Abstract
Rett syndrome (RTT) is characterized by specific motor, cognitive, and behavioral deficits. Because several of these abnormalities occur in other disease states associated with alterations in aminergic neurotransmitters, we investigated the contribution of such alterations to RTT pathogenesis. We found that both individuals with RTT and Mecp2-null mice have lower-than-normal levels of aminergic metabolites and content. Deleting Mecp2 from either TH-positive dopaminergic and noradrenergic neurons or PET1-positive serotonergic neurons in mice decreased corresponding neurotransmitter concentration and specific phenotypes, likely through MeCP2 regulation of rate-limiting enzymes involved in aminergic neurotransmitter production. These data support a cell-autonomous, MeCP2-dependent mechanism for the regulation of aminergic neurotransmitter synthesis contributing to unique behavioral phenotypes.
Keywords: dopamine, norepinephrine, Rett syndrome, serotonin
Rett syndrome (RTT, MIM 312750) is an X-linked neurodevelopmental disorder caused, in the vast majority of cases, by mutations in Methyl-CpG Binding Protein 2 (MECP2) (1). RTT primarily affects females, with a milder clinical phenotype correlating with late truncating and some missense mutations (2, 3). It has been suspected that aminergic neurons malfunction in RTT, because the heightened anxiety, aggression, and mood alterations seen in RTT and MECP2 disorders are associated with abnormalities of the serotonergic system in other human disorders (4–6). Rigidity and movement abnormalities are associated with dysfunction of dopaminergic system (7–9). Autonomic dysfunction underlying breathing irregularities could be attributable to decreased norepinephrine (7, 10). Although clinical studies have explored the hypothesis that the aminergic neurotransmitter systems might be altered in RTT, no solid conclusions have been reached. Early work suggested decreased aminergic metabolite levels in the spinal fluid of individuals with RTT (11, 12), but subsequent reports failed to identify such changes (13, 14). More recently, one study reported instances of both decreased and increased aminergic metabolite levels in a few individuals with RTT (15). The interpretation of these clinical data are hindered by clinical heterogeneity, small sample sizes, and lack of a sufficient number of controls. To circumvent these problems, we explored the role of the aminergic neurotransmitter system by performing both clinical and animal studies. To determine whether the aminergic neurotransmitter system is altered in RTT, we analyzed the levels of the dopamine metabolite homovanillic acid (HVA) and the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in spinal fluid from women who both met the clinical criteria for RTT and had a disease-causing MECP2 mutation. We also evaluated neurotransmitter changes in a RTT murine model and studied the neurochemical, molecular, and behavioral consequences of deleting Mecp2 from either TH-positive dopaminergic and noradrenergic neurons or PET1-positive serotonergic neurons in the mouse.
Results
HVA and 5-HIAA Are Decreased in RTT Individuals and Mecp2null/y Mouse Brain.
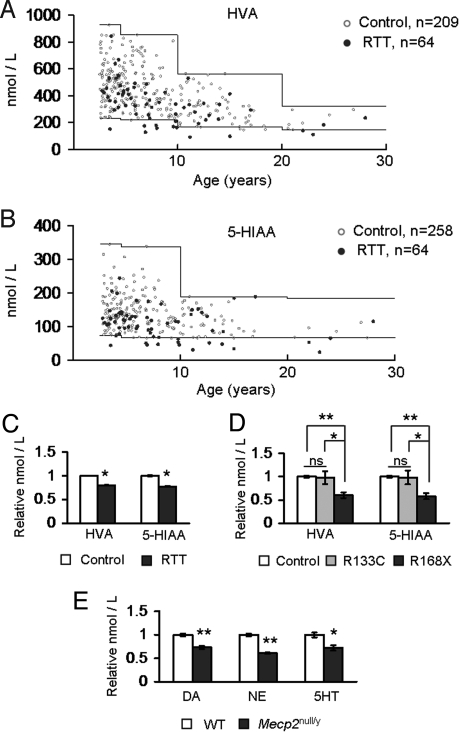
We analyzed the metabolites of aminergic neurotransmitters in spinal fluid from 64 RTT individuals and >200 normative samples used to define the reference ranges using HPLC (16). A significant fraction of RTT individuals had HVA levels below the reference range (12/64, ≈19%, Fig. 1A). Similarly, a significant fraction of individuals with RTT had 5-HIAA levels below the reference range (15/64, ≈23%, Fig. 1B). In contrast, only ≈1% of control individuals (3/209) had low HVA levels, and ≈1% of control individuals (3/258) had 5-HIAA levels that were slightly below the threshold level. These numbers were significantly different from those seen in the RTT group (Fisher's exact test, P < 0.001 for each). Furthermore, analysis of the age-adjusted mean values of HVA and 5-HIAA demonstrated a significant decrease in individuals with RTT compared with controls (Fig. 1C). A comparison of the metabolite levels in RTT individuals carrying two specific mutations that confer either severe (p.Arg168X, n = 12/64 RTT individuals) or mild (p.Arg133Cys, n = 7/64 RTT individuals) phenotypes (2) revealed that 33% (n = 4/12) and 50% (n = 6/12) of the individuals with the p.Arg168X mutation had HVA and 5-HIAA levels below the reference range, respectively, whereas only one person with the p.Arg133Cys mutation had HVA and 5-HIAA levels below the reference range. Interestingly, further comparison of the age-adjusted mean metabolite levels in individuals with these two mutation types showed that individuals with the p.Arg168X mutation had a significantly greater reduction in HVA and 5-HIAA than individuals with the p.Arg133Cys mutation or the normative control samples. In fact, the age-adjusted mean values for the metabolite levels of individuals with the p.Arg133Cys mutations were similar to those of the normative samples (Fig. 1D).
Fig. 1.
Aminergic metabolite levels and content are reduced by the loss of MeCP2 function. (A) Levels of the dopamine and norepinephrine metabolite, HVA, were reduced in RTT. Normative reference ranges are indicated by the solid lines. (B) The levels of the serotonin metabolite, 5-HIAA, were reduced in RTT. The normative reference ranges are indicated by the solid lines. (C) Age-adjusted mean values for both HVA (mean age = 7.38) and 5-HIAA (mean age = 7.78) were reduced in the spinal fluid of girls with RTT. Values were normalized to control samples. (D) Age-adjusted mean values for HVA (mean age = 7.16) and 5-HIAA (mean age = 7.66) in individuals with the p.Arg168X mutation compared to the normative samples were reduced. In contrast, age-adjusted mean values for HVA and 5-HIAA were similar in individuals with the p.Arg133Cys mutation compared with those of the normative samples. Values were normalized to control samples. (E) Reductions in DA, NE, and 5HT levels were detected in the brains of Mecp2null/y animals. Values were normalized to those of wild-type littermate samples. *, P ≤ 0.05; **, P ≤ 0.001. ns, Not significant. Values represent mean ± SEM. Raw HPLC values are provided in Table S1.
To understand the mechanism underlying these neurotransmitter abnormalities, we analyzed aminergic content in brains of male mice that completely lack MeCP2 function (Mecp2null/y). As previously observed (17), their concentrations of dopamine (DA), norepinephrine (NE), and serotonin (5HT) were lower than those of littermate control animals (Fig. 1E). This confirms that the alterations identified in individuals with RTT result from MeCP2 dysfunction and that the reduced levels seen in humans may be caused by decreased biogenic amine production rather than alterations in the degradative pathways for these amines.
Loss of MeCP2 Causes a Decrease in Expression of Biosynthetic Enzymes Th and Tph2.
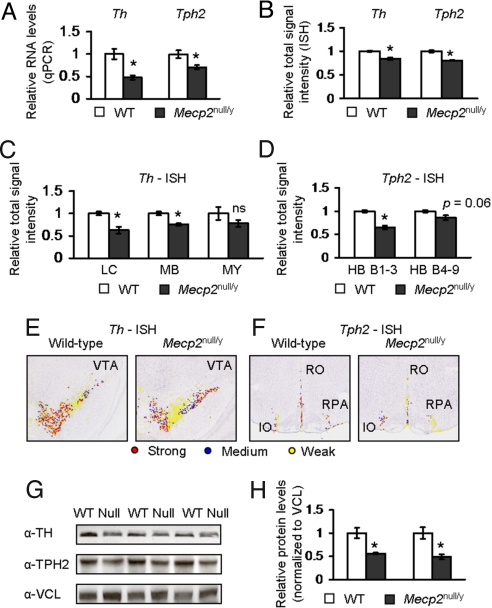
We reasoned that one potential cause of the neurochemical changes that we observed in RTT spinal fluid and Mecp2null/y brain could be defects in the pathways responsible for neurotransmitter synthesis. Tyrosine hydroxylase (Th) and Tryptophan hydroxylase 2 (Tph2) are the key rate-limiting enzymes in the catalysis of tyrosine and tryptophan to produce DA and NE, and 5HT, respectively (16). Defects in the level of these enzymes could cause a concomitant decrease in neurotransmitters; therefore, we assessed the RNA levels of Th and Tph2 by quantitative real-time PCR (qPCR) and quantitative in situ hybridization (ISH), and their corresponding protein levels by Western analysis in Mecp2-null mice. qPCR analysis showed that the levels of Th and Tph2 were reduced in Mecp2null/y animals compared with control littermates (Fig. 2A). Moreover, quantitative ISH analysis (18) of the signal intensity showed a reduction in the total signal intensity of Th and Tph2 in Mecp2null/y whole brain compared with control littermate brain (Fig. 2B). Examination of signal intensities in different brain regions showed that the Th signal intensities in the locus ceruleus and the midbrain region containing the ventral tegmental area and substantia nigra were reduced compared with control littermate samples (Fig. 2C; representative image shown in Fig. 2E). The total Th signal intensity in the medulla was decreased but failed to reach statistical significance (Fig. 2C). Examination of the Tph2 signal intensities in the hindbrain region containing raphe nuclei B1 through B3 (HB B1–3) revealed a significant reduction in signal intensity (Fig. 2D, representative image shown in Fig. 2F). For regions containing the raphe nuclei B4 through B9 (HB B4–9), we detected a trend toward decreased signal intensity of Tph2 (P = 0.06) (Fig. 2D). Similar reductions in Th and Tph2 expression levels in Mecp2null/y animals were observed upon further separation of the total ISH signal intensity into strong, medium, and weak signal intensities (18) [Supporting Information (SI) Text, Fig. S1 and Fig. S2). Furthermore, analysis of TH and TPH2 protein levels by Western blot analysis showed reduced expression of both synthetic enzymes in Mecp2null/y brain compared with those of wild-type controls (Fig. 2 G and H).
Fig. 2.
Expression of Th and Tph2 is reduced in Mecp2null/y animals. (A) qPCR analysis shows that the transcript levels of Th and Tph2 in Mecp2null/y animals were reduced. (B) Quantification of total Th and Tph2 signal intensities throughout the whole Mecp2null/y brain shows a reduction in both Th and Tph2 expression. (C) Quantification of total Th ISH signal intensity in different brain regions. Total Th signal intensity is reduced in the locus ceruleus (LC) and midbrain (MB) but not medulla (MY). (D) Quantification of total Tph2 ISH signal intensity in two regions of the hindbrain. Total Tph2 signal intensity is reduced in the hindbrain region that encompasses B1 through B3 raphe nuclei (HB B1–3). A trend in reduced signal intensity is observed in the hindbrain region that encompasses B4 through B9 raphe nuclei (HB B4–9). (E) Representative pseudocolored images are shown for Th expression in the ventral tegmental area (VTA). (F) Representative pseudocolored images are shown for Tph2 expression in the inferior olivary complex (IO), raphe nucleus pallidus (RPA), and raphe nucleus obscurus (RO). (G) The protein levels of TH and TPH2 are reduced in Mecp2null/y animals compared with controls. (H) Quantification of TH and TPH2 signal relative to VCL (vinculin) shows that TH and TPH2 are reduced in Mecp2null/y animals. *, P ≤ 0.05; **, P ≤ 0.001. ns, Not significant. Values were normalized to those of wild-type samples and represent mean ± SEM.
Deletion of Mecp2 from Either TH-Positive or PET1-Positive Neurons Results in Specific Phenotypes.
An important question is whether the reduction in biogenic amines and their synthetic enzymes is a direct effect of MeCP2 loss-of-function within the cells that produce these amines or whether it is a non-autonomous effect of disrupting MeCP2 function within other cell populations. Another question is whether the neurochemical changes, possibly caused by abnormal cell-autonomous neurotransmitter synthesis, contribute to specific clinical features of RTT. To address these questions, we removed Mecp2 specifically from either TH-expressing dopaminergic and noradrenergic neurons or PC12 ets factor 1 (PET1)-expressing serotonergic neurons using Cre-lox technology to generate conditional knock-out (CKO) animals. The resulting TH-CKO and PET1-CKO mice allowed characterization of the molecular and phenotypic consequences of deleting Mecp2 in these particular aminergic neurons.
We bred male transgenic mice that express Cre recombinase from either the TH or PET1 regulatory regions with female mice that carry a Mecp2 allele flanked by loxP sites to produce male mice that were wild-type, TH- or PET1-Cre, Mecp2flox/y (Flox), or TH-Cre; Mecp2flox/y (TH-CKO) or PET1-Cre; Mecp2flox/y (PET1-CKO). The TH-Cre transgenic mouse line expresses Cre in all TH expressing regions of the brain, including the substantia nigra/ventral tegmental area, locus ceruleus, and medullary norepinephrine neurons, as well as in the peripheral nervous system and the adrenal medulla (19). In contrast, the PET1-Cre transgenic mouse line expresses Cre driven by the PET1 enhancer element in the dorsal and medial raphe nuclei (20). The recombination efficiency of each Cre line was tested by immunofluorescence, showing that Mecp2 was effectively deleted from the specified brain regions (SI Text and Fig. S3).
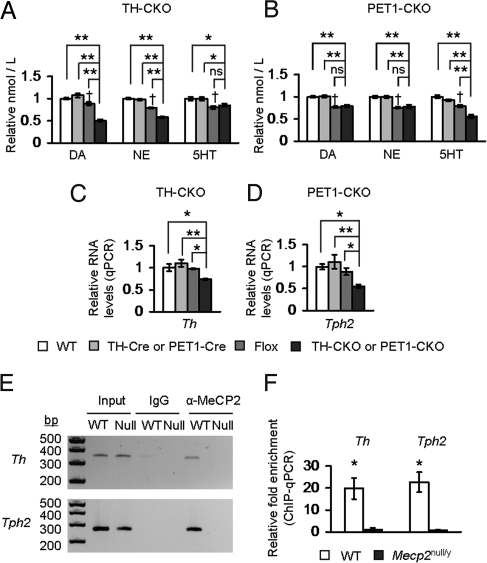
Whole-brain HPLC analysis of neurotransmitter levels in each CKO line showed neurochemical changes influenced by the presence of the Mecp2flox allele. This was expected, given the recent report that the Mecp2flox allele is a hypomorphic allele (21). However, the respective biogenic amines in CKO animals were markedly reduced in comparison to Flox littermate animals. TH-CKO animals displayed reduced DA and NE levels but 5HT levels that were similar to those of Flox animals (Fig. 3A), whereas PET1-CKO animals displayed reduced 5HT levels but DA and NE levels that were similar to those of Flox animals (Fig. 3B). To probe the mechanism underlying these neurochemical changes, we assessed the expression levels of Th and Tph2. Th was reduced in the TH-CKO animals, whereas Tph2 was reduced in the PET1-CKO animals (Fig. 3 C and D). Thus, the loss of MeCP2 causes a cell-autonomous reduction of biogenic amines comparable to the decreased neurotransmitter content observed in Mecp2null/y animals, and this reduction is associated with decreased levels of rate-limiting enzymes responsible for the production of those amines. We also found, by chromatin immunoprecipitation (ChIP) using an anti-MeCP2 antibody, that MeCP2 occupies the promoters of these genes, suggesting that MeCP2 may directly regulate the expression of these genes (Fig. 3 E and F).
Fig. 3.
Removing MeCP2 from TH or PET1-neurons causes cell-autonomous effects on biogenic amines and expression of synthetic enzymes. (A) Selective removal of MeCP2 from TH neurons (TH-CKO) caused a reduction in DA and NE levels. 5HT levels were unchanged compared with Flox animals. (B) Selective removal of MeCP2 from serotonergic neurons (PET1-CKO) caused a reduction in 5HT levels. DA and NE levels were unchanged compared with the Flox animals. Values were normalized to wild-type samples. (C and D) The expression levels of the biosynthetic enzymes (Th and Tph2) involved in the production of DA, NE, and 5HT were reduced in a cell-autonomous fashion. Th levels were decreased in TH-CKO animals (C); Tph2 levels were decreased in PET1-CKO animals (D). Values were normalized to wild-type samples. (E) ChIP-PCR showed that MeCP2 was bound to the Th and Tph2 promoters. Representative gel image is shown. (F) ChIP-qPCR showed that MeCP2 is significantly enriched within 1kb of the promoter regions of Th and Tph2. The fold enrichment of chromatin fragments immunoprecipitated with anti-MeCP2 antibody compared with a control antibody (normal rabbit IgG), relative to input samples is shown (ddCT method). *, P ≤ 0.05; **, P ≤ 0.001; †Flox effect compared with WT or Cre, P ≤ 0.05. ns, Not significant. Values represent mean ± SEM. Raw HPLC values are provided in Table S1.
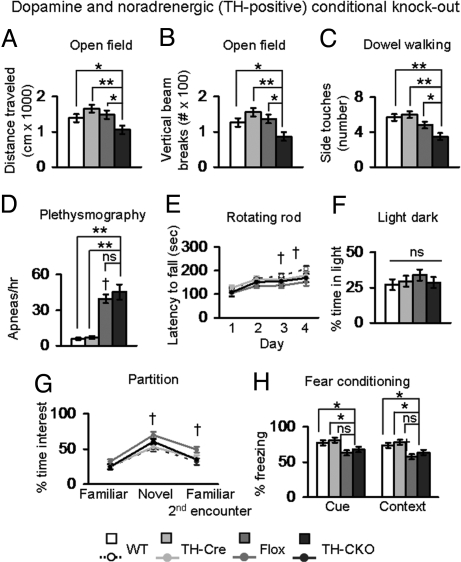
To determine the behavioral consequences of removing MeCP2 from these specific neuronal populations, we performed a detailed behavioral analysis of both the TH-CKO and the PET1-CKO animals, selecting behavioral tests relevant to phenotypes seen in RTT and MeCP2 mouse models (see SI Text and Table S2 for the list of the specific behavioral assays, the age of the animals at the time of each test, and a complete summary of statistical analyses). TH-CKO animals showed a specific alteration in locomotor activity, as demonstrated by decreased total distance and vertical activity in the open field and poor performance on the dowel walking task (Fig. 4 A–C). We investigated the breathing phenotypes of TH-CKO animals because previous work proposed that the breathing abnormalities observed in Mecp2null/y animals were linked to defects in the noradrenergic system (22). The hypomorphic Flox animals had an increased number of apnea episodes (Fig. 4D), possibly because of the decrease in biogenic amine levels (Fig. 3A), but we did not detect a more severe breathing phenotype in the TH-CKO animals beyond those observed in the hypomorphic Flox animals, despite further reduction of NE levels in the TH-CKO animals and deletion of MeCP2 from TH-positive norepinephrine neurons (Fig. S3). We also did not detect abnormalities in motor learning, anxiety, social interaction or learning and memory that were specific to the loss of Mecp2 in TH-positive neurons (Fig. 4 E–H).
Fig. 4.
Removing MeCP2 from TH-neurons causes motor abnormalities. (A) TH-CKO animals traveled less in an open field. (B) TH-CKO animals had less vertical activity in an open field. (C) TH-CKO animals had fewer side touches on a dowel walking task, a measure of motor coordination. (D) Population data of plethysmographic recordings showed a significant increase in the frequency of apneas per hour in Flox animals. No differences were observed between TH-CKO and Flox animals. (E) TH-CKO animals had normal motor learning; a significant Flox effect was observed on days 3 and 4 of testing. (F) No significant differences were observed in the amount of time mice explored the lit side of the light–dark box. (G) TH-CKO animals did not show a significant difference from wild-type and Cre littermate animals in the time spent at the partition (social interest). Flox animals showed a significant increase in the time spent at the partition during a novel encounter and during a second encounter with a familiar partner. (H) TH-CKO animals did not freeze less in the cued stimulus test paradigm. In the context test paradigm, a significant effect of the Flox allele was observed. *, P ≤ 0.05; **, P ≤ 0.001; †, Flox effect compared with WT or Cre, P ≤ 0.05. ns, Not significant. Values represent mean ± SEM.
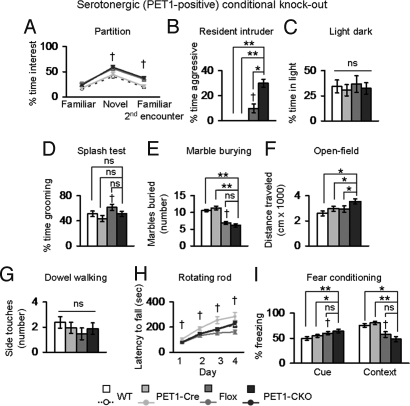
In contrast to TH-CKO animals, PET1-CKO animals did not show decreased motor function. Similar to TH-CKO animals, PET1-CKO animals showed an altered pattern of social interest influenced by the Flox allele (Fig. 5A). Defects in 5HT levels have been linked to aggression in other murine models (4, 6). To test for the presence of increased aggressive behavior, we performed the resident intruder test. PET1-CKO animals were more aggressive when exposed to conspecific partner mice (Fig. 5B). The serotonergic system has also been implicated in anxiety, repetitive behavior, and hyperactivity; therefore, we also tested PET1-CKO animals using assays that would address these behavioral domains (23–25). We found no evidence of altered anxiety in the light–dark box exploration task, altered self-grooming behavior in the splash test, or abnormal repetitive behavior in the marble-burying task that was specific to the deletion of Mecp2 in PET1-positive serotonergic neurons (Fig. 5 C–E). Although the selective deletion of MeCP2 in PET1-positive neurons appeared to increase activity based on total distance traveled, we believe that this resulted from increased baseline activity in mice carrying both the Flox allele and the PET1-Cre transgene. Consistent with this interpretation, a one-way ANOVA among the four genotypes showed a statistically significant increase in distance traveled in PET1-CKO animals compared with littermate control animals, but a two-way ANOVA (MeCP2-Flox allele X PET1-Cre allele) of these data does not support genetic interaction between the two alleles (Fig. 5F, Table S2). Given that the loss of serotonergic neurons causes increased apneas in mice (26), we measured breathing in PET1-CKO animals and their wild-type littermates. We found a significant effect of the Flox allele on the distribution of respiratory frequency with Flox animals showing a greater percentage of time breathing faster than control animals (Fig. S4). However, a further specific reduction of 5HT in the PET1-CKO animals (Fig. 3B) did not worsen the breathing phenotype. PET1-CKO animals showed no specific abnormalities in motor coordination, motor learning or learning and memory (Fig. 5 G–I). Both CKO animals lived until at least 18 months of age; in comparison, the constitutive Mecp2null/y mice on an F1 hybrid background live until 69 days of age (median age, n = 115). This indicates that MeCP2 function is not required in either TH- or PET1-positive neurons for normal lifespan.
Fig. 5.
Removing MeCP2 from PET1-neurons causes aggression. (A) In the partition test for social interaction, PET1-CKO animals, like Flox animals, spent more time interacting with novel mice and when re-exposed to a familiar mouse. (B) PET1-CKO animals were more aggressive in the resident intruder task as measured by the duration of aggression toward intruder conspecific animals. (C) Altered anxiety-like behavior was not observed in the light–dark box exploration task. (D) PET1-CKO animals did not have altered grooming behavior in the splash test; however, Flox animals showed a significant increase in grooming compared with Cre animals. (E) PET1-CKO and Flox animals buried fewer marbles in a task for repetitive behavior compared with littermate controls (F) PET1-CKO animals traveled more in the open field compared to littermate animals because of an additive effect of both the PET1-Cre and Flox alleles. (G) No difference was observed between PET1-CKO and littermate animals in the number of side touches in the dowel walking task. (H) No difference was observed between PET1-CKO and littermate animals in motor learning; Flox animals show a significant decrease in the latency to fall of the rotating rod. (I) No specific effect related to the loss of MeCP2 in PET1-positive neurons was observed in fear conditioning test paradigms. Flox animals froze more in the cued stimulus test paradigm but froze less in the context test paradigm. *, P ≤ 0.05; **, P ≤ 0.001; †, Flox effect compared with WT or Cre, P ≤ 0.05. ns, Not significant. Values represent mean ± SEM.
Discussion
Before the discovery of MECP2 mutations, one of the early hypotheses regarding RTT pathogenesis ascribed a role for defects in aminergic neurotransmitter systems. This was a reasonable hypothesis, as several of the clinical symptoms of RTT overlap with those of clinical disorders associated with abnormalities in DA, NE, and 5HT (4–9, 27). Also, the first study that examined neurotransmitter abnormalities in RTT supported such a hypothesis (11). Subsequent studies ranged from inconclusive to contradictory (13–15). Murine models of MECP2 disorders recapitulated behavioral features that could be linked to neurotransmitter abnormalities (28–32, 22, 33), providing at last the opportunity to address the question in a controlled fashion. In this study, we investigated the role of the aminergic neurotransmitter systems in RTT by first settling the issue on aminergic metabolite levels in human samples and then exploring the biological relevance of these aminergic deficiencies in a RTT murine model. We also investigated the role of MeCP2 in different types of aminergic systems and found that MeCP2 regulates the functionality of aminergic neuronal subtypes in a cell-autonomous manner.
Starting with clinical studies, we show that the loss of MeCP2 function causes a decrease in aminergic metabolites in humans. Because of the large sample size and rigorous methodology used in our analysis, the evidence that both HVA and 5-HIAA are reduced in RTT is strong. Furthermore, we found that individuals carrying the p.Arg168X mutation, who have a more severe RTT phenotype (2), had even more remarkable reductions in HVA and 5-HIAA, whereas individuals with the milder p.Arg133Cys mutation had metabolite levels similar to those of controls. The clinical and aminergic metabolite data support the notion that missense mutations such as the p.Arg133Cys mutation retain some MeCP2 function. Nevertheless, by comparing the age-adjusted mean levels of these metabolites in RTT with reference ranges for the normative samples, we identified an overall significant decrease in the RTT group, indicating a general reduction in aminergic metabolites levels regardless of the type of MECP2 mutation. To determine whether this reduction was caused by a primary defect in the production of amines rather than a secondary defect in aminergic metabolite degradation, we analyzed aminergic neurotransmitter content in Mecp2null/y mouse brain and showed that DA, NE and 5HT are lower than in the brains of control littermates. Our findings in the Mecp2-null mice are concordant with observations made in a previous study (17). Together, the results from both human and murine biological specimens clearly implicate MeCP2 in the regulation of aminergic neurotransmitters.
To gain insight into how MeCP2 dysfunction leads to alterations in aminergic neurotransmitters and their metabolites, we measured the RNA and protein levels of the rate-limiting synthetic enzymes responsible for the production of DA and NE (Th) and 5HT (Tph2). Three different quantitative approaches showed that the expression levels of both synthetic enzymes are indeed reduced in Mecp2null/y brain.
An important question is whether MeCP2 regulates the levels of Th and Tph2 in a cell-autonomous manner. Previous studies have reported both cell-autonomous and non-autonomous effects of MeCP2 in murine models (34–37). To address the role of MeCP2 in aminergic neurons, we selectively deleted MeCP2 in either TH-positive or PET1-positive neurons. Although we cannot fully exclude our results as a consequence of both cell-autonomous and non-autonomous effects, and we cannot exclude the effect of deleting MeCP2 in select neuronal populations in the background of the hypomorphic Flox allele, our data provide strong evidence that the functional consequences of deleting Mecp2 in biogenic amine neurons are specific. A significant finding from our work is that the reduction in the expression levels of Th and Tph2 and their corresponding neurotransmitter levels in the respective aminergic MeCP2-CKO animals are comparable to those observed in Mecp2null/y animals, fully recapitulating the neurochemical and molecular defects. In addition, the unique behavioral phenotypes that we observed in the TH- and PET1-CKO mice are due to the absence of MeCP2 function in either dopaminergic and noradrenergic or serotonergic neurons, respectively, and these phenotypes have been linked to defects in the respective neurotransmitter systems in both humans and mice (4–9, 27). To explore the mechanism underlying the decrease in expression of the synthetic enzymes, we tested whether MeCP2 occupied the Th and Tph2 promoter regions and found that the promoter regions of both neurotransmitter synthesis genes are occupied by MeCP2. These data, combined with our expression data, point to a potential role for MeCP2 in upregulating levels of these genes, which is consistent with recent reports (38, 39, 40). Importantly, these data demonstrate that MeCP2 is critical for the in vivo regulation of neurotransmitter synthesis genes.
A notable observation in the TH-CKO and PET1-CKO animals is the absence of a specific breathing problem. In the hypomorphic Flox animals, decreased NE and 5HT levels may contribute to the breathing phenotype observed in these animals. However, the breathing abnormalities are not worsened in either TH-CKO and PET1-CKO animals that have a further specific reduction of NE or 5HT, respectively. One interpretation is that NE and 5HT levels play little or no role in the control of breathing in the CKO animals. On the other hand, the effect caused by abnormal aminergic neurotransmitter levels is due to the slight reduction in amine concentration seen in the hypomorphic Flox animals but is not worsened by a further reduction. Because breathing defects are prominent in RTT, it will be interesting to determine whether selectively deleting Mecp2 from another neuronal subpopulation or from a combination of various neurons, such as both TH- and PET1-positive neurons, can recapitulate the more robust and complex breathing defects in Mecp2null/y animals. Furthermore, neither the TH-CKO, PET1-CKO nor the Flox animals show the dramatically shortened lifespan seen in the Mecp2-null animals, despite having breathing abnormalities qualitatively similar to the Mecp2null/y animals. We conclude that the reduction in NE and 5HT, and the breathing abnormalities potentially caused by alterations in MeCP2 function is not sufficient to cause death. It is possible that either some secondary phenotypic alterations together with the breathing abnormality are needed to cause death, or that the breathing abnormality is not the underlying cause of death.
The behavioral defects that we observe in the MeCP2 aminergic-CKO mice suggest etiologies for specific symptoms of the human disorder. For example, it is well established that decreased dopamine levels are linked to motor abnormalities in humans and in mice (9, 41). Motor deficits are also prominent in girls and women with RTT (8, 42). Our TH-CKO animals displayed motor defects that are similar to those reported in animals that are deficient in DA (41). Hypoactivity in the open field and abnormal motor coordination on the dowel walking task in TH-CKO animals were independent of innate anxiety-like behavior in a novel environment, as exploratory behavior in the light–dark box exploration task was normal. Our PET1-CKO animals also provide insight into clinical features of RTT. Heightened anxiety and repetitive stereotyped behavior are commonly observed in RTT (43), and a large body of evidence supports the notion that 5HT modulates both of these behaviors (4, 5). Although the levels of 5HT are specifically decreased in PET1-CKO animals, our data suggest that innate anxiety-like and repetitive behaviors in PET1-CKO animals are not altered in comparison with littermate controls. Moreover, in the analysis of hyperactive behavior, a feature also attributed to defects in 5HT levels (41), the increase in distance traveled was significant only in a one-way ANOVA, as the hyperactivity that we observed was an additive effect of both the PET1-Cre and Flox alleles in the PET1-CKO animals. This is a significant finding given that TH-CKO animals display hypoactivity. The presence of opposing phenotypes in the two different aminergic-CKO animals (hypoactivity in TH-CKO animals and hyperactivity in PET1-CKO animals) provides further evidence of a cell-autonomous role for MeCP2 in these particular neurons. The most striking phenotype observed in PET1-CKO animals is the increased aggression toward unfamiliar conspecific animals. Although aggression is not a well-documented symptom of RTT, there are some typically higher-functioning RTT individuals that do display aggressive behavior (44). Our group previously reported aggression in mice that lack MeCP2 in Sim1-positive hypothalamic neurons (45). This study now reveals another anatomical origin of aggression in RTT, the dysfunction of MeCP2 in serotonergic neurons. It is interesting that 5HT reuptake inhibitor treatment in an individual with RTT decreased self-abusive behaviors (15). Formal studies that investigate aggressive behavior in RTT and potential interventions using 5HT reuptake inhibitors might prove clinically useful.
In conclusion, our findings from the genetic studies in aminergic-CKO animals support a critical role for MeCP2 in the aminergic systems and attribute specific behavioral abnormalities seen in RTT to these neurons. We propose that the overall neurochemical and behavioral phenotypes observed in the Mecp2null/y mouse model are likely the cumulative result of MeCP2 dysfunction within a variety of cells. The work reported here underscores the value of the conditional knockout approach in dissecting the role of MeCP2 in normal brain function and indicates that MeCP2 regulates genes and/or molecules in specific neuronal populations to subsequently affect specific behaviors. Further investigation of the loss of MeCP2 function in additional neuronal subtypes will likely yield additional insight into understanding RTT phenotypes such as social behavior deficits, autonomic dysfunction, and impaired learning. Importantly, the discovery that specific behavioral phenotypes in the mouse may be a direct consequence of abnormal MeCP2-dependent regulation of aminergic neurotransmitter levels suggests that targeting therapies to modulate these specific systems may mitigate specific deficits in individuals with RTT.
Materials and Methods
Spinal fluid was collected as previously described (2). Neurotransmitter monoamine metabolites in cerebrospinal fluid were measured after isocratic HPLC separation by electrochemical detection as previously described (16). For qPCR, total RNA was isolated from brain (three to four animals per genotype) using TRIzol (Invitrogen). A 3-μg quantity of RNA was used to synthesize cDNA (Superarray Biosciences). qPCR was performed as previously described (21) using commercially available primers (Superarray Biosciences). Nonradioactive ISH and quantification of ISH signal intensity were performed as previously described (18) on 25-μM, serially sectioned brain samples (three to four animals per genotype). Western blot analyses of Mecp2null/y and wild-type brain protein extracts (three animals per genotype) were performed as previously described (21) using 50 μg of protein per sample and anti-TH (1:500, Abcam), anti-TPH (1:500, Sigma), and anti-VCL (1:2000, Sigma) antibodies. Quantification of signal intensity was performed using ImageJ (rsbweb.nih.gov/ij/). For ChIP-PCR and ChIP-qPCR, chromatin was cross-linked and immunoprecipitated from brain tissue samples from wild-type and Mecp2null/y animals 6–8 weeks of age (three animals per genotype) using either rabbit IgG or anti-MeCP2 antibody (Millipore) as previously described (39). A full description of spinal fluid collection, probe sequences for ISH, primer sequences, conditions for ChIP-PCR/qPCR amplification, and behavioral tests is provided in Materials and Methods in the SI Text Data from statistical analyses for behavioral tests are provided in Table S2.
Supplementary Material
Acknowledgments.
We thank the following individuals for their contributions to this work: the members of the Zoghbi laboratory and Vicky Brandt for critical reading of the manuscript; Evan Deneris for providing the initial founders for the PET1-Cre mouse line; Corinne Spencer and Richard Paylor for advice on neurobehavioral tasks; Agnes Liang, Ying Liu, and Ron Kao for technical assistance with ISH experiments; Chris McGraw for assistance with protein analysis; Melissa Arvide for tissue sectioning; and the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center cores for use of facilities. This work was funded by grants from Autism Speaks (Predoctoral Fellowship to R.S.), grants from the National Institutes of Health (NS057819 and HD024064 [to H.Z.], NS052240 [to J.N.], and MH078678 [to H.T.C.]), the International Rett Syndrome Foundation (to J.N.), and the Simons Foundation (to H.Z.). Huda Zoghbi is a Howard Hughes Medical Institute investigator.
Footnotes
Conflict of interest statement: Keith Hyland is co-owner of Medical Neurogenetics, L.L.C. The authors do not advocate or recommend ongoing cerebrospinal fluid (CSF) analysis in girls with Rett syndrome, as it does not contribute to the treatment or diagnosis of the disorder.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912257106/DCSupplemental.
References
- 1.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 2.Neul JL, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebbington A, et al. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008;70:868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- 4.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 5.Gordon JA, Hen R. The serotonergic system and anxiety. Neuromol Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- 6.Popova NK. From gene to aggressive behavior: The role of brain serotonin. Neurosci Behav Physiol. 2008;38:471–475. doi: 10.1007/s11055-008-9004-7. [DOI] [PubMed] [Google Scholar]
- 7.Segawa M. Pathophysiology of Rett syndrome from the stand point of clinical characteristics. Brain Dev. 2001;23(Suppl 1):S94–S98. doi: 10.1016/s0387-7604(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DD. Neuropathology of Rett syndrome. J Child Neurol. 2005;20:747–753. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Mov Disord. 2008;23(Suppl 3):S497–S508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 10.Katz DM, Dutschmann M, Ramirez J, Hilaire G. Breathing disorders in Rett syndrome: Progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoghbi HY, Percy AK, Glaze DG, Butler IJ, Riccardi VM. Reduction of biogenic amine levels in the Rett syndrome. N Engl J Med. 1985;313:921–924. doi: 10.1056/NEJM198510103131504. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi HY, et al. Cerebrospinal fluid biogenic amines and biopterin in Rett syndrome. Ann Neurol. 1989;25:56–60. doi: 10.1002/ana.410250109. [DOI] [PubMed] [Google Scholar]
- 13.Perry TL, Dunn HG, Ho HH, Crichton JU. Cerebrospinal fluid values for monoamine metabolites, gamma-aminobutyric acid, and other amino compounds in Rett syndrome. J Pediatr. 1988;112:234–238. doi: 10.1016/s0022-3476(88)80060-x. [DOI] [PubMed] [Google Scholar]
- 14.Lekman A, et al. CSF and urine biogenic amine metabolites in Rett syndrome. Clin Genet. 1990;37:173–178. doi: 10.1111/j.1399-0004.1990.tb03499.x. [DOI] [PubMed] [Google Scholar]
- 15.Temudo T, et al. Evaluation of CSF neurotransmitters and folate in 25 patients with Rett disorder and effects of treatment. Brain Dev. 2009;31:46–51. doi: 10.1016/j.braindev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Hyland K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin Chem. 2008;54:633–641. doi: 10.1373/clinchem.2007.099986. [DOI] [PubMed] [Google Scholar]
- 17.Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neurosci Lett. 2005;386:14–17. doi: 10.1016/j.neulet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Carson JP, Eichele G, Chiu W. A method for automated detection of gene expression required for the establishment of a digital transcriptome-wide gene expression atlas. J Microsc. 2005;217:275–281. doi: 10.1111/j.1365-2818.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindeberg J, et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 20.Scott MM, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaco RC, et al. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viemari J, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducottet C, Belzung C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav. 2004;81:417–426. doi: 10.1016/j.physbeh.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Spencer CM, et al. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1984–1994. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- 25.Thomas A, et al. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura Y, Segawa M, Higurashi M. Rett syndrome—an early catecholamine and indolamine deficient disorder? Brain Dev. 1985;7:334–341. doi: 10.1016/s0387-7604(85)80040-1. [DOI] [PubMed] [Google Scholar]
- 28.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 29.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 30.Shahbazian M, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 31.McGill BE, et al. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 33.Moretti P, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braunschweig D, Simcox T, Samaco RC, LaSalle JM. X-chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2/+ mouse brain. Hum Mol Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- 35.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belichenko NP, Belichenko PV, Mobley WC. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol Dis. 2009;34:71–77. doi: 10.1016/j.nbd.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Taneja P, et al. Pathophysiology of Locus Ceruleus Neurons in a Mouse Model of Rett Syndrome. J Neurosci. 2009;29:12187–12195. doi: 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui DH, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci USA. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viggiano D. The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008;194:1–14. doi: 10.1016/j.bbr.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Temudo T, et al. Movement disorders in Rett syndrome: An analysis of 60 patients with detected MECP2 mutation and correlation with mutation type. Mov Disord. 2008;23:1384–1390. doi: 10.1002/mds.22115. [DOI] [PubMed] [Google Scholar]
- 43.Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Huppke P, et al. Very mild cases of Rett syndrome with skewed X inactivation. J Med Genet. 2006;43:814–816. doi: 10.1136/jmg.2006.042077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fyffe SL, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.