Abstract
In mammals, hypocretin/orexin (HCRT) neuropeptides are important sleep–wake regulators and HCRT deficiency causes narcolepsy. In addition to fragmented wakefulness, narcoleptic mammals also display sleep fragmentation, a less understood phenotype recapitulated in the zebrafish HCRT receptor mutant (hcrtr−/−). We therefore used zebrafish to study the potential mediators of HCRT-mediated sleep consolidation. Similar to mammals, zebrafish HCRT neurons express vesicular glutamate transporters indicating conservation of the excitatory phenotype. Visualization of the entire HCRT circuit in zebrafish stably expressing hcrt:EGFP revealed parallels with established mammalian HCRT neuroanatomy, including projections to the pineal gland, where hcrtr mRNA is expressed. As pineal-produced melatonin is a major sleep-inducing hormone in zebrafish, we further studied how the HCRT and melatonin systems interact functionally. mRNA level of arylalkylamine-N-acetyltransferase (AANAT2), a key enzyme of melatonin synthesis, is reduced in hcrtr−/− pineal gland during the night. Moreover, HCRT perfusion of cultured zebrafish pineal glands induces melatonin release. Together these data indicate that HCRT can modulate melatonin production at night. Furthermore, hcrtr−/− fish are hypersensitive to melatonin, but not other hypnotic compounds. Subthreshold doses of melatonin increased the amount of sleep and consolidated sleep in hcrtr−/− fish, but not in the wild-type siblings. These results demonstrate the existence of a functional HCRT neurons-pineal gland circuit able to modulate melatonin production and sleep consolidation.
Keywords: pineal gland, sleep consolidation
Hypocretin 1 and 2 (HCRT 1 and 2, also known as orexin A and B) are two neuropeptides originally isolated in rats, that are derived from a single gene precursor (Hcrt/Orx) (1, 2). HCRT preproprotein is exclusively expressed in neurons restricted to the lateral hypothalamus (LH) organized as a single compact cluster in each hemi-brain (1–3). HCRT neuron number may vary from a few thousand in a rodent LH to 50,000–80,000 in the human LH. This cluster organization is conserved in all mammals investigated (4). Despite its restricted expression, HCRT is a critical regulator of the sleep–wake cycle and is further implicated in food intake regulation, energy homeostasis, arousal, drug addiction, stress, and cardiovascular function. Interestingly, the complexity of HCRT physiological function is reflected in the diversity of HCRT anatomic projections and HCRT receptor expression sites in the central nervous system. From their discrete location in the LH, HCRT neurons send widespread projections throughout the brain and the spinal cord (3, 5). This broad fiber distribution is consistent with the diffuse expression patterns of the two HCRT G protein-coupled receptors (HCRTR1/OX1R and HCRTR2/OX2R) (2, 6).
HCRT deficiencies produce narcolepsy, a disorder characterized in mammals by excessive sleepiness during the normal wake periods, direct transitions from wake to REM sleep, and sudden loss of muscle tonus while still being awake (cataplexy) (4, 7). Moreover, HCRT is crucial to maintain wakefulness and has been extensively shown to have a wake-promoting role. HCRT neurons are active during wakefulness (8) and intracerebroventricular administration of HCRT induces a dose-dependent increase of time spent awake (9). Furthermore, HCRT also appears to play an important role in sleep regulation and consolidation. Individuals with narcolepsy and similarly affected dogs and mice also suffer sleep fragmentation (10, 11), even when pharmacologically kept awake during the daytime to increase homeostatic pressure for sleep (12).
We, and others, have previously characterized the HCRT system in zebrafish (13–16), a simpler model to understand the anatomy and function of the HCRT neuronal network and its evolution across vertebrates. Unlike mammals, zebrafish have only one HCRT receptor gene (hcrtr) (16). Despite the close similarity of this receptor with the mammalian HCRTR2, zebrafish hcrtr−/− null mutants do not have a fragmented wake during the daytime or cataplexy-like behavior but only possess the sleep fragmentation phenotype (16). Moreover, in contrast to the mammalian HCRT system involving thousands of neurons in a complex neuronal circuit, the larval and adult zebrafish hypothalamus contain approximately 20 and 60 HCRT-positive neurons, respectively (13–16). In this study, we show a neuroanatomical and functional connection between the HCRT and melatonin systems, supporting a role for a HCRT/melatonin pathway in sleep consolidation.
Results
Zebrafish HCRT Neurons Are Mostly Glutamatergic.
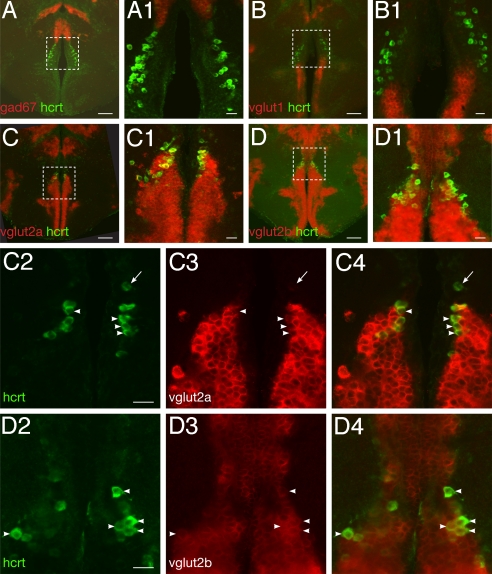
In mammals, the majority of HCRT neurons are glutamatergic, a phenotype consistent with the wake-promoting influence and neuroexcitatory nature of HCRT neuropeptides (17). However, HCRT signaling deficiency in zebrafish failed to impact wake behavior (16). This led us to examine the excitatory or inhibitory nature of the zebrafish HCRT neurons. We studied their fast neurotransmitter phenotype using double in situ hybridization (ISH) of hcrt with vesicular glutamate transporter genes vglut1, vglut2a, vglut2b (markers of excitatory glutamate), and glutamate decarboxylase gene gad67 (marker of inhibitory GABA). Colocalization with hcrt was determined by confocal microscopy. We found that no HCRT neurons express gad67 (Fig. 1A, close-up A1) suggesting that HCRT neurons are not GABAergic. Similarly, HCRT neurons in majority do not express vglut1 (Fig. 1 B and B1), however occasional coexpression in a few ventralmost HCRT cells was observed. In contrast, a large majority of the HCRT neurons express one of the vglut2 genes suggesting a glutamatergic phenotype (Fig. 1 C and D, and associated close-up panels C1–C4 and D1–D4). Of note, vglut2a- or 2b-negative HCRT neurons were sometimes observed dorsally (Fig. 1 C2–C4, arrow). These results are reminiscent of the rat, where none of the HCRT neurons express gad67, a minority expresses vglut1 and a majority expresses vglut2 (17), suggesting a conservation of the developmental program underlying the HCRT cell identity across vertebrates. Functionally, the glutamatergic phenotype of HCRT neurons in zebrafish suggests a conservation of the excitatory nature of these neurons between fish and mammals.
Fig. 1.
Zebrafish HCRT neurons are glutamatergic. (A–D) and close-ups (A1–D1) Double fluorescent ISH between hcrt mRNA and fast neurotransmitter phenotype markers as visualized using confocal microscopy on adult brain sections (reconstructed stacks of 0.5- or 1-μm sections). (C2–C4) and (D2–D4): single-plane, high-magnification pictures of hcrt cells (green, C2, D2), vglut2a or -b cells (red, C3, D3), and merged views (C4, D4). Arrowheads indicate cells coexpressing hcrt and vglut2a or vglut2b. Note the frequent colocalization. Absence of coexpression is occasionally observed (arrow). [Scale bar, 100 μm (A–D), 20 μm (A1–D1, C2–C4, and D2–D4).]
Architecture of the Larval and Adult Zebrafish HCRT System.
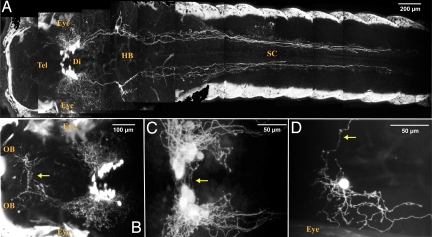
We isolated the zebrafish hcrt gene promoter (14) and generated a specific stable hcrt:EGFP transgenic line (Fig. S1) to perform high resolution imaging of the zebrafish HCRT circuit, and its potential connectivity with sleep/wake-regulating brain regions. Two-photon microscopy analysis of living hcrt:EGFP larvae revealed the ipsilateral track projecting to the spinal cord (14, 15) and bilateral projections proceeding anteriorly through the telencephalon toward both olfactory bulbs (OB, Fig. 2A and close up in B), reminiscent of the rat dorsal descending and ventral ascending pathways respectively. In addition, contralateral projections were observed ventrally to the HCRT cell bodies (Fig. 2 C and D, arrow) and in the commissura anterior to the OB (Fig. 2B, arrow).
Fig. 2.
A stable hcrt:EGFP transgenic line reveals the entire HCRT circuit of a living larva. (A–C) Two-photon imaging of a 7dpf stable transgenic larva with head to the left, dorsal views. (A and close-up B) Composite picture showing the HCRT cell bodies in the diencephalon (Di) and their processes in the hindbrain (HB) and spinal cord (SC) and in the telencephalon (Tel) directed toward the olfactory bulbs (OB). In A and B, white areas on both sides of the larva correspond to skin autofluorescence. Commissural projections are observed ventrally to the HCRT cell body clusters (C, arrow) and in the anterior telencephalon (B, arrow, commissura anterior). (D) Mosaic expression of a nonintegrated hcrt:EGFP transgene allowing the observation of a single HCRT neuron harboring both commissural (arrow) and ipsilateral processes.
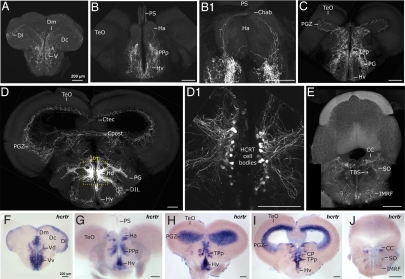
In adult hcrt:EGFP zebrafish (Fig. 3), HCRT fibers are found in the dorsal telencephalic, homologous to the mammalian, and in the subpallium midline (Fig. 3A), similar to the rat dorsal and ventral ascending pathways respectively (3). As in mammals, the highest density of axonal projections is detected in the hypothalamic periventricular region (Fig. 3 B–D) and HCRT cell bodies are organized as two compact clusters facing each other without direct contralateral projection to their counterpart (Fig. 3 D and D1). Commissural projections are clearly visible along at least four levels, the habenular commisure (Fig. 3B1), the commissura tecti, the commissura posterior, and through the most ventral enclosure of the periventricular hypothalamus (Fig. 3D). Fibers were also observed around the tectal ventricule in the periventricular gray zone of the optic tectum (Fig. 3 C and D), a structure homologous to the superficial layers of the superior coliculli in mammals where HCRT fibers have also been described in rats (3). Finally, posterior projections are sent dorsally and ventrally in the subventricular region of the rhombencephalon (Fig. 3E) similar to that observed in rat (3, 5).
Fig. 3.
Adult zebrafish HCRT circuit. (A–E) Confocal imaging of 100 μm transversal brain sections from a stable hcrt:EGFP transgenic adult fish (reconstructed stacks of 0.5- or 1-μm sections). Note the compact organization of the HCRT cell bodies in the periventricular hypothalamus (D and dashed-box close up in D1). (F–J) hcrtr mRNA ISH pattern in equivalent brain sections to A–E. Note the similar distribution of HCRT fibers and hcrtr mRNA. Compare sections of telencephalon (A vs. F), telencephalon-midbrain boundary (B and B1 vs. G), anterior diencephalon and mesencephalon (C vs. H), mid diencephalon and mesencephalon (D vs. I), rhombencephalon (E vs. J). Chab, commissura habenularum; Ctec, commissura tecti; Cpost, commissura posterior; CC, crista cerebellaris; CP, central posterior thalamic nucleus; D, dorsal telencephalic area; Dm, medial zone of D; Dc, central zone of D; Dl, lateral zone of D; DIL, diffuse nucleus of the inferior lobe; Ha, habenula; Hv, ventral zone of the periventricular hypothalamus; Hd, dorsal zone of the periventricular hypothalamus; IMRF, intermediate reticular formation; PG, preglomerular nucleus; PGZ, periventricular gray zone of the optic tectum; PPp, parvocellular preoptic nycleus, posterior part; PS, pineal stalk; SO, secondary octaval population; TBS, tractus bulbospinalis; TeO, optic tectum; TPp, periventricular nucleus of posterior tuberculum; V, ventral telencephalic area; Vv, ventral nucleus of V; Vd, dorsal nucleus of V.
We next examined the distribution of HCRT receptor expression to confirm the projection architecture observed in our hcrt:EGFP transgenics. Consistently, the fiber distribution matched the HCRT receptor mRNA expression profile (Fig. 3, compare panels A vs. F, B vs. G, C vs. H, D vs. I, and E vs. J), except in the hypothalamus. Indeed, abundant projections around the HCRT cell bodies do not have corresponding receptor expression (see Fig. 3 D vs. I) suggesting those processes are likely dendritic. Overall, our stable hcrt:EGFP transgenic line documents considerable conservation of the HCRT circuit architecture across vertebrates and is therefore a valuable tool for identifying HCRT circuitry involved in sleep–wake regulation.
HCRT Neurons Project to the Pineal Gland Where hcrtr Is Expressed.
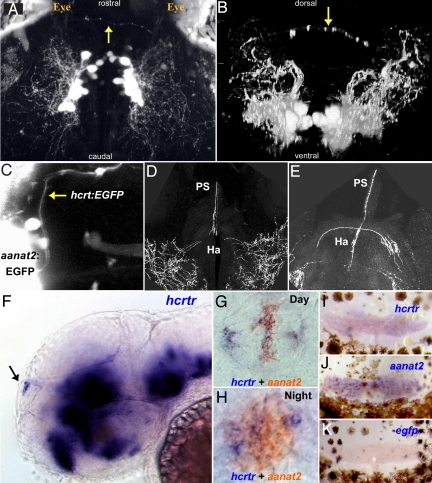
Through 2-photon imaging of hcrt:EGFP larvae, we identified axons emerging from both HCRT cell clusters and forming an arch reaching the most dorsal region of the midbrain-telencephalon boundary (Fig. 4A, dorsal view and B, frontal view). These fibers were located superficially, just underneath the skull, suggesting an innervation of the pineal gland. In all vertebrates, the pineal gland influences daily and annual physiological changes mediated by the secretion of melatonin at night (18, 19). In zebrafish, melatonin mediates the circadian clock output and also promotes sleep (20, 21). To demonstrate that HCRT neurons project into the pineal gland, we crossed hcrt:EGFP and aanat2:EGFP transgenic fish. The aanat2:EGFP fish expresses EGFP specifically in melatonin producing photoreceptors of the pineal gland (22). In the resulting double transgenic fish, HCRT axons were indeed found to project into the pineal gland (Fig. 4C). This observation was confirmed in adult brains, where, in addition to the commissural innervation of the habenula (Ha, Fig. 3B1), HCRT neurons innervate the pineal gland stalk (PS, Fig. 4 D and E). This HCRT-pineal connection is an exceptional case of a direct neuropeptidergic innervation of the fish pineal gland. The importance of this projection was substantiated by the detection of hcrtr mRNA in the pineal gland of both larvae (Fig. 4 F–H) and adults (Fig. 4 I–K), strongly suggesting that pineal gland activity could be modulated by HCRT circuitry.
Fig. 4.
The HCRT-pineal gland circuit. (A and B) Dorsal and frontal views of the brain of a 7 dpf hcrt:EGFP transgenic larva imaged by two-photon microscopy. HCRT axons (arrows) projecting toward the pineal gland are observed. (C) A dorsal image of 6 dpf transgenic larva carrying two transgenes; an EGFP reporter driven by hcrt (hcrt:EGFP) and the pineal-specific aanat2 (aanat2:EGFP) promoters, demonstrate direct axon projection (arrow) to the pineal gland. (D and E) Close-ups of two adjacent transversal hcrt:EGFP adult brain sections showing HCRT projections to the habenula and the pineal gland stalk. (F) Lateral and (G and H) dorsal views of whole-mount in situ hybridization of 2-dpf embryos. (F) hcrtr mRNA is expressed in several regions of the brain (16) including the pineal gland (arrow). Double ISH experiment with aanat2 demonstrates that hcrtr is expressed in the pineal gland during the day (G) and the night (H). Similarly, in adult animals, hcrtr is expressed in the pineal gland (I). aanat2 (J) and egfp (K) probes were used as positive and negative controls, respectively. Adult pineal glands (I–K) were removed with the upper skull and skin hence presence of brown melanophores cells in the preparations.
HCRT Increases Melatonin Production in Cultured Pineal Glands.
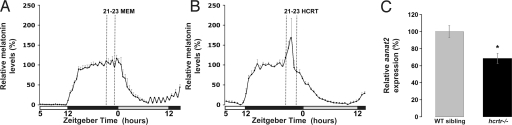
To test whether HCRT peptides affect melatonin production in the pineal gland, we used a well-established perfusion assay of adult zebrafish pineal glands (SI Materials and Methods and Fig. S2). Pineal glands were removed and placed in a flow-through perfusion system for 3 days. As expected, isolated pineal glands of fish displayed rhythmic production of melatonin under light–dark (LD) and dark–dark (DD) cycles (Fig. 5A and B). Custom-made mature zebrafish HCRT-1 peptide (10−6 M) (16) was applied to individual pineal glands for 2 h during the second night at ZT (zeitgeber time) 21–23, when melatonin level is declining. HCRT-1 application led to a significant increase in melatonin production (up to 70%, P < 0.05, n = 8), which lasted for the duration of HCRT application (Fig. 5B), indicating a stimulating effect of HCRT-1 on melatonin production. In contrast, minimum essential medium (MEM) application did not affect melatonin production in control pineal glands (n = 12, Fig. 5A). These results demonstrate that the zebrafish pineal gland can respond to HCRT excitatory input and suggest the existence of a functional HCRT/pineal gland neuronal circuit in zebrafish.
Fig. 5.
HCRT modulates pineal gland melatonin production. (A and B) HCRT induces melatonin release from cultured zebrafish pineal glands. (A) Normal circadian rhythm of melatonin release of melatonin from zebrafish pineal glands cultured in constant darkness. MEM medium application does not affect melatonin production in 12 control pineal glands. (B) zebrafish HCRT-1 application (10−6 M) stimulates melatonin production (ZT 21–23) (n = 8, P < 0.05). The production lasts for the duration of HCRT application. (C) Quantitative PCR analysis of aanat2 mRNA level in six wild-type sibling and 6 hcrtr−/− pineal glands collected during the night (ZT 16). Note the significant decrease (31%, P < 0.05) of aanat2 expression in pineal glands devoid of functional HCRT input.
aanat2 Expression Is Down-Regulated in hcrtr−/− Pineal Gland.
We next tested whether this putative circuit is functional in vivo and analyzed whether the absence of HCRT input in hcrtr−/− background could affect pineal gland melatonin production. To do so, we studied the level of expression of AANAT the key enzyme of melatonin production (19). In zebrafish pineal glands, aanat2 mRNA level is rhythmic, and is a robust indicator of melatonin production (23). aanat2 mRNA levels from six wild-type sibling and six hcrtr−/− adult pineal glands collected at 1:00 AM (ZT16) were measured using quantitative PCR. Strikingly, we observed a significant reduction of 31% of aanat2 expression level in hcrtr−/− pineal glands (P < 0.05, Fig. 5C), indicating that melatonin production is decreased in absence of HCRT signal. This result demonstrates the existence of a functional HCRT-pineal gland circuit and may explain the fragmented sleep phenotype of hcrtr−/− fish in the dark.
hcrtr−/− Larval and Adult Mutants Are Hypersensitive to the Sleep Inducing Effects of Melatonin.
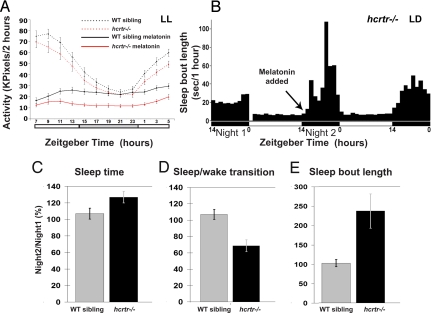
To further investigate whether melatonin signaling down-regulation is responsible for the hcrtr−/− mutant sleep phenotype, we next examined the effects of exogenous melatonin and other hypnotic drugs on hcrtr−/− fish and wild-type siblings. We treated larvae with melatonin and compounds from four different classes of hypnotics (barbiturate, benzodiazepine, anti-histaminergic, and α2 adrenergic agonist) and monitored their activity over 24 h under constant dim light conditions (Fig. 6 and Fig. S3). No significant differences were found under drug-free conditions between genotypes (Fig. 6A). However, with melatonin exposure, hcrtr−/− larvae were more sedated than the wild-type siblings (P < 0.0001, Fig. 6A). In contrast, hcrtr−/− and wild-type siblings showed no differences upon exposure to the other hypnotics (Fig. S3), demonstrating a specific hypersensitivity to melatonin and suggesting, in this mutant, an up-regulation of the actors downstream of melatonin, which might reflect a reduction of endogenous melatonin signaling (24).
Fig. 6.
hcrtr−/− fish are hypersensitive to the melatonin sleep-promoting effect. (A) Larvae were kept under constant dim light conditions (LL, <10lux) in the monitoring system (white bars represent the subjective day period). hcrtr−/− and wild-type sibling 5 dpf larvae demonstrate similar rhythmic activity that peak during the day. hcrtr−/− larvae were significantly more sensitive to melatonin's (1 μM) hypnotic effect. (B–E) Adult fish were kept under LD cycle (represented by white and black bars) in AFSRS [SI Materials and Methods and (16)] and fine sleep architecture was analyzed. (B) Representative sleep bout pattern of an adult hcrtr−/− mutant. Subthreshold dose of melatonin (1 μM) was added at the beginning of the second night (arrow) and sleep parameters were compared to first night. hcrtr−/− adults were more sensitive to melatonin as, after its administration, total sleep time increased (C), the number of sleep/wake transition decreased (D) and sleep bout length increased (E), as compared to wild-type siblings.
We next analyzed the impact of melatonin application on the sleep dynamics of adult hcrtr−/− and wild-type sibling fish. Based on the hypersensitivity observed in larvae and to test whether a very small concentration of melatonin could abrogate the sleep fragmentation phenotype, we used a subthreshold dose of melatonin: the highest dose that could be administered to wild-type adults without inducing hypnotic effects; that is, 1 μM. Sleep time, sleep bout length, and sleep transition number were monitored under LD cycle in wild-type and mutant adult fish using our previously described Adult Fish Sleep Recording System (AFSRS) (16). For each individual fish, sleep patterns in the presence of melatonin were compared to records obtained during the previous night without exogenous melatonin. Although, as expected, wild-type fish were not affected by this low dose of melatonin, we found that hcrtr−/− fish were strongly sedated. They displayed an approximate 30% increase in sleep (P < 0.01) (Fig. 6C), and a strong increase of sleep consolidation indicated by a 40% decrease in the number of sleep–wake transitions (P < 0.005) (Fig. 6D), and a 100–150% increase in mean sleep bout length (P < 0.005) during the night (Fig. 6 B and E). Together, these data indicate that hypersensitivity to melatonin is a strong and specific feature of hcrtr−/− fish. Further, it demonstrates a behavioral-level of interaction between the HCRT and melatonin systems and suggests that the absence of functional HCRT pathway affects sleep-promoting melatonin signaling at a level yet to be discovered.
Discussion
In mammals, HCRT has a wake-promoting role (4). In addition to this function, evidence supports a major role of HCRT in the consolidation not only of wake state but also of sleep state. In human, dog, and mouse, HCRT deficiency is associated with both wake and sleep fragmentation (10, 11). Moreover, sleep fragmentation persists in narcoleptic patients even when pharmacologically kept awake during the daytime (12), excluding a simple wake rebound hypothesis. Furthermore, mice with HCRT neurons devoid of GABA-B receptors also display both fragmented sleep and wake without reduction of total sleep amount (25). Finally, although studies have shown that HCRT neurons decrease firing during sleep, they do not completely cease to discharge during sleep (8). This is also reflected at the extracellular levels, where HCRT levels stay high during sleep (26) and could regulate it (27). We therefore propose that HCRT has a dual function on promoting wake and on consolidating sleep, thus explaining daytime sleepiness and insomnia in narcoleptic patients. In zebrafish, this dual function is very likely conserved. Ubiquitous HCRT overexpression in transgenic fish increases wakefulness (15), while hcrtr−/− mutants display sleep fragmentation (16). Surprisingly, hcrtr−/− mutants do not have wake fragmentation or cataplexy phenotype suggesting the existence of compensatory system(s) or wiring differences. Nevertheless commonalities observed in these experiments (15, 16) suggest that HCRT function is conserved in zebrafish and can be implicated in both wake promotion and sleep consolidation.
Here, we demonstrate conservation of glutamatergic phenotype of HCRT neurons across vertebrates, and we further show fish HCRT circuit homology with the mammalian system (3, 5), including the direct HCRT innervation of the pineal gland. In sheep (28), rat (29), and pig (30), HCRT fibers and/or HCRT receptors were indeed also found in the pineal gland, demonstrating conservation across diurnal and nocturnal vertebrates. In all vertebrates, diurnal or nocturnal, melatonin is secreted in the dark by the pineal gland and controls daily and seasonal changes in physiology (18, 19). Melatonin is also a well-established sleep inducer in zebrafish (21), albeit a weaker one in humans. In mammals, light acts via retinohypothalamic projections to entrain the central oscillator located in the suprachiasmatic nucleus (SCN). In turn, the SCN synchronizes rhythmic melatonin production through sympathetic innervation of the pineal gland. In contrast, in teleosts, phototransduction, oscillator, and melatonin production are located in the photoreceptor cells of the pineal gland. Thus, the fish pineal gland is considered as an autonomous organ able to rhythmically produce melatonin (19, 20). There are only a few described examples of pineal innervations in teleosts (20, 31). In this study, we show HCRT innervation of the pineal gland and that HCRT signal modulates pineal melatonin production. This is an example of peptidergic innervation and functional control of the fish pineal gland. Thus, the dogma that pineal melatonin production in teleost is totally independent of CNS regulation may need to be revisited.
This study not only describes a neuroanatomical connection between two major sleep/circadian systems, it also demonstrates functional relevance of this sleep regulatory circuit at the molecular and behavioral levels. Application of zebrafish HCRT on wild-type pineal glands consistently stimulates the release of melatonin. This promoting effect was confirmed in vivo by the down-regulation of aanat2 in hcrtr−/− fish pineal glands, suggesting that the HCRT signaling pathway likely modulates melatonin production by acting upstream of the transcriptional cascade controlling aanat2 expression (32). Furthermore, hcrtr−/− fish are strikingly hypersensitive to the hypnotic effects of melatonin. This effect is specific, as four other classes of hypnotics (Fig. S3) show no differences between hcrtr−/− fish and wild-type siblings. This hypersensitivity may be interpreted in different ways. A first possibility could be that hcrtr−/− fish are sleepier, and thus more sensitive to sleep induction by melatonin. In this case, however, we would have expected similar results with other hypnotics. An attractive alternative hypothesis supported by our results, could be that HCRT promotes, at least partially, sleep consolidation in the dark through the stimulation of the melatonin sleep-promoting system. This would explain sleep disruption in the hcrtr−/− fish when in the dark, but not the light that is a suppressor of melatonin release of its own. Furthermore, in the absence of HCRT signaling, the downstream melatonin pathway and/or circuit could be up-regulated to compensate for the decrease of melatonin production, thus explaining the hypersensitivity to melatonin of hcrtr−/− fish. Such a regulatory loop has been previously reported in mammals and birds where melatonin receptor numbers increased after pinealectomy (24). Moreover, interestingly, HCRT fiber and hcrtr mRNA distributions (Fig. 3) strongly resemble melatonin receptors' expression pattern (Fig. S4) especially in the periventricular gray zone of the optic tectum, and the periventricular thalamus and hypothalamus. Thus, HCRT and melatonin pathways could also interact at downstream levels in brain regions where both HCRT and melatonin receptors are coexpressed. These potential downstream interconnections might also be responsible for the hcrtr−/− melatonin hypersensitivity phenotype. Overall, future studies will be required to investigate the full extent of melatonin and HCRT pathway collaboration (synergy or compensation) and to further test the wake-promoting and sleep-consolidating dual function of HCRT.
Whether or not HCRT promotion of melatonin release and sleep is relevant to humans is unknown at this stage. Studies in narcoleptics have not shown dramatic differences in melatonin release, although light regime was not controlled (33). In addition, humans display variable sensitivity to exogenous melatonin's hypnotic effects. Melatonin acts as a mild hypnotic when administered during the daytime and can cause small shifts in the timing of the circadian clock (34). Hypnotic effects of melatonin at night are more debated, although notably in Smith Magenis syndrome strong beneficial effects on disturbed nocturnal sleep have been documented (35). The use of melatonin for the treatment of insomnia in narcolepsy has only been reported in a single case, with a dramatic REM sleep promoting effect (36). As this is not in line with clinical impression, a more systematic evaluation is needed.
In summary, because HCRT is not present in nonvertebrate lineages, zebrafish provide an excellent simplified system in which to dissect how HCRT neurons and their projections may affect the sleep/wake cycle and other behaviors. This work reveals a sleep consolidating circuit and shows that melatonin can act as one of the mediators of the HCRT system in regulating sleep.
Materials and Methods
The methods are described in detail in SI Materials and Methods. Imaging was performed using a custom-made two-photon laser-scanning microscope and Zeiss LSM 510 META confocal microscope. hcrt:EGFP stable transgenic lines were established with the tol2 technique. Cultured pineal assay was done in Dr. Gothilf laboratory. Behavior was monitored with either Adult Fish Sleep Recording System or ViewPoint system.
Supplementary Material
Acknowledgments.
The authors thank Dr. David C. Klein for fruitful discussion, Dr. Jamie M. Zeiter for his advices in statistical analysis, Drs. Matthew P. Klassen and Shawn M. Burgess for critical reading of the manuscript, Laura Alexander, Gemini Skariah, and Drs. Sarit Lampart and Oren Levy for their technical assistance. This work was supported by Howard Hughes Medical Institute and National Institutes of Health Grant R01 NS062798 (to E.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906637106/DCSupplemental.
References
- 1.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lecea L, Sutcliffe JG. The hypocretins and sleep. FEBS J. 2005;272:5675–5688. doi: 10.1111/j.1742-4658.2005.04981.x. [DOI] [PubMed] [Google Scholar]
- 5.van den Pol AN. Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus JN, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 7.Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci. 2006;27:368–374. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishino S, et al. Is narcolepsy a REM sleep disorder? Analysis of sleep abnormalities in narcoleptic Dobermans. Neurosci Res. 2000;38:437–446. doi: 10.1016/s0168-0102(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 11.Willie JT, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 12.Arnulf I, Mignot E. Sodium oxybate for excessive daytime sleepiness in narcolepsy-cataplexy. Sleep. 2004;27:1242–1243. [PubMed] [Google Scholar]
- 13.Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–2689. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraco JH, et al. Regulation of hypocretin (orexin) expression in embryonic zebrafish. J Biol Chem. 2006;281:29753–29761. doi: 10.1074/jbc.M605811200. [DOI] [PubMed] [Google Scholar]
- 15.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokogawa T, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:2379–2397. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 18.Arendt J, Deacon S, English J, Hampton S, Morgan L. Melatonin and adjustment to phase shift. J Sleep Res. 1995;4:74–79. doi: 10.1111/j.1365-2869.1995.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 19.Klein DC, et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 2009;52:307–357. 1997; discussion 357–308. [PubMed] [Google Scholar]
- 20.Falcon J. Cellular circadian clocks in the pineal. Prog Neurobiol. 1999;58:121–162. doi: 10.1016/s0301-0082(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 22.Gothilf Y, et al. Pineal-specific expression of green fluorescent protein under the control of the serotonin-N-acetyltransferase gene regulatory regions in transgenic zebrafish. Dev Dyn. 2002;225:241–249. doi: 10.1002/dvdy.10152. [DOI] [PubMed] [Google Scholar]
- 23.Gothilf Y, et al. Zebrafish serotonin N-acetyltransferase-2: Marker for development of pineal photoreceptors and circadian clock function. Endocrinology. 1999;140:4895–4903. doi: 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Cassone VM. Pineal regulation of circadian rhythms of 2-deoxy[14C]glucose uptake and 2[125I]iodomelatonin binding in the visual system of the house sparrow, Passer domesticus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1993;173:765–774. [Google Scholar]
- 25.Matsuki T, et al. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci USA. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeitzer JM, et al. Circadian and homeostatic regulation of hypocretin in a primate model: Implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi MC, Chase MH. Neuronal mechanisms of active (rapid eye movement) sleep induced by microinjections of hypocretin into the nucleus pontis oralis of the cat. Neuroscience. 2006;140:335–342. doi: 10.1016/j.neuroscience.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, et al. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 2005;124:81–87. doi: 10.1016/j.regpep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Mikkelsen JD, et al. Hypocretin (orexin) in the rat pineal gland: A central transmitter with effects on noradrenaline-induced release of melatonin. Eur J Neurosci. 2001;14:419–425. doi: 10.1046/j.0953-816x.2001.01655.x. [DOI] [PubMed] [Google Scholar]
- 30.Fabris C, Cozzi B, Hay-Schmidt A, Naver B, Moller M. Demonstration of an orexinergic central innervation of the pineal gland of the pig. J Comp Neurol. 2004;471:113–127. doi: 10.1002/cne.20007. [DOI] [PubMed] [Google Scholar]
- 31.Falcon J, Besseau L, Sauzet S, Boeuf G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol Metab. 2007;18:81–88. doi: 10.1016/j.tem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Appelbaum L, Anzulovich A, Baler R, Gothilf Y. Homeobox-clock protein interaction in zebrafish. A shared mechanism for pineal-specific and circadian gene expression. J Biol Chem. 2005;280:11544–11551. doi: 10.1074/jbc.M412935200. [DOI] [PubMed] [Google Scholar]
- 33.Blazejova K, Illnerova H, Hajek I, Nevsimalova S. Circadian rhythm in salivary melatonin in narcoleptic patients. Neurosci Lett. 2008;437:162–164. doi: 10.1016/j.neulet.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan V, et al. Melatonin and melatonergic drugs on sleep: Possible mechanisms of action. Int J Neurosci. 2009;119:821–846. doi: 10.1080/00207450802328607. [DOI] [PubMed] [Google Scholar]
- 35.De Leersnyder H, et al. Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2003;40:74–78. doi: 10.1136/jmg.40.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavel S, Goldstein R, Petrescu M. Vasotocin, melatonin, and narcolepsy: Possible involvement of the pineal gland in its patho-physiological mechanism. Peptides. 1980;1:281–284. doi: 10.1016/0196-9781(80)90003-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.