359–377. doi: 10.1113/jphysiol.1983.sp014945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winklbauer R, Nagel M. Directional mesoderm cell migration in the Xenopus gastrula. Dev Biol. 1991;148:573–589. doi: 10.1016/0012-1606(91)90275-8. [DOI] [PubMed] [Google Scholar]
- 25.Winklbauer R, Selchow A, Nagel M, Stoltz C, Angres B. In: Gastrulation: Movements, Patterns, and Molecules. Keller R, Clark WH, Griffin F, editors. New York: Plenum Press; 1991. pp. 147–168. [Google Scholar]
- 26.Brickman MC, Gerhart JC. Heparitinase inhibition of mesoderm induction and gastrulation in Xenopus laevis embryos. Dev Biol. 1994;164:484–501. doi: 10.1006/dbio.1994.1218. [DOI] [PubMed] [Google Scholar]
- 27.Van Stry M, McLaughlin KA, Ataliotis P, Symes K. The mitochondrial-apoptotic pathway is triggered in Xenopus mesoderm cells deprived of PDGF receptor signaling during gastrulation. Dev Biol. 2004;268:232–242. doi: 10.1016/j.ydbio.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Itoh K, Sokol SY. Heparan sulfate proteoglycans are required for mesoderm formation in Xenopus embryos. Development. 1994;120:2703–2711. doi: 10.1242/dev.120.9.2703. [DOI] [PubMed] [Google Scholar]
- 29.Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 30.Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 31.Chua CC, Rahimi N, Forsten-Williams K, Nugent MA. Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ Res. 2004;94:316–323. doi: 10.1161/01.RES.0000112965.70691.AC. [DOI] [PubMed] [Google Scholar]
- 32.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantation reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 33.Keller R. Cell migration during gastrulation. Curr Opin Cell Biol. 2005;17:533–541. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Wacker S, Brodbeck A, Lemaire P, Niehrs C, Winklbauer R. Patterns and control of cell motility in the Xenopus gastrula. Development. 1998;125:1931–1942. doi: 10.1242/dev.125.10.1931. [DOI] [PubMed] [Google Scholar]
- 35.Yost HJ. Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature. 1992;357:158–161. doi: 10.1038/357158a0. [DOI] [PubMed] [Google Scholar]
- 36.Nagel M, Winklbauer R. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development. 1999;126:1975–1984. doi: 10.1242/dev.126.9.1975. [DOI] [PubMed] [Google Scholar]
- 37.Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci. 1997;110:1413–1419. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- 38.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–124. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 39.Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983;258:14539–14544. [PubMed] [Google Scholar]
- 40.Williams EC, Janmey PA, Ferry JD, Mosher DF. Conformational states of fibronectin. Effects of pH, ionic strength, and collagen binding. J Biol Chem. 1982;257:14973–14978. [PubMed] [Google Scholar]
- 41.Rocco M, Carson M, Hantgan R, McDonagh J, Hermans J. Dependence of the shape of the plasma fibronectin molecule on solvent composition. Ionic strength and glycerol content. J Biol Chem. 1983;258:14545–14549. [PubMed] [Google Scholar]
- 42.Bergkvist M, Carlsson J, Oscarsson S. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of atomic force microscopy. J Biomed Mater Res A. 2003;64:349–356. doi: 10.1002/jbm.a.10423. [DOI] [PubMed] [Google Scholar]
- 43.Andersson M, Ostman A, Westermark B, Heldin CH. Characterization of the retention motif in the C-terminal part of the long splice form of platelet-derived growth factor A-chain. J Biol Chem. 1994;269:926–930. [PubMed] [Google Scholar]
- 44.Teel AL, Yost HJ. Embryonic expression patterns of Xenopus syndecans. Mech Dev. 1996;59:115–127. doi: 10.1016/0925-4773(96)00584-9. [DOI] [PubMed] [Google Scholar]
- 45.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signaling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 46.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 47.Moreno M, et al. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development. 2005;132:4621–4633. doi: 10.1242/dev.02042. [DOI] [PubMed] [Google Scholar]
- 50.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol. 2001;12:107–116. doi: 10.1006/scdb.2000.0239. [DOI] [PubMed] [Google Scholar]
- 52.Matthews HK, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 53.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 54.Goerges AL, Nugent MA. Regulation of vascular endothelial growth factor binding and activity by extracellular pH. J Biol Chem. 2003;278:19518–19525. doi: 10.1074/jbc.M211208200. [DOI] [PubMed] [Google Scholar]
- 55.Peng HB. In: Xenopus laevis: Practical Uses in Cell and Molecular Biology. Kay BK, Peng HB, editors. Vol 36. San Diego, CA: Academic; 1991. pp. 657–662. [Google Scholar]
- 56.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- 57.Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 58.Sasai Y, et al. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith JC, Price BMJ, Green JBA, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 60.Vize PD, Melton DA. In: Xenopus laevis: Practical Uses in Cell and Molecular Biology. Kay BK, Peng HB, editors. Vol 36. San Diego, CA: Academic; 1991. pp. 367–387. [Google Scholar]
- 61.Winklbauer R, Keller RE. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev Biol. 1996;177:413–426. doi: 10.1006/dbio.1996.0174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.