Abstract
The arterial response to injury is dominated by proliferation of smooth muscle cells and infiltration of blood-borne cells in the vascular intima. Arterial smooth muscle cell proliferation is under growth factor control, but how this regulation operates in vivo is unclear. We studied the effect on arterial response to mechanical injury of cyclosporin A, a drug that inhibits T-lymphocyte activation. Cyclosporin A treatment at surgery caused a persistent inhibition of the intimal proliferative lesion. Cyclosporin A also inhibited expression of Ia antigens on smooth muscle cells in situ but had no direct effects on smooth muscle cell proliferation in culture. Therefore, the inhibition of intimal cell proliferation appears to be mediated via the immune system.
Full text
PDF
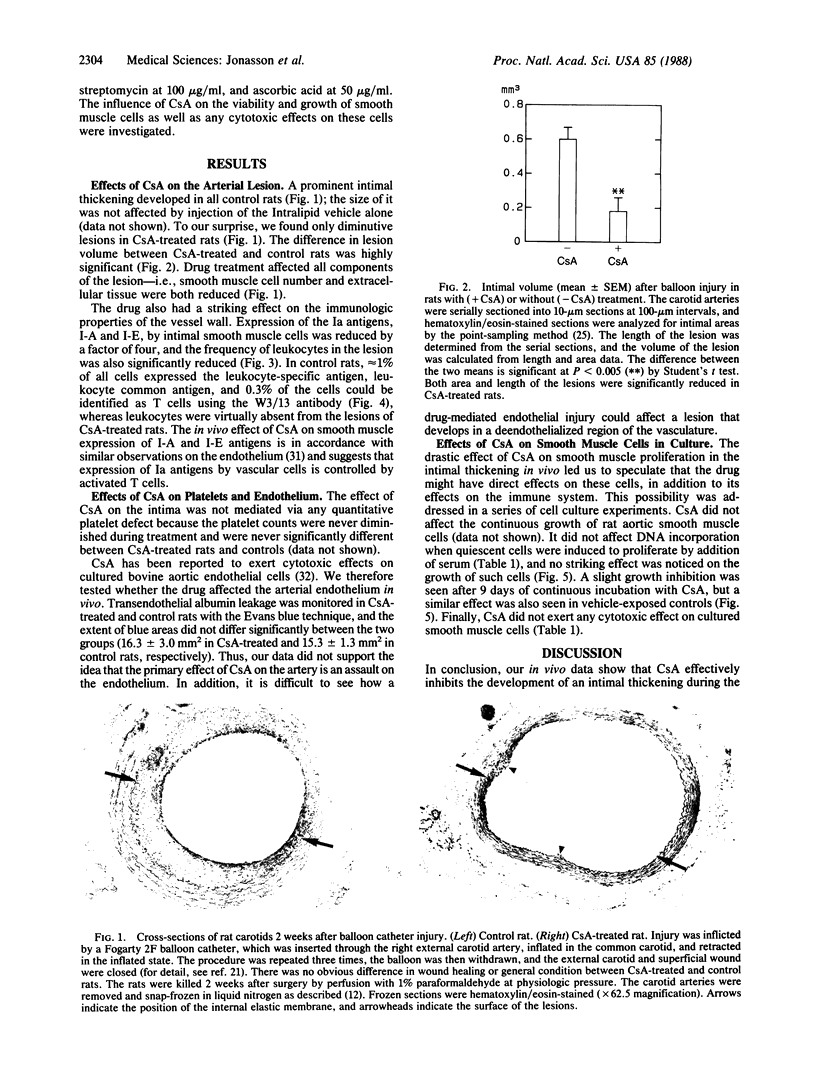
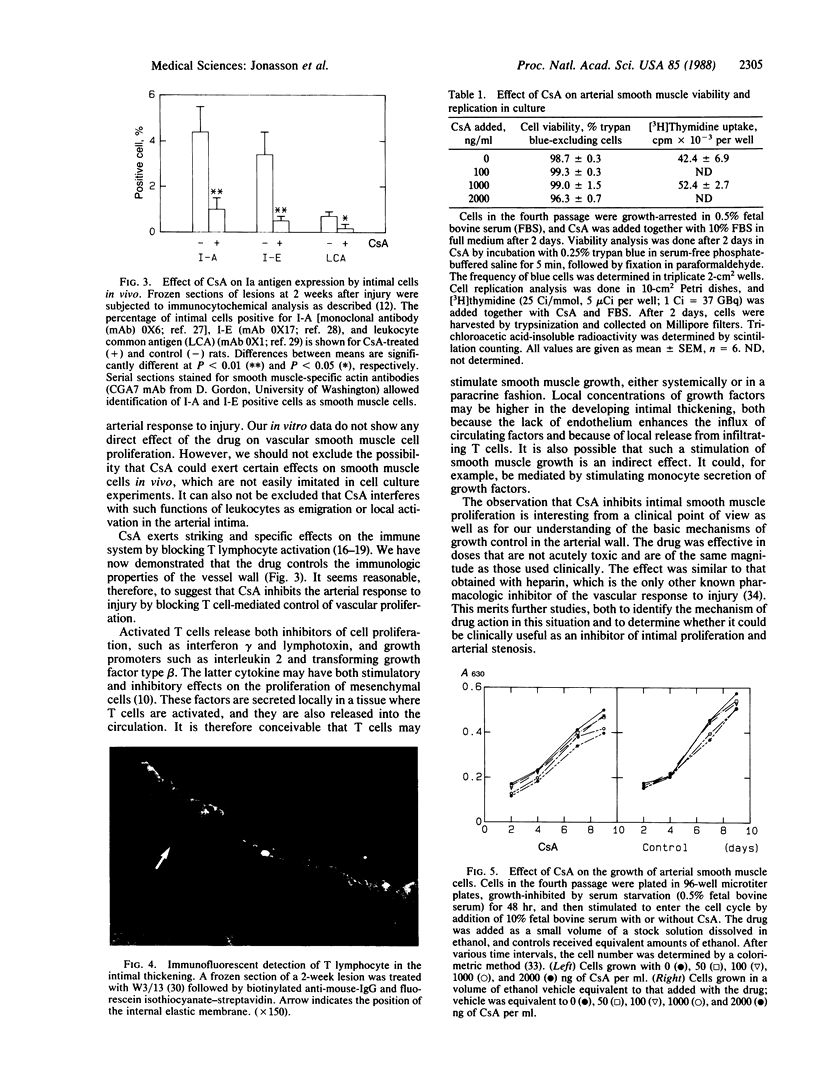

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autenried P., Halloran P. F. Cyclosporine blocks the induction of class I and class II MHC products in mouse kidney by graft-vs-host disease. J Immunol. 1985 Dec;135(6):3922–3928. [PubMed] [Google Scholar]
- BAUMGARTNER H. R. EINE NEUE METHODE ZUR ERZEUGUNG VON THROMBEN DURCH GEZIELTE UBERDEHNUNG DER GEFAESSWAND. Z Gesamte Exp Med. 1963 Sep 12;137:227–247. [PubMed] [Google Scholar]
- Benveniste E. N., Merrill J. E. Stimulation of oligodendroglial proliferation and maturation by interleukin-2. Nature. 1986 Jun 5;321(6070):610–613. doi: 10.1038/321610a0. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Britton S., Palacios R. Cyclosporin A--usefulness, risks and mechanism of action. Immunol Rev. 1982;65:5–22. doi: 10.1111/j.1600-065x.1982.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Khandjian E. W., Weil R. Cyclosporin A prevents induction of the interleukin 2 receptor gene in cultured murine thymocytes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6430–6434. doi: 10.1073/pnas.83.17.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G. Endothelial proliferation and atherogenesis in rabbits with moderate hypercholesterolemia. Artery. 1980;7(4):316–329. [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Burckart G. J., Venkataramanan R. Cyclosporine. Drug Intell Clin Pharm. 1985 Feb;19(2):90–100. doi: 10.1177/106002808501900202. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Selden S. C., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Whiting P. H., Simpson J. G. Cyclosporine: immunology, toxicity and pharmacology in experimental animals. Agents Actions. 1984 Oct;15(3-4):306–327. doi: 10.1007/BF01972366. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Zoja C., Furci L., Ghilardi F., Zilio P., Benigni A., Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986 Oct;55(4):455–462. [PubMed] [Google Scholar]