Abstract
Micronutrients such as siderophore-bound iron and vitamin B12 cross the outer membrane of Gram-negative bacteria through a group of 22-stranded β-barrel proteins. They share the unusual feature that their N-terminal end inserts from the periplasmic side into the β-barrel and plugs the lumen. Transport results from energy-driven movement of TonB protein, which either pulls the plug out of the barrel or causes it to rearrange within the barrel. Attempts to reconstitute native plugged channels in an ion-conducting state in lipid bilayer membranes have so far been unsuccessful. We, however, have discovered that if the cis solution contained 4 M urea, then, with the periplasmic side of the channel facing that solution, macroscopic conductances and single channel events could be observed. These results were obtained with FhuA, Cir, and BtuB; for the former two, the channels were closed by removing the 4 M urea. Channels generated by 4 M urea exposure were not a consequence of general protein denaturation, as their ligand-binding properties were preserved. Thus, with FhuA, addition of ferrichrome (its siderophore) to the trans, extracellular-facing side reversibly inhibited 4 M urea-induced channel opening and blocked the channels. With Cir, addition of colicin Ia (the microbial toxin that targets Cir) to the trans, extracellular-facing side prevented 4 M urea-induced channel opening. We hypothesize that 4 M urea reversibly unfolds the FhuA and Cir plugs, thereby opening an ion-conducting pathway through these channels, and that this mimics to some extent the in vivo action of TonB on these plugs.
Keywords: BtuB, Cir, FhuA, urea-denaturation
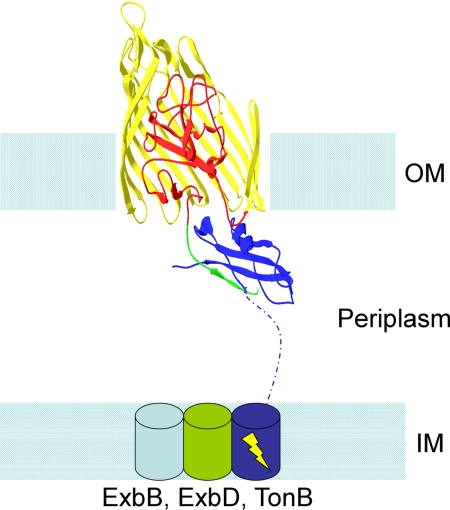
For a Gram-negative bacterium to transport micronutrients such as iron and vitamin B12 across its plasma membrane, it must first get them across its outer membrane. In general, these molecules are too large or, like Fe3+, are bound to a carrier molecule that is too large, to pass through the various porins in the outer membrane. Instead they are transported through an unusual group of 22-stranded β-barrel proteins that require energy input via the TonB complex to unplug the channel (hence the name TonB-dependent transporters; for a general review of this subject, see ref. 1). The TonB complex consists of three proteins that traverse the plasma membrane, ExbB, ExbD, and TonB. TonB itself is inserted in the plasma membrane and extends across the periplasmic space to make contact with the β-barrel proteins via their “TonB box” (Fig. 1). The crystal structure of a number of these β-barrels has been determined (2–8), and in addition to being 22-stranded, they share the unusual feature that their N-terminal end inserts from the periplasmic side into the β-barrel and plugs the lumen (Fig. 1). The picture of transport through this ostensibly occluded β-barrel pore is as follows (Fig. 1); (i) the appropriate siderophore (or vitamin B12) binds to extracellular loops of the barrel and to an extracellularly exposed part of the plug; (ii) this leads to a movement of the TonB box of the plug into the periplasm, where it makes contact with TonB; (iii) this in turn results in an energy-driven movement of TonB, which either pulls the plug out of the barrel or causes it to rearrange within the barrel to open a space wide enough for the siderophore to diffuse through (6, 9–11).
Fig. 1.
Schematic model of the TonB-receptor interaction. Structures of TonB interacting with the siderophore receptor FhuA (36) and vitamin B12 receptor BtuB (37) have been solved. In this example, FhuA is shown in the outer membrane (OM). Its β-barrel is colored yellow, with residues 181–480 cut away for clarity and to reveal its N-terminal plug domain, shown here in red. Residues 8–18 (which contain the TonB box, residues 8–13) are colored green and shown interacting with the C terminus of TonB (colored solid blue). The ribbon structures of FhuA and the C terminus of TonB are taken from PDB file 2GRX (36). The rest of the figure is a diagram representation, and therefore is not representative of actual structures. The inner membrane is shown with ExbB, ExbD and the inner-membrane-anchored part of TonB depicted as cylinders, with a dashed blue line connecting the TonB inner-membrane cylinder to its C-terminal domain from 2GRX. The yellow lightning bolt indicates that the system is energy-driven.
Given, as indicated above, that these channels are plugged, it is not surprising that, with the exception of two FhuA deletion mutants that displayed aberrant properties (12, 13), no one has thus far reconstituted them in an ion-conducting state in lipid bilayer membranes (14–16). [The binding of T5 bacteriophage to FhuA generated approximately 1 nS channels (16), but it is not clear that these resulted from opening of the FhuA channel (17, 18)]. Our own initial attempts with several of these proteins were equally unsuccessful. Recently, however, we have discovered and report here that if the cis solution contains 4 M urea, then macroscopic conductances and single-channel events can be observed with the 22-stranded β-barrel proteins FhuA, Cir, and BtuB. Moreover, FhuA and Cir channels retain their biological responses to appropriate substrates; that is, the siderophores, colicins, and bacteriophages that interact with them.
Results
Inducing Channel Activity.
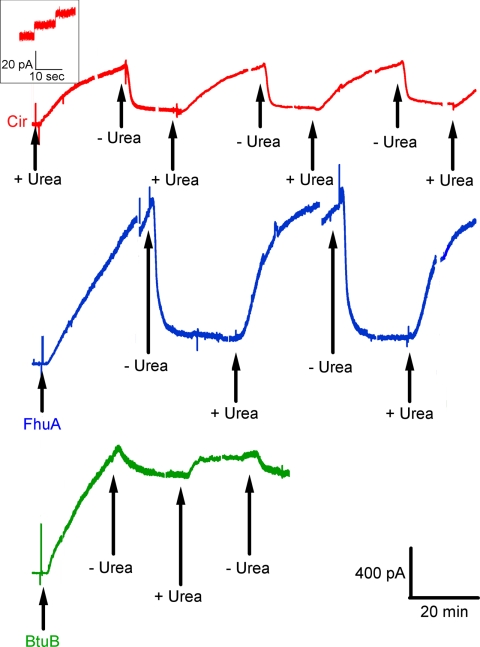
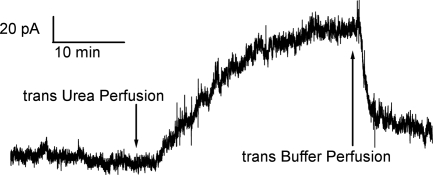
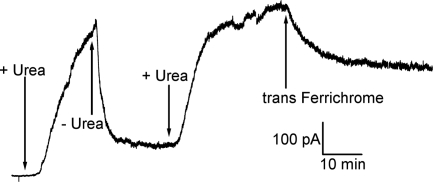
When either FhuA, Cir, or BtuB was added to the cis compartment with the membrane separating symmetric solutions (100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM Hepes, pH 7.2), no conductance increase occurred over periods of tens of minutes. This was not surprising, since the conduction pathway through these β-barrels was presumably blocked by their N-terminal plugs. To loosen or remove these plugs, we thought to unfold them with a denaturant such as urea. And indeed, if the cis solution contained 4 M urea and either Cir, BtuB, or FhuA, a continuous rise in conductance ensued for tens of minutes, with some discernible single-channel events of 0.1–1 nS (Fig. 2). This result was independent of the order of addition, i.e., urea followed by protein or protein followed by urea. However, in the latter case, the protein is being perfused out of the compartment concurrently with urea perfusion into the compartment; hence, the magnitude of the response was more variable. Upon removal of the 4 M urea, the FhuA- and Cir-induced conductances fell 60–90%, and this rise and fall of conductance could be repeated several times upon addition and removal of 4 M urea from the cis compartment (Fig. 2). Higher concentrations of urea up to 8 M gave similar results, whereas 3 M urea and lower were ineffective. In the case of BtuB, removal of the 4 M urea terminated the conductance rise, but caused minimal conductance decline (Fig. 2). Because of the essentially irreversible nature of the BtuB-induced conductance, all subsequent experiments reported here were with FhuA and Cir.
Fig. 2.
Current traces obtained when membranes exposed to the specified TonB-dependent receptors were repeatedly cycled between cis 4 M urea and cis buffer exposure. Greater than 60% loss of conductance was consistently observed upon removal of urea (by perfusion) in the case of FhuA and Cir. With BtuB, we saw little reversal, generally <20%. For BtuB and FhuA, the transporters were added to the cis compartment (first arrows) with 4 M urea already perfused in. For Cir, the urea was perfused into the cis compartment (first arrow) 5 min after the addition of Cir. All traces conform to the same time and current scale, shown at the lower right. “+ Urea” indicates times when the receptor began its exposure to urea (via perfusion), whereas “− Urea” indicates the beginning of urea removal (by buffer perfusion). In all traces, perfusions were into the cis compartment at a rate of 2 mL/min over a period of 8 min. Breaks in the current traces are positions where voltage was switched to 0 or + 20 mV; those resulting current traces have been removed to simplify the figure. Note that with FhuA, there is some loss of conductance after such a protocol. The inset shows a magnification of the early minutes of the Cir current trace to display an example of the single channel conductances observed in the system for all receptors reported. Except for the short breaks in the traces, the voltage was held at cis −20 mV. The concentrations of Cir, FhuA, and BtuB in the cis compartment were approximately 14 nM, 1.4 nM, and 13 nM, respectively. Note also that after the initial exposure to urea, the free receptor in the cis compartment is washed out along with the urea; consequently, there is no free receptor in solution for the remainder of the experiment.
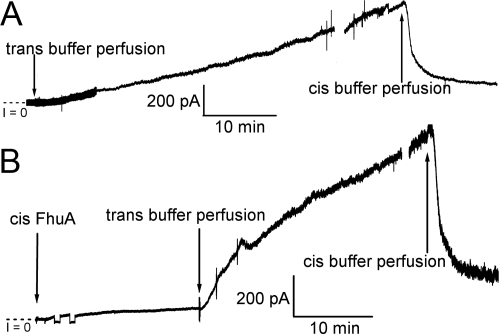
With 4 M urea or 3 M glycerol [which has approximately the same osmolality as 4 M urea and like urea is not very membrane-permeant (19)] in the trans solution and 4 M urea in the cis solution, the introduction of FhuA or Cir to the cis solution did not result in a significant conductance increase across the membrane. If, however, the 4 M urea (or 3 M glycerol) was subsequently removed from the trans solution by perfusion, the reversible 4 M urea-induced conductance ensued (Fig. 3). These experiments suggest that cis 4 M urea performs two functions in opening the channels: (i) it unfolds the plugs, and (ii) it establishes an osmotic pressure gradient across the membrane. We have been able to dissect these two aspects of its action through experiments conducted with 3 M glycerol, but to explain these experiments requires the following digression.
Fig. 3.
In the absence of an osmotic gradient, cis 4 M urea has little effect on membrane conductance. With 4 M urea in the cis solution, if FhuA is introduced to the cis compartment (final concentration ≈1.4 nM) in the presence of trans 4 M urea (A) or trans 3 M glycerol (B), little or no conductance increase is observed across the membrane. (In A, FhuA was introduced 15 min before the start of the record.) However, when the trans urea or glycerol is replaced by buffer, thus establishing an osmotic gradient across the membrane, a rapid rise in conductance across the membrane occurs. This conductance is reversible, as shown when the substitution of 4 M urea in the cis solution with buffer results in a decrease in conductance across the membrane. The breaks in the record indicate preparations for perfusing the opposite compartment of the chamber. Except in B, where the voltage polarity was switched twice for ≈1 min early in the record, the voltage was held at cis −20 mV.
FhuA is the receptor for the T5 bacteriophage (20); it is a TonB-independent phage that has been shown to extrude its DNA upon contact with FhuA (18). Bonhivers et al. (16) have reported that the binding of the phage to FhuA in planar phospholipid bilayers resulted in the appearance of approximately 1 nS channels in 0.1 M KCl. We have not been able to reproduce this finding under the conditions described by these authors, but as we shall see below, we have also established conditions for observing phage-induced channels. It is not clear whether the phage has opened up the FhuA channel, as those authors then proposed, or, as the same group more recently proposed, that the channel is formed from the insertion of the phage tail into the membrane, subsequent to its binding to FhuA (17, 18); for our present purposes, this is irrelevant. What is relevant is that we can use the phage-induced channels as an assay for determining if FhuA channels have been inserted into the membrane in their closed (plugged) state. The following experiment demonstrates that such an insertion can be effected by 3 M glycerol.
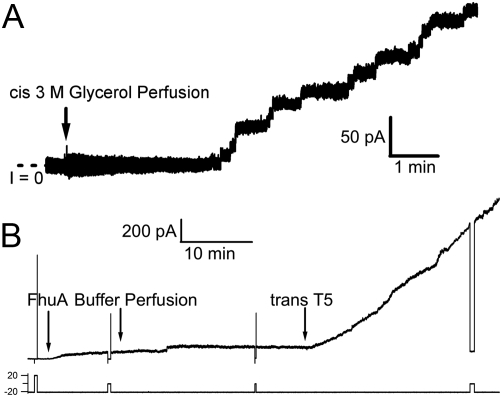
After FhuA was added to the solution on one side of the bilayer (the cis side) and then perfused out of the cis solution, there was, as expected, no channel activity. If at this point T5 phage was added to either the trans or cis solution, there was still no channel activity. This indicates that the FhuA was not properly inserted in the membrane to expose the phage binding site, on its extracellular-facing side, to either solution. One might assume that this is because perfusion of free FhuA from the cis solution caused it to be no longer associated with the membrane. This, however, was not the case. If after FhuA was perfused out of the cis solution, 3 M glycerol was added to the cis solution and then subsequently perfused out, there was still no channel activity. Now, however, the addition of T5 phage to either the trans or cis side resulted in the appearance of numerous 0.1 to 1 nS channels, leading to large macroscopic conductances (Fig. 4 A and B). Neither experiments using bacteriophage BF23 in place of T5 phage nor experiments exposing T5 phage directly to 3M glycerol in the absence of FhuA produced any channel activity. If, on the other hand, 3 M glycerol was added to the trans solution instead of to the cis solution, or if 3 M glycerol was added first to the trans solution, then to the cis solution, and then perfused out of both solutions, cis side first, so that at no time was the cis solution hyperosmotic to the trans solution, the subsequent addition of T5 phage to either solution was ineffective in producing channels. Similar results to those obtained with 3 M glycerol were obtained with 2.6 M glucose, which has approximately the same osmolality (Fig. S1).
Fig. 4.
An osmotic gradient is needed for proper insertion of the β-barrel proteins into the membrane; 3 M glycerol can create the osmotic gradient necessary to insert and orient FhuA. (A) Before the start of the record, FhuA (1.4 nM) was added to and then perfused out of the cis compartment, followed by the addition of T5 (1 × 1011 particles, ≈55 pM) to the same compartment, producing no conductance. The trace begins (50 min after the addition of FhuA) with the perfusion (at a rate of 2 mL/min for 8 min) of 3 M glycerol into the cis compartment, and we have focused on the early minutes of the glycerol perfusion to allow easy visualization of the single channel events. Although perfusion of the cis compartment with 3 M glycerol simultaneously removes T5 phage from that compartment, it has not removed all of the phage. Current trace was obtained at −20 mV. (B) Addition of FhuA (1.4 nM) to the cis compartment with 3 M glycerol already present, and then removal of the glycerol (and FhuA) by perfusion, produced very little increase in conductance. However, addition of phage T5 (1 × 1011 particles, ≈55 pM) to the trans solution caused a rapid increase in the conductance. (In comparable experiments in which the 3 M glycerol was omitted, trans T5 phage had no effect.) Arrows indicate changes to the cis solution unless otherwise specified. Current trace was obtained at cis voltages shown on the chart. These data in A and B indicate that FhuA was inserted into the membrane, with its T5 phage binding site facing either solution.
We interpret these data as follows. We believe that after free FhuA was perfused out of the cis solution, there still remained FhuA adsorbed to the cis membrane/solution interface, but it was not oriented (i.e., inserted) properly in the membrane to expose the phage binding site to either solution. The osmotic gradient created by cis 3 M glycerol resulted in the proper insertion of FhuA, with some oriented extracellular side (containing the phage binding site) facing the trans solution and some extracellular side facing the cis solution.
Consistent with these phage experiments, if, after the 3 M glycerol protocol (exposure of FhuA to cis 3 M glycerol, then removal via perfusion), 4 M urea was added to the trans solution, a continuous rise in conductance occurred, with some discernable channels of 0.1 nS to 1 nS, leading to large macroscopic conductances. Thus, the 3 M glycerol treatment led to the insertion of FhuA channels in their closed state, with some of these oriented with their periplasmic side facing the trans solution. Without the 3 M glycerol protocol, the trans 4 M urea had no effect. Removal of the 4 M urea by perfusion resulted in the closure of these channels (Fig. 5).
Fig. 5.
Three molar glycerol can insert FhuA to allow urea to induce conductance. The current trace begins after FhuA (1.4 nM) was added to the cis compartment with 3 M glycerol present. Later, the cis compartment was perfused with buffer, removing both FhuA and glycerol. The first arrow of the trace is the beginning of a trans 4 M urea perfusion; the rise in conductance indicates that FhuA was inserted into the membrane. The second arrow is the beginning of the urea perfusion out of the system, again demonstrating the reversibility of the conductance. (Similar results were obtained if 4 M urea was perfused into the cis rather than the trans compartment). The experiment was performed at cis +20 mV. Here, cis positive voltage was used to keep the side with the urea at negative voltage, to be consistent with the rest of the experiments shown. All perfusions in this experiment were 8 min long with a flow rate of 2 mL/min.
Preservation of Function.
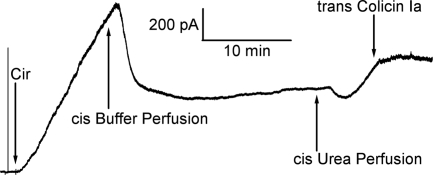
It might be thought that the channels generated by protein exposure to 4 M urea in these experiments were caused by general denaturation of the proteins, and therefore that their structures bear no resemblance to native forms. We found, however, that both Cir and FhuA channels could interact with their native ligands after exposure to 4 M urea. Colicin Ia is a channel-forming colicin that has parasitized Cir, using it as its receptor (21). When added to the trans (corresponding to the extracellular) side at nanomolar concentrations, it dramatically slowed or stopped the 4 M urea-induced rise in conductance produced by Cir (Fig. 6); as a control, the addition of a comparable amount of colicin E3, which does not bind to Cir, had no effect (Fig. S2). [One may be concerned that colicin Ia would complicate the experiments by being an additional channel-forming entity in the system. In the decane-containing diphytanoylphosphatidylcholine membranes used in these experiments, however, colicin Ia channel-forming activity is essentially nonexistent, and therefore is not a complicating factor. Moreover, we obtained the same results using a construct that lacked the channel-forming domain of colicin Ia and contained only the receptor-binding (R) and translocation (T) domain (Fig. S3)]. Similarly, when ferrichrome (the cognate siderophore of FhuA) was added at nanomolar concentrations to the trans (corresponding to the extracellular) side, it stopped, and even partially reversed, the 4 M urea-induced conductance produced by FhuA (Fig. 7). Thus, the 4 M urea treatment did not compromise Cir's ability to bind the R domain of colicin Ia, nor FhuA's ability to bind ferrichrome.
Fig. 6.
Cir is responsive to its cognate colicin. The current trace begins with the addition of Cir (≈14 nM) to 4 M urea. The 4 M urea-induced Cir-mediated membrane conductance is seen to be halted soon after addition of trans colicin Ia (≈15 nM). The experiment was performed with the cis compartment held at −20 mV.
Fig. 7.
FhuA is responsive to its natural ligand, ferric-ferrichrome. The current trace begins after FhuA (1.4 nM) was added to the cis compartment and washed out 10 min later by perfusion with buffer. Then, at the first arrow, 4 M urea was perfused into the cis compartment (at a rate of 2 mL per min for 8 min) and removed by a buffer perfusion (second arrow). Urea was then reintroduced, and following the second urea perfusion, the addition of ferric-ferrichrome (330 nM) to the trans compartment resulted in partial conductance decrease. The current shown was obtained at cis −20 mV, and the break in the trace indicates the time during which the voltage had been switched from −20 mV to + 20 mV.
Discussion
Transport of micronutrients across the outer membrane of Gram-negative bacteria occurs via a class of 22-stranded β-barrel channels whose lumens are occluded by a plug, formed from their N-terminal ends, that inserts into them from the periplasmic side (Fig. 1). Given this picture, it is not surprising that the simple addition of the proteins that form these channels to the solution on one side of a planar lipid bilayer membrane does not lead to an increase in conductance and the appearance of ion-conducting channels. We have shown here, however, that if the solutions to which these proteins are added contain 4 M urea, large increases in conductance occur, composed of ion-conducting channels with partly unresolvable (continuous) conductance and partly resolvable (discrete) conductance in the range of 0.1–1 nS. In particular, we have demonstrated this for the TonB-dependent transporters BtuB, FhuA, and Cir; in the case of the latter two, the channels can be reversibly closed and reopened by removal and reexposure to 4 M urea (Fig. 2). These channels are not manifestations of 4 M urea-denatured proteins, but rather they retain the native channel properties of binding, at nanomolar concentrations, their appropriate micronutrient [the siderophore ferrichrome in the case of FhuA (Fig. 7)] or the appropriate agents that parasitize them [colicin Ia in the case of Cir (Fig. 6) and the bacteriophage T5 in the case of FhuA (Fig. 4); the FhuA-T5 interaction was described in ref. 16]. The ability of colicin Ia to halt the Cir-induced conductance rise across the membrane (Fig. 6) can be seen as a functional example of what was observed previously in the literature: namely, neither the R-domain of colicin E2 in complex with BtuB (22) nor the R domain of colicin Ia in complex with Cir (3) resulted in any displacement of the plug domains from their respective barrels. In addition, both of the crystal structures show hydrogen bond interactions between the plug domain of the receptor and the R-domain of the respective colicin. What we believe our observed phenomena represent, therefore, is the binding of colicin Ia to the extracellular side of still closed Cir transporters, thus stabilizing the “closed” state of the protein against the denaturing properties of the urea.
The reconstitution of these channels in an ion-conducting state is apparently a two-step process, requiring both an osmotic gradient across the membrane and a denaturant. Thus, with the denaturant 4 M urea in the solution on both sides of the membrane, the addition of FhuA to one side of the membrane does not result in the appearance of channels in either an open state (Fig. 3A) or a closed state (as evidenced by the failure of T5 phage to produce channels, as discussed in Inducing Channel Activity). On the other hand, the application of an osmotic gradient generated by 3 M glycerol, a nondenaturant, across the membrane results in the insertion of FhuA channels in the closed state (Fig. 4B), but not in the open state. When 4 M urea is used alone on the cis side, which is the case in most of the experiments we report, it produces channel opening through two effects: its osmotic effect, which causes proper insertion of the channels, and its denaturing effect on the plug (see below), which opens the inserted channels. The 3 M glycerol treatment deconvolutes these two actions of 4 M urea. How an osmotic gradient causes the insertion of detergent-bound FhuA is not clear, but it is probably in some ways related to the mechanism by which vesicle-containing channels can be incorporated into planar bilayers by osmotic gradient-induced fusion of these vesicles into that membrane (23).
We note that although only a cis hyperosmotic gradient is capable of inserting these proteins in the membrane, the proteins appear to insert in both possible orientations—with the side of the protein that faces the outside of the E. coli cell facing both the cis side and the trans side—as shown by the response of FhuA to phage T5 from both cis and trans sides (Results and Fig. 4). This contrasts with the trimeric porin, maltoporin, which inserts spontaneously in planar lipid bilayer membranes with its outside-facing side oriented toward the cis side of the membrane. Conductance through that porin is blocked by phage lambda, for which it is the cell surface receptor, added to the cis side, but not to the trans side (24). In contrast, colicin E3 occludes the OmpF porin only from the trans side (15) of planar lipid bilayer membranes, implying the opposite orientation of OmpF porin to that of maltoporin. There is, in any case, no reason to expect that a trimeric porin, which inserts spontaneously in the bilayer, and the TonB-dependent receptors, which require an osmotic gradient to insert, should necessarily behave the same way in an in vitro system such as ours.
Based on the work of others in the field, our picture of what happens as urea opens the inserted channels is as follows (although it should be noted, that the primary purpose of this work is to present a technique for inserting functional plugged channels into planar lipid bilayers, not to directly address the critical questions in the field). We believe that 4 M urea (but not 3 M urea or less) partially or fully unfolds the N-terminal part of the protein that forms the plug of these channels, thereby opening an ion-conducting pathway. It is generally assumed that during transport (of siderophores or colicins) the plug domain either comes out of its barrel (11, 25) or rearranges within the barrel (9, 10). A general unfolding of the plug domain could be a mechanism in either theory. When urea is removed, the N-terminal part of the protein refolds to form a functional plug that closes the channels.
The fact that not all of the channels (10–40%) close upon removal of the 4 M urea may reflect irreversible unfolding of a subpopulation of plugs, or perhaps refolding of plugs that have been completely pulled out of the channel but cannot readily reinsert into them. We hypothesize that there is a continuum of structures between the folded native plug and its completely denatured unfolded state, and that this accounts for the unresolvable increments in conductance and for the dispersion of single-channel conductances, both of which contribute to the macroscopic conductance. Given, however, that loss of an external loop can convert FhuA into an open, TonB-independent channel (12), we cannot preclude the possibility that urea is somehow causing that loop to move aberrantly and thereby opening the channel. However, the stability and independence of the plug domain when apart from the barrel has been demonstrated, allowing us to suggest that the unfolding happens only to the plug domain. Thus, Braun et al. (26) showed in vivo that separately expressed FhuA plugs and barrels can complement each other to form functional units, and Oke et al. (27) showed the stability of the plug domain of TbpA from N. meningitidis when expressed and purified alone in E. coli, independent of the barrel. Most compelling, Bonhivers et al. (28) demonstrated, using differential scanning calorimetry, that the plug and barrel domains of FhuA behave as autonomous domains and unfold at different temperatures, with the plug domain unfolding before the barrel domain.
We should like to believe that the action of 4 M urea on the plug mimics to some extent that of TonB (i.e., urea either changes the conformation of the plug within the barrel or pulls it out of the barrel, or both); whether this is true or not will depend on further experiments both with this model bilayer system and with complete TonB-coupled channel opening. It would seem that the incomplete reversibility of conductance, resulting from subpopulations of receptors with misfolded plugs, is an artifact of the use of urea instead of TonB to affect the plug. For of course in a cell, the plugs would return to their original state lest a large permeation pathway be left opened in the bacterial outer membrane.
Materials and Methods
Planar Lipid Bilayer Experiments.
Planar bilayers were formed at room temperature by the brush technique (29) across a 0.5-mm hole in a 125 μm thick Teflon partition. Membranes separated two Lucite compartments, each containing 3 mL of 100 mM KCl, 1 mM EDTA, 5 mM CaCl2, 20 mM Hepes, pH 7.2, which could be stirred by small magnetic stir bars. The membrane-forming solution was 3% diphytanoylphosphatidylcholine in n-decane. Unless otherwise specified, the following reagents were added at the indicated final concentrations: FhuA at ≈1.4 nM, Cir at ≈14 nM, BtuB at ≈13 nM, ferrichrome at ≈330 nM, phage T5 or BF23 at 1 × 1011 PFU (≈55 pM assuming one particle per PFU), and colicin Ia at ≈15 nM. Perfusions of each compartment were performed via a polystaltic pump (Buchler Instruments) at a flow rate of 2 mL/min. All experiments were done under voltage-clamp conditions; voltages are those of the cis solution (to which protein was added) with respect to that of the trans solution, which was held at virtual ground. Current responses were filtered at 10 Hz and recorded either on a Narco physiograph chart recorder or by an NI6211 Data Acquisition Board (National Instruments) and stored digitally. Each of the experiments reported here was performed at least three times with similar results.
Reagents.
FhuA with a D336C mutation, which was introduced in anticipation of future experiments, was expressed and purified according to James et al. (30), who showed it to behave identically to wild type FhuA; Cir was expressed and purified according to Buchanan et al. (3); BtuB was a generous gift from William Cramer (Purdue University, West Lafayette, IN); colicin Ia was expressed and purified according to Kienker et al. (31). Stock solutions of FhuA (0.33 mg/mL in 50 mM ammonium acetate, pH 8.0, 250 mM NaCl, 70 mM imidazole, 0.1% LDAO), Cir (3.5 mg/mL in 20 mM Tris-Cl, pH 7.3, 200 mM NaCl, 1% octyl glucoside, 0.02% sodium azide), BtuB (3 mg/mL in 20 mM Tris-Cl, pH 8.0, 0.1% LDAO), and colicin Ia (0.79 mg/mL in 50 mM boric acid, pH 9.0 (NaOH), 300 mM NaCl, 2 mM EDTA) were stored at −80 °C; working solutions were kept stable at 4 °C for at least 6 months.
Ferrichrome was purified from 5 L of culture supernatant of Ustilago sphaerogena grown in modified Grimm-Allen medium (32) without added iron, by modifications of the procedure of Neilands (33). The ferrichrome-containing aqueous solution from benzyl alcohol/diethyl ether extractions of ferrous sulfate-treated culture media was concentrated by rotary evaporation, and chromatographed over DEAE cellulose in 0.05 M Tris-Cl, pH 7.5, to separate ferrichrome and trace amounts of anionic ferrichrome A, whose identities were confirmed by their visible absorption spectra (ferrichrome: εmM425 nm = 2.9; ferrichrome A: εmM440 nm = 3.7). Ferrichrome was crystallized from anhydrous methanol (33), and stored in desiccated form.
Phage BF23 was a gift of the late Robert Kadner. High-titer stocks of both BF23 and T5 were prepared by adapting protocols described by Yamamoto et al. (34), and Zweig and Cummings (35). Phage were propagated on W3110 rpsL in Difco nutrient broth containing 1 mM CaCl2. One liter of phage lysate was sterilized with 0.1% CHCl3, treated for 30 min with 300 μg DNase (Worthington DPRF) and 50 μg RNase (Worthington RPDF). Phage from two successive precipitations in 7.5% PEG 6000 (34) were gently resuspended in 9 mL of sterile phage buffer (1 mM sodium phosphate, pH 7.2, 0.9% NaCl, 2 mM MgSO4, 1 mM CaCl2) and finally purified by loading onto a CsCl gradient (35) and centrifuging for 3.5 h at 104,000 × g. The sharp blue band of purified phage was removed with a syringe and needle from the side of the tube. Purified phage were dialyzed into 10 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM MgSO4, 1 mM CaCl2, stored at 4 °C. in the presence of 0.05% sodium azide, and each had a titer of approximately 5 × 1012 PFU/mL.
Supplementary Material
Acknowledgments.
We thank Dr. James W. Coulton for his generous gift of FhuA protein and phage T5 and his helpful discussions throughout this work, and Dr. William Cramer for the gift of BtuB. This work was supported by National Institutes of Health Grants GM29210 (to A.F.), GM53836 (to P.E.K.) and GM008572 (to E.U.); National Science Foundation Grant MCB-0414694 (to P.E.K.); Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (S.K.B.); Canadian Institutes of Health Research Grants MOP 14133 to J. W. Coulton (K.J.J.) and PRG 82674 (to K.J.J.); and the Canada Foundation for Innovation (K.J.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910023106/DCSupplemental.
References
- 1.Faraldo-Gomez JD, Sansom MS. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol. 2003;4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: Crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan SK, et al. Structure of colicin I receptor bound to the R-domain of colicin Ia: Implications for protein import. EMBO J. 2007;26:2594–2604. doi: 10.1038/sj.emboj.7601693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan SK, et al. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 5.Chimento DP, Mohanty AK, Kadner RJ, Wiener MC. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat Struct Biol. 2003;10:394–401. doi: 10.1038/nsb914. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson AD, et al. Structural basis of gating by the outer membrane transporter FecA. Science. 2002;295:1715–1719. doi: 10.1126/science.1067313. [DOI] [PubMed] [Google Scholar]
- 7.Locher KP, et al. Transmembrane signaling across the ligand-gated FhuA receptor: Crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 8.Cherezov V, et al. In meso structure of the cobalamin transporter, BtuB, at 1.95 A resolution. J Mol Biol. 2006;364:716–734. doi: 10.1016/j.jmb.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty R, Storey E, van der Helm D. Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli. Biometals. 2007;20:263–274. doi: 10.1007/s10534-006-9060-9. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer HA, Shames S, Pawelek PD, Coulton JW. Siderophore transport through Escherichia coli outer membrane receptor FhuA with disulfide-tethered cork and barrel domains. J Biol Chem. 2005;280:30574–30580. doi: 10.1074/jbc.M506708200. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, et al. Evidence of ball-and-chain transport of ferric enterobactin through FepA. J Biol Chem. 2007;282:397–406. doi: 10.1074/jbc.M605333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killmann H, Benz R, Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993;12:3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun M, Killmann H, Maier E, Benz R, Braun V. Diffusion through channel derivatives of the Escherichia coli FhuA transport protein. Eur J Biochem. 2002;269:4948–4959. doi: 10.1046/j.1432-1033.2002.03195.x. [DOI] [PubMed] [Google Scholar]
- 14.Zakharov SD, Cramer WA. On the mechanism and pathway of colicin import across the E. Coli outer membrane. Front Biosci. 2004;9:1311–1317. doi: 10.2741/1334. [DOI] [PubMed] [Google Scholar]
- 15.Zakharov SD, et al. Colicin occlusion of OmpF and TolC channels: Outer membrane translocons for colicin import. Biophys J. 2004;87:3901–3911. doi: 10.1529/biophysj.104.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonhivers M, Ghazi A, Boulanger P, Letellier L. FhuA, a transporter of the Escherichia coli outer membrane, is converted into a channel upon binding of bacteriophage T5. EMBO J. 1996;15:1850–1856. [PMC free article] [PubMed] [Google Scholar]
- 17.Letellier L, Boulanger P, de Frutos M, Jacquot P. Channeling phage DNA through membranes: From in vivo to in vitro. Res Microbiol. 2003;154:283–287. doi: 10.1016/S0923-2508(03)00072-X. [DOI] [PubMed] [Google Scholar]
- 18.Bohm J, et al. FhuA-mediated phage genome transfer into liposomes: A cryo-electron tomography study. Curr Biol. 2001;11:1168–1175. doi: 10.1016/s0960-9822(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 19.Orbach E, Finkelstein A. The nonelectrolyte permeability of planar lipid bilayer membranes. J Gen Physiol. 1980;75:427–436. doi: 10.1085/jgp.75.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantke K, Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978;135:190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konisky J, Liu CT. Solubilization and partial characterization of the colicin I receptor of Escherichia coli. J Biol Chem. 1974;249:835–840. [PubMed] [Google Scholar]
- 22.Sharma O, et al. Structure of the complex of the colicin E2 R-domain and its BtuB receptor. The outer membrane colicin translocon. J Biol Chem. 2007;282:23163–23170. doi: 10.1074/jbc.M703004200. [DOI] [PubMed] [Google Scholar]
- 23.Niles WD, Cohen FS, Finkelstein A. Hydrostatic pressures developed by osmotically swelling vesicles bound to planar membranes. J Gen Physiol. 1989;93:211–244. doi: 10.1085/jgp.93.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurnev PA, Oppenheim AB, Winterhalter M, Bezrukov SM. Docking of a single phage lambda to its membrane receptor maltoporin as a time-resolved event. J Mol Biol. 2006;359:1447–1455. doi: 10.1016/j.jmb.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Devanathan S, Postle K. Studies on colicin B translocation: FepA is gated by TonB. Mol Microbiol. 2007;65:441–453. doi: 10.1111/j.1365-2958.2007.05808.x. [DOI] [PubMed] [Google Scholar]
- 26.Braun M, Endriss F, Killmann H, Braun V. In vivo reconstitution of the FhuA transport protein of Escherichia coli K-12. J Bacteriol. 2003;185:5508–5518. doi: 10.1128/JB.185.18.5508-5518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oke M, et al. The plug domain of a neisserial TonB-dependent transporter retains structural integrity in the absence of its transmembrane beta-barrel. FEBS Lett. 2004;564:294–300. doi: 10.1016/S0014-5793(04)00196-6. [DOI] [PubMed] [Google Scholar]
- 28.Bonhivers M, et al. Stability studies of FhuA, a two-domain outer membrane protein from Escherichia coli. Biochemistry. 2001;40:2606–2613. doi: 10.1021/bi001725i. [DOI] [PubMed] [Google Scholar]
- 29.Mueller P, Rudin DO. Induced excitability in reconstituted cell membrane structure. J Theor Biol. 1963;4:268–280. doi: 10.1016/0022-5193(63)90006-7. [DOI] [PubMed] [Google Scholar]
- 30.James KJ, Hancock MA, Moreau V, Molina F, Coulton JW. TonB induces conformational changes in surface-exposed loops of FhuA, outer membrane receptor of Escherichia coli. Protein Sci. 2008;17:1679–1688. doi: 10.1110/ps.036244.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kienker PK, Jakes KS, Finkelstein A. Identification of channel-lining amino acid residues in the hydrophobic segment of colicin Ia. J Gen Physiol. 2008;132:693–707. doi: 10.1085/jgp.200810042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimm PW, Allen PJ. Promotion by zinc of the formation of cytochromes in ustilago sphaerogena. Plant Physiol. 1954;29:369–377. doi: 10.1104/pp.29.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neilands JB. A crystalline organo-iron pigment from a rust fungus (ustilago sphaerogena)1. J Am Chem Soc. 1952;74:4846–4847. [Google Scholar]
- 34.Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970;40:734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- 35.Zweig M, Cummings DJ. Structural proteins of bacteriophage T5. Virology. 1973;51:443–453. doi: 10.1016/0042-6822(73)90443-1. [DOI] [PubMed] [Google Scholar]
- 36.Pawelek PD, et al. Structure of TonB in complex with FhuA, E. coli outer membrane receptor. Science. 2006;312:1399–1402. doi: 10.1126/science.1128057. [DOI] [PubMed] [Google Scholar]
- 37.Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: Structure of the BtuB:TonB complex. Science. 2006;312:1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.