Abstract
The onset of walking is a fundamental milestone in motor development of humans and other mammals, yet little is known about what factors determine its timing. Hoofed animals start walking within hours after birth, rodents and small carnivores require days or weeks, and nonhuman primates take months and humans approximately a year to achieve this locomotor skill. Here we show that a key to the explanation for these differences is that time to the onset of walking counts from conception and not from birth, indicating that mechanisms underlying motor development constitute a functional continuum from pre- to postnatal life. In a multiple-regression model encompassing 24 species representative of 11 extant orders of placental mammals that habitually walk on the ground, including humans, adult brain mass accounted for 94% of variance in time to walking onset postconception. A dichotomous variable reflecting species differences in functional limb anatomy accounted for another 3.8% of variance. The model predicted the timing of walking onset in humans with high accuracy, showing that this milestone in human motor development occurs no later than expected given the mass of the adult human brain, which in turn reflects the duration of its ontogenetic development. The timing of motor development appears to be highly conserved in mammalian evolution as the ancestors of some of the species in the sample presented here diverged in phylogenesis as long as 100 million years ago. Fundamental patterns of early human life history may therefore have evolved before the evolution of primates.
Keywords: brain, development, locomotion, evolution, life history
Translating developmental timescales between mammals is a complex issue of wide biological interest, with implications for the interpretation of research data from animal models of human development (1, 2) and, potentially, for the understanding of the evolution of human life history (3–5). The complexity lies partly in the vast interspecies differences in the timing of fundamental developmental milestones, such as walking onset, and in that the mechanisms determining the timing of such milestones are poorly understood (6–9). Humans appear to differ from other walking mammals, including other primates, with respect to a number of factors of potential relevance for locomotor development. The most important, perhaps, are that humans have a bipedal gait (10), a large brain relative to body mass (11, 12), a high rate of postnatal brain growth (13), and a protracted postnatal period during which adaptive neuronal mechanisms may operate (8). It is often argued that, taken together, these factors render the time course of human locomotor development qualitatively different from that of other mammals (14). Here we challenge this notion and test the hypothesis that, with regard to walking onset, differences between humans and other mammals are in fact quantitative and hence in essence predictable.
In ontogenetic terms, the most straightforward explanation for interspecies differences in timing of walking onset would be that they reflect different time courses of brain development in general and of maturation of motor systems in particular. In support of this notion, recent studies indicate that the relative time courses of motor development in two mammalian species may be very closely related to the relative time courses of their cerebellar development (15, 16). Because the brains of different mammals appear to develop at similar rates (17), it may be assumed that the time during which the brain develops in a given species will be reflected in its adult brain mass. Indeed, adult brain mass and also body mass have been proposed as important determinants of mammalian life history (18, 19), although this hypothesis is not uncontroversial (19). These variables are therefore “prime candidates” as predictors for the timing of walking onset.
To test our hypothesis we have evaluated and present here a multiple-regression model that encompasses data from 24 species representative of a wide taxonomic range of placental mammals (20, 21), including humans (Fig. 1; Table S1). Contrary to convention, but in accordance with indications that prenatal and postnatal motor developmental processes represent a functional continuum (22, 23), we measured time to walking onset from the point of conception and not from birth. In view of their potential relevance for motor development, but also for life history, we considered neonatal brain mass, gestation time, brain advancement at birth, and relative adult brain mass as continuous independent variables in addition to adult brain mass and body mass (5, 6, 18, 19, 24, 25). Two dichotomous independent variables were also included. One reflects maturity at birth, categorizing species as precocial or altricial, and has been proven useful in previous theories for variation in the timing of general development in mammals (23, 24). The other reflects limb biomechanics and is therefore of potential importance for motor development in particular. It categorizes species on the basis of whether they can or cannot assume a plantigrade standing position of the hindlimb (lower extremity in humans), i.e., stand on the full length of their hind foot including tarsal and metatarsal bones (26). All data were obtained from the literature (see Table S2).
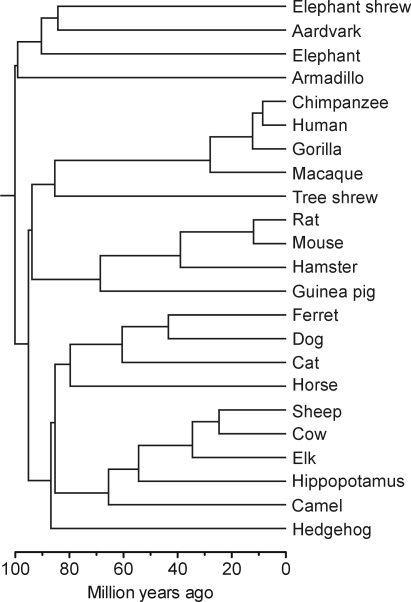
Fig. 1.
The phylogenetic relatedness of species in the present sample illustrated by a chronogram based on divergence time estimates from phylogenomic analysis (48) complemented with mitogenomic analysis (49).
Results
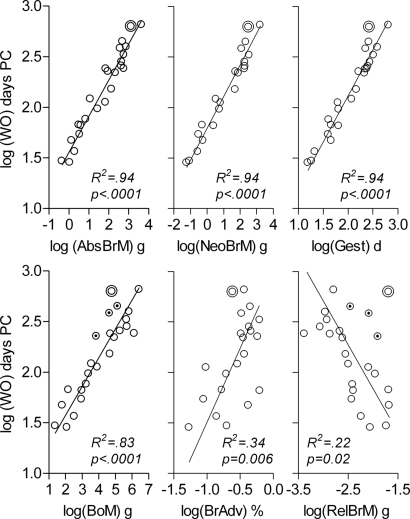
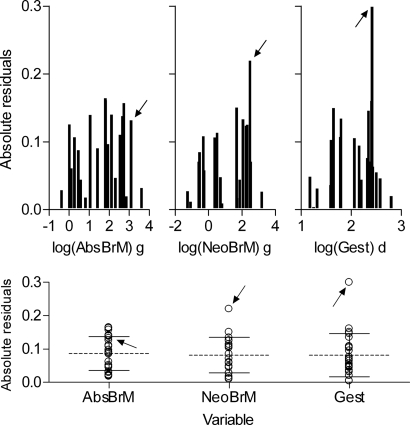
In a first round of analysis, we examined the individual relationships between each of the continuous variables and time to walking onset. Fig. 2 shows linear regressions of walking onset on each of the individual continuous variables alone. Fig. 3 shows the extent to which residuals deviated from the mean for the first three variables in Fig. 2. Absolute brain mass accounted for 94% of variance in time to walking onset and residuals of all individual species were distributed within 1.5 SD from the mean. Neonatal brain mass and gestation time accounted for as much variance as adult brain mass for the sample as a whole, but the residuals of humans for these two variables were conspicuous by their deviation from the mean, by >2.5 SD and >3.0 SD, respectively (Fig. 3). Body mass, brain advancement at birth, and relative brain mass produced progressively lower R2-values, again with humans as an outlier (Fig. 2). Following humans, the three nonhuman primates in our sample had the highest positive residuals with regard to body mass and relative brain mass. One reason could be that humans, and other primates to a varying degree, display a retardation in early postnatal body growth in comparison to other mammals (27), resulting in a lower than expected adult body mass. In support of this explanation, the relative size of positive residuals for humans, chimpanzee, macaque, and gorilla (from high to low values) replicated exactly the relative order from lowest to highest values of body growth constants for these four species (28), despite the overall similarity in body growth rates among the great apes (29).
Fig. 2.
Continuous independent variables considered as predictors of time to walking onset. Time to walking onset, log(WO), is shown as a function of absolute adult brain mass, log(AbsBrM), neonatal brain mass, log(NeoBrM), gestation time, log(Gest), adult body mass, log(BoM), brain advancement at birth (neonatal brain mass/adult brain mass), log(BrAdv), and adult relative brain mass (adult brain mass/body mass), log(RelBrM). PC, postconception; g, grams; d, days. Sample is shown as in Tables S1 and S2. Double circle, humans; dotted circles (in BoM and RelBrM), nonhuman primates; solid lines, model II linear regression (reduced major axis) on all species. R2 and P values for linear regressions are given in diagrams.
Fig. 3.
Residuals from regression analyses in Fig. 2. (Upper) Absolute values of residuals as a function of, from left to right, absolute adult brain mass, log(AbsBrM), neonatal brain mass, log(NeoBrM), and gestation time, log(Gest). (Lower) Based on the same data as Upper, but with means and 1.0 SD indicated by horizontal longer dashed lines and shorter solid lines, respectively. Data points from humans are indicated by arrows in all diagrams.
In view of the debate concerning the roles of adult brain mass and body mass as potential determinants of mammalian life history (19), it should be noted that the partial correlation between adult brain mass and timing of walking onset was still highly significant when we controlled for body mass in the present sample (original r(24) = 0.97, P < 0.0001, rpartial = 0.83, P < 0.0001). On the contrary, when controlling for adult brain mass, the correlation between body mass and timing of walking onset was reduced to nonsignificance (original r(24) = 0.91, P < 0.0001, rpartial = −0.33, P = 0.13).
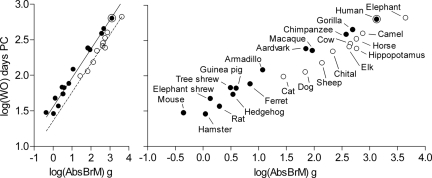
Colinearity analysis carried out as a first step toward a multiple-regression model for the prediction of timing of walking onset eliminated neonatal brain mass and body mass from the set of independent variables (tolerance set at a lenient 0.01 limit was exceeded). The remaining six variables (adult brain mass, gestation time, brain advancement at birth, relative brain mass, maturity at birth, and hindlimb standing position) were entered by using the stepwise method in a multiple regression with time to walking onset as an independent variable. Three variables individually accounted each for >1% of variance in time to walking onset postconception: adult brain mass (94%), hindlimb standing position (3.9%), and gestation time (1.2%). In a model selection procedure (30), in effect weighing the contribution to the variance in time to walking onset accounted for by the individual variables against the principle of parsimony, the three-variable model was discarded in favor of a two-variable model that excluded gestation time. (Note also that it is difficult to interpret the biological significance of gestation time as a model variable given that a key to the translatability of motor developmental time courses between mammals was to actually disregard the timing of birth and hence gestation time; see Fig. S1.) The two-variable model, illustrated in Fig. 4, was highly significant (adj R2 = 0.978, F(2,23) = 504.3, P < 0.0001). The model equation was
where WO is time to walking onset in days postconception, AbsBrM is absolute adult brain mass in grams, and HSP is an indicator with the value 1 or 0 for species that can or cannot, respectively, assume a plantigrade hindlimb standing position (Table S2).
Fig. 4.
Absolute adult brain mass and hindlimb standing position as predictors of time to walking onset. (Left) Time to walking onset, log(WO), as a function of absolute adult brain mass, log(AbsBrM), and hindlimb standing position. Solid symbols and solid regression line represent species that can assume a plantigrade hindlimb standing position; open symbols and dashed regression line represent species that cannot assume a plantigrade hindlimb standing position (Table S2). Analysis of variance with covariance (ANCOVA) confirmed the overall robust main effect of adult brain mass as a covariate (F(1,24) = 820.51, P < 0.0001, η2 = 0.97) and revealed a main effect of hindlimb standing position as a grouping variable (F(1,24) = 40.39, P < 0.0001, η2 = 0.66). For the full model equation derived from multiple-regression analysis, R2 values, and P values, see main text. The 95% confidence interval for R2 was 0.95–0.99. (Right) The same as the Left panel, but with species indicated next to each data point for clarity. The Pearson correlation coefficient was 0.97 (two-tailed P < 0.0001; cf. Fig. S3). Axis units are as in Left but the x axis is expanded.
The predictive accuracy of the model above was evaluated by using an iterative leave-one-out procedure, i.e., for each given species calculating walking onset from adult brain mass and hindlimb standing position on the basis of data from all other species. The prediction error was calculated for each species as
On the basis of the other 23 species, our model predicted walking onset in humans with an error of ≈10%, which was just below the mean error in predictions made for all individual species in the sample. In absolute terms, the age predicted by the model was ≈2 months more than the actual mean age, a modest error compared to the normal range of 9–17 postnatal months for walking onset in humans (Table S2).
Discussion
Our findings demonstrate that in a sample representative of a wide range of mammals, adult brain mass accounts for a remarkably large amount of variance in time to walking onset. Because brains of different mammals develop at similar rates (17), adult brain mass may be assumed to reflect the time during which the brain has developed. Therefore, although we have not measured time courses of brain development, our model in essence tests and supports the hypothesis that brain development determines the timing of walking onset.
The identification of this proximate cause, pertaining to the ontogenetic level of analysis, does not challenge the possibility of an ultimate cause for the pace of motor development, pertaining to the evolutionary level of analysis. These levels of explanation are in fact complementary, capturing the different perspectives of different research fields (31, 32). Whereas a functional biologist would regard the pace of brain development as a causal factor for the pace of motor development, an evolutionary biologist might instead regard it as a product of selection on the age at motor maturity, reflecting an evolutionary adaptation to juvenile mortality rates (33, 34). Because slow development implies an extended period of nonindependence and vulnerability to predators and intraspecific competition, selection will always favor rapid development, particularly during stages associated with high mortality. A longer time to motor maturity must therefore be considered the price paid for some other evolutionarily more advantageous feature, such as a large complex brain (33, 34). Ontogenetically, the long time necessary for the development of a large brain requires parental care and would therefore not have been possible in species such as lizards, turtles, and most invertebrates.
The notion that adult brain mass and, by association, brain development are a major determinant of walking onset in a wide range of mammals is not unexpected, because adult brain mass has been proposed to be a key determinant of mammalian life history (18, 19). It has also been suggested that the slow postnatal human development is due to “secondary altriciality,” a consequence of the evolution of the large human brain (35). What is surprising in view of the literature on human life history (3–5, 13) and motor development (7, 8), however, is the degree to which walking onset in humans is quantitatively predictable from the corresponding milestone in other mammals. In more general terms, our findings indicate that timescales of behavioral development are translatable across mammalian species independently of previous classifications associated with differences in developmental timing. Independently of whether species are born precocial or altricial (23, 24), whether they have slow or fast life histories (19, 36), or how they lie along the r–K continuum (describing the extent to which species are subject to density-independent vs. density-dependent mortality; cf. refs. 19 and 35), they can be fitted into a relatively simple model accounting for a large proportion of the variance in the time to walking onset.
The ontogenetic regularity in behavioral development across species (37) strongly emphasized by our findings indicates that walking onset has a specific neural basis that is similar across mammals (6, 38) and functional at a similar relative time point in their development. This would certainly be in line with the striking regularities across mammalian species with respect to brain morphogenesis (39) and neurogenesis (1, 2, 40) and ultimately with the substantial homologies between mammalian genomes (41). Indeed, the developmental similarities between different mammalian brains (17, 18) suggest that the key change underlying their variation may have a relatively restricted genetic basis (41), such as regulating the number of rounds of symmetrical mitotic divisions during early brain growth (42–44).
Conspicuously, brain development does not appear to be the only factor that determines the ontogenetic timing of walking onset. Species that cannot assume a plantigrade hindlimb standing position appear to systematically start walking at a relatively earlier stage compared to species that can assume this position. There are general biomechanical differences between plantigrade and nonplantigrade hindlimbs (45), regardless of whether or not a given species that can assume a plantigrade hindlimb standing position actually walks or runs in a plantigrade fashion. These differences pertain to the relative length of the hind foot (26), but also to the mobility of the limb and, therefore, to its biomechanical degrees of freedom (45). It is possible that for adequate motor control, the smaller range of possible movements in hindlimbs that cannot assume a plantigrade standing position requires learning or adaptation of fewer muscle synergies in the course of ontogenetic development. If that is the case, a given level of motor performance should hypothetically take relatively less time to achieve in species with such hindlimbs. Note also that the plantigrade standing position of the hindlimb is closer to the primitive mammalian pattern (26), as indicated also by the pentadactyly (five digits) of the hind foot displayed by all species in this group (21). Their relative position on the evolutionary timescale suggests that in cursorial species (46), characterized by hindlimbs that cannot assume a plantigrade standing position, adaptations of motor control for fast running are associated with a systematic shift toward an ontogenetically earlier walking onset.
The multiple-regression model presented here concerns specifically the timing of walking onset, which—although a fundamental milestone in ontogenetic development—has not been commonly used in analyses of life history variation in mammals. Any implications of our findings for life history variation among mammals in general and for human life history in particular should therefore be stated with caution. Our findings do suggest, nevertheless, that a strong link between the timing of brain development and the timing of motor development may constitute the basis for a fundamental ontogenetic pattern of early life history shared by a wide range of placental mammals, some of which diverged in phylogenesis as long as 100 million years ago. This pattern, which therefore may be traced back before the evolution of primates, appears to be recognizable also in humans despite the fact that humans differ from other mammals in so many respects.
Materials and Methods
To ensure a wide taxonomic range and taxonomic independence between the individual species (20), the sample was drawn from 19 families in 11 orders (15 suborders) of mammals and encompassed no more than 1 species from any given genus; the 24 species in the sample represented 24 genera (21). These 11 orders constitute a high proportion of a total of 14 extant orders of placental mammals that habitually walk on the ground and that were therefore relevant to the present study. Several other orders were not of immediate relevance because they are (mainly or entirely) aquatic (Cetacea, Sirenia), arboreal (Dermoptera, many species of the Pilosa), or flying (Chiroptera) or have a half-bound gait (Lagomorpha), whereas only Pholidota, Soricomorha, and Afrosoricida were not represented as we could not find the necessary data. The cape/rock hyrax, Procavia capensis, a representative of the Hyracoidea, has been previously pointed out as an extreme outlier with respect to brain growth in relation to gestation time (18) and was therefore not suitable for our model.
The model was approximately balanced among the four orders representing large numbers (>200) of species: Rodentia, Carnivora, Primates, and Artiodactyla, in addition to a series of single representatives of orders with relatively few (<20) species (Table S1). Primates were approximately matched for brain mass by ungulates; camel and elephant, having the largest and second largest brains among unguligrade mammals, together were the closest possible approximation of size match to the human brain. For Rodentia, Carnivora, and Primates, the selection of species was in essence random, but limited by the availability of reliable published data on walking onset. Species representing these three orders were those commonly used in fundamental biological and applied biomedical research, toxicology, and primatology (Table S2) (2, 47). Notably, adding more species that start walking briefly after birth, from orders already represented in the sample, would disturb the balance of the sample with regard to phylogenetic relatedness between species (20), but have otherwise very minor effects on the model. For instance, increasing sample size from n = 24 to n = 40 by adding Artiodactyla for which data were available (18) resulted in a change from 94% to 89% in variance of walking onset accounted for by adult brain mass, with virtually no change in slope or Y-intercept of the regression line (Fig. S2).
Phylogenetic relatedness between the species in the sample was determined on the basis of divergence time estimates from phylogenomic analysis (48) complemented with mitogenomic analysis (49). The 12 H-strand-encoded protein-coding genes from mitochondrial genomes of 23 mammalian species were aligned manually (the chital was not included, as the mitochondrial genome was not available; for another 4 species genomes of closely related species were used). The opossum Didelphis virginiana was included to root the tree. After removing gaps and third codon positions, 7,120-nt sites remained for phylogenetic analysis. For calculating branch lengths, the tree topology was constrained to the topology of the placental mammal tree that has been calculated in a phylogenomic study from 3 Mnt of protein-coding data (48). Branch lengths were estimated by maximum-likelihood analysis in Treefinder (TF) (50), using the GTR + 4G + I model of sequence evolution. Tree topology and branch lengths were subsequently used in analyses evaluating the influence of phylogenetic relatedness on the statistical significance of the findings illustrated in Fig. 4. For independent contrasts (20), the Mesquite software (51) and the PDAP:PDTREE package were used (52). The analysis revealed modest influence of phylogenetic relatedness on the correlation between time to walking onset and absolute adult brain mass (r = 0.959; P < 0.0001; Fig. S3). The separation between the plantigrade and the nonplantigrade group was assessed by the PDSIMUL/PDANOVA method, a type of parametric bootstrap (53), and was found to be highly significant also when taking phylogenetic relatedness into consideration.
Supplementary Material
Acknowledgments.
We are grateful to Axel Janke (Lund University) for the analysis of branch lengths in the phylogenetic tree and the draft for Fig. 1, to Peter E. Midford (University of Kansas) for analysis of independent contrasts and PDSIMUL/PDANOVA (Fig. 4 and Fig. S3), and to Julie Heiner (David Fitzpatrick's laboratory, Duke University Medical Center) for data on walking onset in tree shrews. We also thank Daniel Garwicz for comments on previous versions of this paper, Jonas Björk for input regarding statistics at an early stage of the project, and Andrea Nord for comments on matters of language and style. This work was funded by the Swedish Research Council, projects 14015 (M.G.) and 60012701 (a Linné Grant to the Neuronano Research Center).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905777106/DCSupplemental.
References
- 1.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 2.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BH, Tompkins RL. Toward a life history of the Hominidae. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 4.Hawkes K. Slow life histories and human evolution. In: Hawkes K, Paine RR, editors. The Evolution of Human Life History. Santa Fe: School of American Research Press; 2006. pp. 95–126. James Currey, Oxford. [Google Scholar]
- 5.Robson SL, Wood B. Hominin life history: Reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- 7.Thelen E. Motor development. A new synthesis. Am Psychol. 1995;50:79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- 8.Zelazo PR. McGraw and the development of unaided walking. Dev Rev. 1998;18:449–471. [Google Scholar]
- 9.Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Dev. 2003;74:475–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- 10.Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002;25:370–376. doi: 10.1016/s0166-2236(02)02173-2. [DOI] [PubMed] [Google Scholar]
- 11.Kaas J. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain Mind. 2000;1:7–23. [Google Scholar]
- 12.Kaas JH. From mice to men: The evolution of the large, complex human brain. J Biosci. 2005;30:155–165. doi: 10.1007/BF02703695. [DOI] [PubMed] [Google Scholar]
- 13.Leigh SR. Brain growth, life history, and cognition in primate and human evolution. Am J Primatol. 2004;62:139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- 14.Touwen B. The brain and development of function. Dev Rev. 1998;18:504–526. [Google Scholar]
- 15.Christensson M, Garwicz M. Time course of postnatal motor development in ferrets: Ontogenetic and comparative perspectives. Behav Brain Res. 2005;158:231–242. doi: 10.1016/j.bbr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Christensson M, Broman J, Garwicz M. Time course of cerebellar morphological development in postnatal ferrets: Ontogenetic and comparative perspectives. J Comp Neurol. 2007;501:916–930. doi: 10.1002/cne.21291. [DOI] [PubMed] [Google Scholar]
- 17.Passingham RE. Rates of brain development in mammals including man. Brain Behav Evol. 1985;26:167–175. doi: 10.1159/000118773. [DOI] [PubMed] [Google Scholar]
- 18.Sacher GA, Staffeldt EF. Relation of gestation time to brain weight for placental mammals: Implications for the theory of vertebrate growth. Am Nat. 1974;108:593–615. [Google Scholar]
- 19.Harvey PH, Purvis A. Understanding the ecological and evolutionary reasons for life history variation: Mammals as a case study. In: McGlade J, editor. Advanced Ecological Theory: Principles and Applications. Oxford: Blackwell; 1999. pp. 232–248. [Google Scholar]
- 20.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 21.Hutchins M, et al., editors. Grzimek's Animal Life Encyclopedia. Mammals I–V. 2nd Ed. Vols 13–16. Detroit: Gale; 2003. [Google Scholar]
- 22.Prechtl HFR. Continuity of Neural Functions from Prenatal to Postnatal Life. Lavenham, UK: Lavenham Press; 1984. [Google Scholar]
- 23.Muir GD. Early ontogeny of locomotor behaviour: A comparison between altricial and precocial animals. Brain Res Bull. 2000;53(5):719–726. doi: 10.1016/s0361-9230(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin RD, MacLarnon AM. Gestation period, neonatal size and maternal investment in placental mammals. Nature. 1985;313:220–223. [Google Scholar]
- 25.Pagel MD, Harvey PH. How mammals produce large-brained offspring. Evolution. 1988;42:948–957. doi: 10.1111/j.1558-5646.1988.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 26.Radinsky LB. The Evolution of Vertebrate Design. Chicago: Univ of Chicago Press; 1987. [Google Scholar]
- 27.Vinicius L. Human encephalization and developmental timing. J Hum Evol. 2005;49:762–776. doi: 10.1016/j.jhevol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Mumby H, Vinicius L. Primate growth in the slow lane: A study of inter-species variation in the growth constant A. Evol Biol. 2008;35:287–295. [Google Scholar]
- 29.Blurton Jones N. Contemporary hunter-gatherers and human life history evolution. In: Hawkes K, Paine RR, editors. The Evolution of Human Life History. Santa Fe, NM: School of American Research Press; 2005. pp. 231–266. James Currey, Oxford. [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 31.Mayr E. Cause and effect in biology. Science. 1961;134:1501–1506. doi: 10.1126/science.134.3489.1501. [DOI] [PubMed] [Google Scholar]
- 32.Sherman PW. The levels of analysis. Anim Behav. 1988;36:616–619. [Google Scholar]
- 33.Williams GC. Adaptation and Natural Selection. Princeton, NJ: Princeton Univ Press; 1966. pp. 87–91. [Google Scholar]
- 34.Carrier DR. Ontogenetic limits on locomotor performance. Physiol Zool. 1996;69:467–488. [Google Scholar]
- 35.Martin RD. Human Brain Evolution in an Ecological Context. New York: American Museum of Natural History; 1983. [Google Scholar]
- 36.Charnov EL, Berrigan D. Why do primates have such long lifespans and so few babies? Evol Anthropol. 1993;1:191–194. [Google Scholar]
- 37.Wood SL, Beyer BK, Cappon GD. Species comparison of postnatal CNS development: Functional measures. Birth Defects Res. 2003;68:391–407. doi: 10.1002/bdrb.10037. [DOI] [PubMed] [Google Scholar]
- 38.Forssberg H. Evolution of plantigrade gait: Is there a neuronal correlate? Dev Med Child Neurol. 1992;34:916–925. doi: 10.1111/j.1469-8749.1992.tb11391.x. [DOI] [PubMed] [Google Scholar]
- 39.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 40.Finlay BL, Hersman MN, Darlington RB. Patterns of vertebrate neurogenesis and the paths of vertebrate evolution. Brain Behav Evol. 1998;52:232–242. doi: 10.1159/000006566. [DOI] [PubMed] [Google Scholar]
- 41.Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- 42.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 43.Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- 44.Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein N. The Coordination and Regulation of Movements. New York: Pergamon; 1967. [Google Scholar]
- 46.Alexander RM. Principles of Animal Locomotion. Princeton, NJ: Princeton Univ Press; 2003. Walking, running and hopping; pp. 103–145. [Google Scholar]
- 47.Anonymous. Statistics of Scientific Procedures on Living Animals: Great Britain 2006. London: Stationery Office; 2007. Available at www.homeoffice.gov.uk/rds/pdfs07/spanimals06.pdf. [Google Scholar]
- 48.Hallström BM, Janke A. Resolution among major placental mammal interordinal relationships with genome data imply that speciation influenced their earliest radiations. BMC Evol Biol. 2008;8:162. doi: 10.1186/1471-2148-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnason U, et al. Mitogenomic relationships of placental mammals and molecular estimates of their divergences. Gene. 2008;421:37–51. doi: 10.1016/j.gene.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Jobb G, von Haeseler A, Strimmer K. TREEFINDER: A powerful graphical analysis environment for molecular phylogenetics. BMC Evol Biol. 2004;4:18. doi: 10.1186/1471-2148-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Maddison WP, Maddison DR. Mesquite: A Modular System for Evolutionary Analysis, Version 2.01. 2007. Available at http://mesquiteproject.org.
- 52.Midford PET, Garland T, Jr, Maddison WP. PDAP:PDTREE Package for Mesquite, Version 1.11. 2008. Available at http://mesquiteproject.org/pdap_mesquite/
- 53.Garland T, Jr, Dickerman AW, Janis CM, Jones JA. Phylogenetic analysis of covariance by computer simulation. Syst Biol. 1993;42:265–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.