Abstract
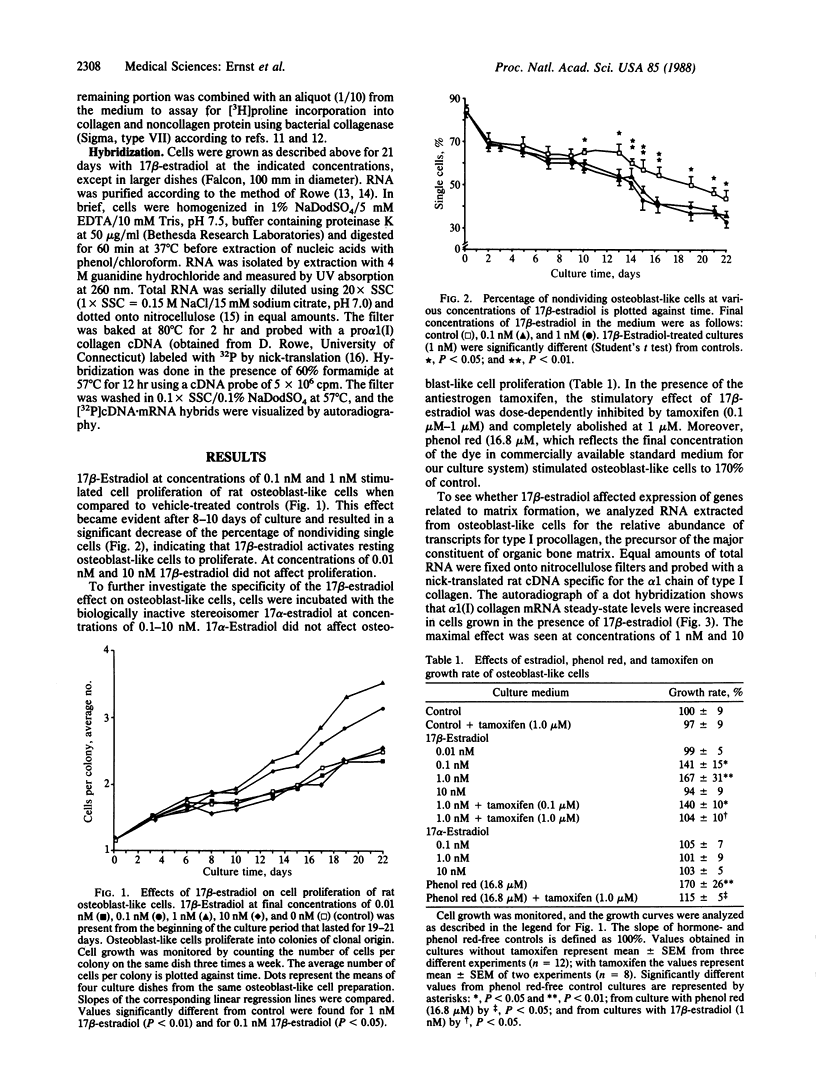
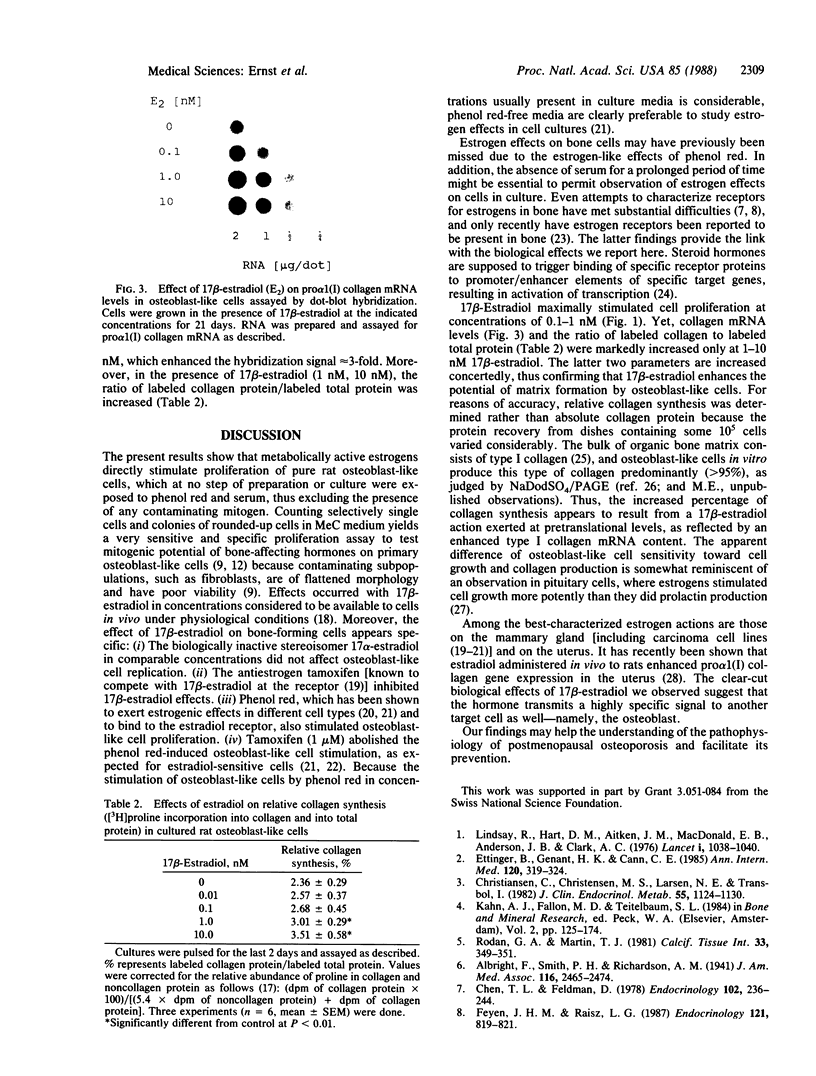
Estrogens play a crucial role in the development of postmenopausal osteoporosis. However, the mechanism by which estrogens exert their effects on bone is unknown. To examine possible direct effects of 17 beta-estradiol on bone-forming cells, we used pure rat osteoblast-like cells in vitro as a model. Osteoblast-like cells prepared from calvaria of newborn rats were cultured serum-free in methylcellulose-containing medium for 21 days. Osteoblast-like cells proliferate selectively into clonally derived cell clusters of spherical morphology. 17 beta-Estradiol at concentrations of 0.1 nM and 1 nM enhanced osteoblast-like cell proliferation by 41% and 68% above vehicle-treated controls. The biologically inactive stereoisomer 17 alpha-estradiol (same concentrations) had no effect. Moreover, the antiestrogen tamoxifen abolished the stimulation of osteoblast-like cell proliferation by 17 beta-estradiol. After 21 days of culture, RNA was prepared and analyzed in a dot-hybridization assay for the abundance of pro alpha 1(I) collagen mRNA. Steady-state mRNA levels were increased in cultures treated with 17 beta-estradiol in a dose-dependent manner with maximal stimulation at 1 nM and 10 nM. At the same concentrations, the percentage of synthesized protein (labeled by [3H]proline pulse) that was digestible by collagenase was increased, indicating that 17 beta-estradiol acts at pretranslational levels to enhance synthesis of bone collagen. These data show that the osteoblast is a direct target for 17 beta-estradiol.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara J. F., Van Itallie C., Dannies P. S. Regulation of prolactin production and cell growth by estradiol: difference in sensitivity to estradiol occurs at level of messenger ribonucleic acid accumulation. Endocrinology. 1987 Jan;120(1):264–271. doi: 10.1210/endo-120-1-264. [DOI] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Distinction between alpha-fetoprotein and intracellular estrogen receptors: evidence against the presence of estradiol receptors in rat bone. Endocrinology. 1978 Jan;102(1):236–244. doi: 10.1210/endo-102-1-236. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Christensen M. S., Larsen N. E., Transbøl I. B. Pathophysiological mechanisms of estrogen effect on bone metabolism. Dose-response relationships in early postmenopausal women. J Clin Endocrinol Metab. 1982 Dec;55(6):1124–1130. doi: 10.1210/jcem-55-6-1124. [DOI] [PubMed] [Google Scholar]
- Coezy E., Borgna J. L., Rochefort H. Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982 Jan;42(1):317–323. [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Collagen biosynthesis during connective tissue development in chick embryo. Dev Biol. 1972 Jul;28(3):443–453. doi: 10.1016/0012-1606(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Ernst M., Froesch E. R. Osteoblastlike cells in a serum-free methylcellulose medium form colonies: effects of insulin and insulinlike growth factor I. Calcif Tissue Int. 1987 Jan;40(1):27–34. doi: 10.1007/BF02555725. [DOI] [PubMed] [Google Scholar]
- Ernst M., Froesch E. R. Triiodothyronine stimulates proliferation of osteoblast-like cells in serum-free culture. FEBS Lett. 1987 Aug 10;220(1):163–166. doi: 10.1016/0014-5793(87)80896-7. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Genant H. K., Cann C. E. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985 Mar;102(3):319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- Feyen J. H., Raisz L. G. Prostaglandin production by calvariae from sham operated and oophorectomized rats: effect of 17 beta-estradiol in vivo. Endocrinology. 1987 Aug;121(2):819–821. doi: 10.1210/endo-121-2-819. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D., Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Komm B. S., Frankel F. R., Myers J. C., Lyttle C. R. Estrogen regulation of alpha 1(I)-procollagen messenger ribonucleic acid in the rat uterus. Endocrinology. 1987 Apr;120(4):1403–1410. doi: 10.1210/endo-120-4-1403. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Aitken J. M., MacDonald E. B., Anderson J. B., Clarke A. C. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976 May 15;1(7968):1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Clark G. M., Takatani O., Wakabayashi Y., Hayward J. L., Bulbrook R. D. Distribution of 17 beta-estradiol in the sera of normal British and Japanese women. J Natl Cancer Inst. 1983 Oct;71(4):749–754. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rajendran K. G., Lopez T., Parikh I. Estrogenic effect of phenol red in MCF-7 cells is achieved through activation of estrogen receptor by interacting with a site distinct from the steroid binding site. Biochem Biophys Res Commun. 1987 Feb 13;142(3):724–731. doi: 10.1016/0006-291x(87)91474-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner M., Fischer S., Dessau W., Müller P. K. Collagen types synthesized by isolated calvarium cells. Exp Cell Res. 1981 May;133(1):115–125. doi: 10.1016/0014-4827(81)90362-1. [DOI] [PubMed] [Google Scholar]




