Abstract
Nonhuman primates posses a highly developed capacity for face recognition, which resembles the human capacity both cognitively and neurologically. Face recognition is typically tested by having subjects compare facial images, whereas there has been virtually no attention to how they connect these images to reality. Can nonhuman primates recognize familiar individuals in photographs? Such facial identification was examined in brown or tufted capuchin monkeys (Cebus apella), a New World primate, by letting subjects categorize facial images of conspecifics as either belonging to the in-group or out-group. After training on an oddity task with four images on a touch screen, subjects correctly identified one in-group member as odd among three out-group members, and vice versa. They generalized this knowledge to both new images of the same individuals and images of juveniles never presented before, thus suggesting facial identification based on real-life experience with the depicted individuals. This ability was unexplained by potential color cues because the same results were obtained with grayscale images. These tests demonstrate that capuchin monkeys, like humans, recognize whom they see in a picture.
Keywords: face recognition, individual discrimination, oddity, primate, visual discrimination
Whereas human face recognition is well studied, we are only beginning to understand the extent of this capacity in other primates. Nonhuman primate face recognition seems highly accurate (1–6), sensitive to the configuration of facial elements as reflected in the so-called “inversion effect” (7–14) and “Thatcher effect” (15), and dependent on similar neural substrates as in humans (16–21). In one study, chimpanzees matched faces of unfamiliar individuals on the basis of kin resemblance (22), and in another they matched faces with a different bodily view of the same individual provided they were familiar with the depicted individual (23). The latter capacity has also been suggested for macaques (24), but absent rigorous methodology and successful replication, the data remain inconclusive. Together with cross-modal identity matching of conspecifics (24–27), the above results offer a first hint that nonhuman primates not only discriminate faces but connect two-dimensional facial representations with actual individuals that they know, the way we recognize whom we see in a photograph.
There is debate as to how exactly animals perceive pictorial stimuli (28–30). For monkeys, similar to humans, the eyes are the most salient feature of faces (31–33), and some studies report socially appropriate behavior, such as lip smacking, toward facial images (8, 34). Chimpanzees, furthermore, show differential heart rate responses (35) and are able to correctly assign names to depictions of known individuals (36). Evidence for such facial identification is much scarcer for monkeys, however, and to our knowledge has never been tested with faces per se. Monkeys are often assumed to process faces as complex stimuli that they merely match and compare, whereas individual identification is seen as part and parcel of human face recognition. This issue was addressed here by testing whether brown capuchin monkeys can apply real-life social knowledge to facial images of group members vs. outsiders.
In addition, this study addresses a larger issue of nonhuman primate understanding of the representational nature of two-dimensional images. Researchers use pictures for the amount of control it offers over the stimulus. Although widely used, there is debate as to what animals truly understand about pictures in general (29, 30), and specifically with respect to face perception (37). Fagot et al. (38) outline three ways in which animals can perceive a picture: (i) independence: there is no association between the image and the object it represents, so all that matters are features present in the image; (ii) confusion: the picture and the object remain undistinguished so that subjects respond to images the way in which they would respond to the real object, such as displaying an emotional reaction; or (iii) equivalence: subjects associate the picture with the object but do not confuse the two. A few studies have demonstrated picture equivalence in chimpanzees (36, 39) and capuchins (40), but for other nonhuman primates, results may fall under confusion (41, 42). If capuchin monkeys in the present study demonstrate differentiation between facial images of in-group and out-group conspecifics, as they do in real life (43–46), and fail to show confusion (e.g., hostile reactions to out-group members), we may conclude that this species interprets two-dimensional images as representing reality.
In-group/out-group distinctions are critical for the survival of many animals, because the out-group typically poses a threat to a group's food and mating resources. Capuchins live in social groups of ≈14–17 individuals and regularly come into both visual and physical contact with neighboring groups (43, 47). Both in captivity and in the wild, capuchin monkeys typically are hostile to outsiders (43–46). We do not know, however, what information they use to discriminate between in-group and out-group members. There is nothing visually apparent that would indicate an individual's group membership. In humans, faces provide a rapid mechanism for determining the identity of individuals, as well as their age, sex, and emotional state. Because nonhuman primates, too, possess an extensive repertoire of facial expressions to communicate emotional states (48–51), the face is a highly salient stimulus that they probably also rely on for individual identification. Capuchin monkeys have previously been shown to recognize faces (3) and to show the inversion effect (14) and hence seem an excellent candidate for tests of facial identification.
All subjects in this study live socially and therefore have experience interacting with a relatively small number of group members (≈14 individuals over the course of testing). They were trained on an oddity task, which was used to let subjects select from among four two-dimensional photographic portraits: either one in-group face against three out-group faces or one out-group face against three in-group faces. Performance was assessed by transferring subjects to a brand-new set of facial stimuli and comparing transfer performance with the 25% chance level.
If subjects perform above chance on this task, this strongly suggests that they classify facial images on the basis of their familiarity with and/or closeness to the depicted individuals, which task cannot be accomplished without facially identifying them. It is important, however, to rule out alternative explanations for positive performance. First, even though the transfer set contained new images, the individuals depicted were the same as those represented during training. Is it possible that subjects categorized the new images on the basis of previous associations learned during testing, thus making their choices independent of real-life experience with these individuals? To rule this out, subjects were presented with novel individuals whom they had never before seen in a two-dimensional image. Testing had been done using images of all available adult monkeys, but for this control test, images were introduced of in-group vs. out-group juveniles. Successful performance would confirm that subjects connect these images to real conspecifics with whom they are familiar.
A possible alternative explanation for positive transfer is that the two groups differ in hue—on average and perhaps only slightly—owing to the genetic influence of one or two breeding males. This might permit subjects to make an in-group vs. out-group distinction according to color cues rather than individual recognition. Therefore, subjects were presented with stimulus sets converted to grayscale, to remove color information. Successful performance with these stimulus sets would again confirm that subjects operate on the basis of individual recognition and are able to connect the facial images to actual conspecifics regardless of color information.
Results
Transfer Test.
This task presented two conditions: (i) In-group Odd: three facial images represented individuals from the out-group, and one represented an individual from the in-group relative to the subject; and (ii) Out-group Odd: three images from the in-group and one from the out-group relative to the subject (Fig. 1). Conditions were presented in blocks, such that the In-group Odd condition was presented for several days, and then the Out-group Odd condition was presented for an equivalent number of days. Once subjects reached a performance criterion of 60% correct choices for two consecutive sessions on the given condition, they were transferred to a new set of stimuli, and performance was assessed on the first 40 transfer trials with this set.
Fig. 1.
Subjects need to select the odd facial image from among four. On this trial, the odd image is a member of group 1 (Top Left) compared with three members of group 2. For monkeys living in group 1 this trial represents the In-group Odd condition, but for those living in group 2 it is the Out-group Odd condition.
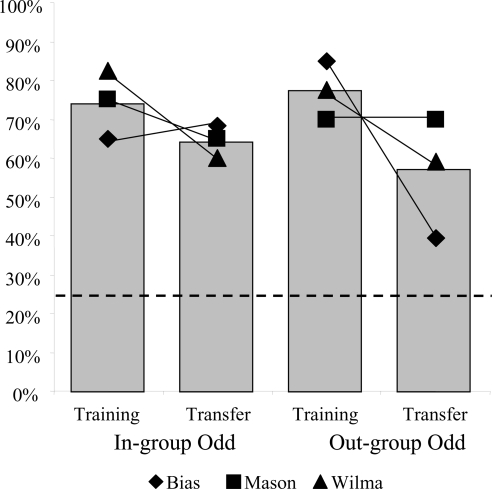
As a group, the three subjects performed above 25% chance upon transfer under both conditions [In-group Odd: Gh = nonsignificant (NS), Gp = 81.24, df = 1, P < 0.001; Out-group Odd: Gh = 7.05, df = 2, P < 0.05, Gp = 59.14, df = 1, P < 0.001). The significant heterogeneity in the Out-group Odd condition is due to one subject's (Bias) poor performance [mean (M) = 39.4%] compared with the better performance of the other two, although even this one subject still performed significantly above chance (z = 1.67, P = 0.048, binomial test). We conducted individual McNemar tests to determine whether there was a difference in performance between the two conditions, In-group Odd and Out-group Odd, suggesting an ease to select one over the other. Results showed that only Bias (McNemar test, n = 33, P = 0.049) showed a difference between conditions, performing better on the In-group Odd condition (In-group Odd: M = 68.4%; Out-group Odd: M = 39.4%; Mason: McNemar test, n = 40, P = 0.815; Wilma: McNemar test, n = 39, P = 1.000). See Fig. 2 for individual performances. Latencies were also analyzed and can be found in the supporting information (SI).
Fig. 2.
Percentage correct choices during the last 40 trials of training and upon transfer to new images (first 40 trials) for both conditions (In-group Odd and Out-group Odd). Gray bars represent the mean performance, whereas each shape indicates the individual performance of three subjects. The horizontal dotted line specifies the chance level (25%).
Juvenile Probe Trials.
To determine whether the above performance could be explained by previous training and exposure to images of the same adult faces, even during transfer, subjects were presented with 40 probe trials containing novel images of juveniles within their normal testing sessions. They had never before been tested on images of these particular individuals. Subjects again performed significantly above chance in both conditions (In-group Odd: Gh = NS, Gp = 46.34, df = 1, P < 0.001; Out-group Odd: Gh = 8.63, df = 2, P = 0.013, Gp = 68.76, df = 1, P < 0.001). The significant heterogeneity in the Out-group Odd condition was due to one subject's (Mason) performance (M = 42.5%) being considerably lower than that of the other two, even though when tested individually this subject did perform significantly above chance (z = 2.26, P = 0.012, binomial test). We tested for performance differences between condition types and found that Bias performed better on Out-group Odd (M = 67.5%) than In-group Odd trial types (M = 40.0%; McNemar test, n = 40, P = 0.027), whereas Mason showed the opposite result (McNemar test, n = 40, P = 0.049). Wilma (McNemar test, n = 40, P = 0.286) showed no difference. See Fig. 3 for individual performances on juvenile probe trials. Latency analyses can be found in SI Text.
Fig. 3.
Performance on novel juvenile probe trials (first 40 trials). Gray bars indicate the mean percentage correct, whereas shapes represent individual performance. The horizontal dotted line designates chance performance (25%).
Grayscale Original Probe Trials.
Subjects were tested with the original stimulus set converted to grayscale to assess whether prior performance could have been due to color differences between the two groups. We report the results of measured hue differences between the two groups for the initial stimulus set and the transfer stimulus set in SI Text. Forty trials were presented as probes within normal testing sessions with the original colored stimulus set. As a group, performance was above chance in both conditions on the grayscale probe trials (In-group Odd: Gh = NS, Gp = 110.93, df = 1, P < 0.001; Out-group Odd: Gh = NS, Gp = 123.23, df = 1, P < 0.001). No subject performed differently according to condition type (Bias: McNemar test, n = 40, P = 0.238; Mason: McNemar test, n = 40, P = 0.754; Wilma: McNemar test, n = 40, P = 0.118). See also Fig. 4. SI Text contains the results of latency analyses.
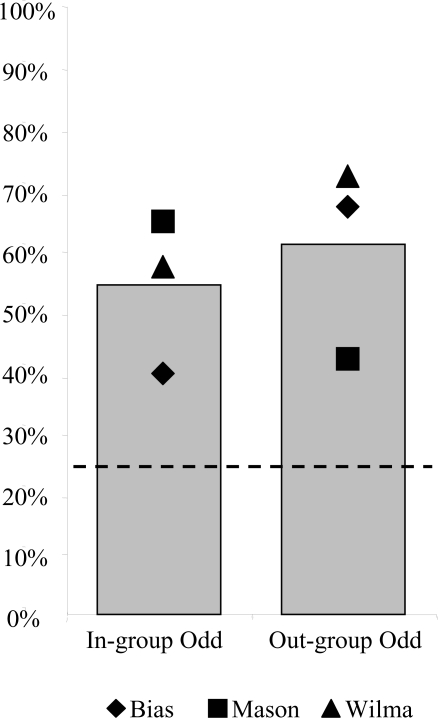
Fig. 4.
Performance by three capuchin monkeys on the two grayscale sets of stimuli. The original (color) training set was turned into grayscale (i.e., Grayscale original, 40 trials, Left) as was the original (color) transfer set (i.e., Grayscale transfer, 40 trials, Right) for both the In-group Odd and Out-group Odd conditions. The shapes designate individual performance on each condition, whereas the gray bars represent the mean. The horizontal dotted line at 25% indicates chance level.
Grayscale Transfer Probe Trials.
To further rule out the possible role of color cues, subjects were presented with the transfer stimulus set converted to grayscale within their normal testing sessions. Subjects performed above chance in both conditions on the 40 grayscale transfer probe trials (In-group Odd: Gh = NS, Gp = 26.27, df = 1, P < 0.001; Out-group Odd: Gh = NS, Gp = 41.41, df = 1, P < 0.001). Again, subjects showed no difference depending on condition type (Bias: McNemar test, n = 40, P = 0.503; Mason: McNemar test, n = 40, P = 1.000; Wilma: McNemar test, n = 40, P = 0.286). See also Fig. 4. Latency analyses can be found in SI Text.
Because this stimulus set was the same as the set used in the transfer session but converted to grayscale, we were able to assess whether removing the color information impaired performance by comparing performance when the trials were initially presented in color (Transfer test) and then when they were presented here in grayscale. Results revealed that only Mason performed significantly poorer when the images were converted to grayscale than when initially presented in color [χ2(1, n = 80) = 4.06, P = 0.007].
Discussion
Socially living capuchin monkeys successfully selected the image of an individual who did not belong to the same social group as three other individuals depicted on the same computer screen. This required them to discriminate among similar-looking faces and apply their personal knowledge of group membership. Given their high performance under a variety of experimental conditions, we conclude that capuchins can individually identify known conspecifics from two-dimensional images of their faces, similar to how humans identify friends and family in photographs.
Presenting the images in grayscale ruled out that slight color differences between the two groups cued the subjects' ability to categorize the facial images as in-group vs. out-group. This strengthens the claim that subjects were selecting the odd individual on the basis of personal knowledge obtained through interactions with in-group members rather than according to visual cues. Our experiment also ruled out that knowledge of group membership was built up during the training and testing itself, because when subjects were presented with a brand new set of images of individuals never presented before (i.e., juveniles born in both groups), they still performed above chance on the task.
Group membership can be known only through personal experience, because it has no obvious facial correlates as opposed to other dimensions, such as sex or age (52–55). Our results strongly suggest a role of personal experience, thus ascribing the outcome to the same learned associations thought to underlie performance in cross-modal studies in which subjects need to match the voice and face of familiar individuals (24–26), which also requires knowledge of familiar individuals.
Many researchers use two-dimensional images for the control they offer over the stimulus. The underlying assumption is that subjects understand what the image represents. Direct tests of picture–item equivalence have typically been performed using tasks such as food vs. nonfood items (41, 42), and in some cases, primates were found to confuse a food image with the real object. In the present study, we did not observe subjects reacting socially to the images, which would suggest confusion. Furthermore, the task required subjects to touch the face image, a behavior not shown toward live conspecifics, further ruling out confusion. We also can rule out independence, whereby animals do not have any association between the image and the object, because there is nothing visibly different about the images that would indicate group membership and allow them to categorize the images appropriately. Given that our subjects successfully categorized the visually diverse facial images as either in-group or out-group, corresponding to their experience in daily life, and that we ruled out alternatives for how they could have perceived the images, we conclude that capuchins understand the representational nature of two-dimensional facial images. They process images of faces not just as complex stimuli but in the same way we do, as faces of individuals they know or do not know.
Materials and Methods
Subjects.
Subjects were three adult brown capuchin monkeys, aged 8 through 19 years, from two separately housed social groups at Yerkes National Primate Research Center. All three subjects (Bias, Mason, and Wilma) participated in earlier studies on face recognition (3). Training and testing began in September 2006, at which time both groups had 15 individuals, and lasted until August 2007. The two groups are housed in the same facility with differently sized indoor and outdoor areas (25 m2 and 31 m2, respectively) that the subjects have access to. Monkey chow and water were continuously available, and supplemental food was supplied in the later afternoon, after testing sessions completed. Tests were conducted between 1000 and 1700 hours, approximately 5 days per week.
Setup and Stimuli.
The apparatus (test chamber and touch-sensitive monitor) that was used to test the monkeys has been described in detail by Pokorny and de Waal (3). Briefly, subjects were moved into a mobile test chamber located in front of their indoor enclosure, and a cart with a touch-sensitive computer monitor (43-cm-diameter Elo Entuitive Touchmonitor) was positioned within arms reach. A computer controlled the display presentation, reward delivery, auditory feedback, and data collection.
Stimuli used as baseline were three-dimensional clip art images sized 300 × 300 dpi. The facial images used in training and testing were digital photographs taken with a Konica Minolta Maxxum 7D digital camera, of all individuals in both group 1 and group 2. These served as both the in-group and out-group stimuli for each subject. In-group refers to individuals who live in the same social group as the subject, whereas out-group individuals are those from the other social group. The two groups do not have visual access to one another, although four of the older adult females (including one subject, Bias) were housed together more than 16 years prior. The terms in-group and out-group are used to denote the current living situation of subjects, whether they had at one point in the past been familiar with one another. The initial, original, stimulus set consisted of six different views of nine individuals from group 1 and six views of eight individuals and three views from two individuals from group 2, for a total of 108 pictures.
Photographs were edited using Adobe Photoshop 6.0 and cropped to only include the head, face, and neck. The monkeys were photographed from a variety viewpoints and gaze orientations. The background was normalized by filling in the remaining area around the face with a solid gray color. Brightness and contrast were adjusted to control for differences in lighting conditions. Images presented in grayscale were converted using Adobe Photoshop. All images were sized to 8.4 cm2 with a resolution of 300 pixels per inch.
Procedure.
This experiment used an oddity paradigm. Subjects were to start the trial by making contact with a colored square located at the center of the screen. After starting the trial, the center square was removed and four images appeared simultaneously on the screen in either a diamond or square layout. Three of the images were related according to group membership (e.g., all three were from group 1), and one was from the other group (e.g., group 2), this being the correct choice. Typical trials consisted of presenting subjects with either (i) three different in-group individuals and one out-group individual (Out-group Odd), or (ii) three different out-group individuals and one in-group individual (In-group Odd; Fig. 2). The two trial types were presented in blocked sessions such that the Out-group Odd condition was presented for several consecutive days before switching to the In-group Odd condition for an equivalent number of days. The layout and location of the odd stimulus was randomly chosen at the beginning of each trial. If subjects contacted the correct image, all images disappeared, a high tone was played, and subjects received a food reward via the pellet dispenser. When the incorrect image was selected, all images disappeared, a low tone was played, and four seconds was added to the intertrial interval. Subjects were given 30 s to make their selection or the trial ended and was recorded as aborted. Aborted trials were not included in the data analysis. A correction procedure was used for regular trials, such that when an incorrect selection was made the trial was repeated four times or until the subject selected the correct response, whichever occurred first. Only the first presentation was included in data analysis.
Transfer Test.
After training on the group membership oddity (subjects attained a performance criterion or 60% correct for two consecutive sessions on the given condition type), subjects were presented with a transfer test using a new stimulus set. This set consisted of three new photographs taken of all adults and subadults in the two groups, prepared as was described for the initial stimulus set. Sessions included 40 transfer trials and 35 clip art trials, used as a baseline measure of attention.
Probe Tests: Juvenile, Grayscale Original, and Grayscale Transfer.
After the transfer test, three more experiments were conducted. The first one (Juvenile) used a new stimulus set consisting of four photographs of eight juveniles each, four from each group for a total of 32 images. The second test (Grayscale original) used the original stimulus set, but converted to grayscale. The last test (Grayscale transfer) used the transfer stimulus converted to grayscale. All experimental trials followed the same procedure, but no correction procedure was used. Instead, subjects were rewarded for any response on probe trials so we could assess performance without contamination of reinforcement history. Sessions consisted of 20 probe trials, 35 original trials (original stimulus set), and 20 clip art trials.
Data Collection and Analysis.
The computer controlling the stimulus presentation also was responsible for data collection. Data recorded for each trial included subject, experimenter, date, type of test, trial condition (In-group Odd/ Out-group Odd), trial number, the images presented at each location, the odd stimulus location, the latency to start the trial and make a response (in milliseconds), the image and location selected by the subject, and whether the trial was correct, incorrect, or aborted. Data were analyzed by using SPSS 16.0. To assess performance above chance level (25%), heterogeneity G tests were conducted. Heterogeneity G tests compare performance with random chance, similar to a χ2, but the G test takes into account individual contributions. Reported results of the G test are Gh, which measures whether the data are homogeneous, and Gp, which indicates whether the pooled data fit the expected ratio. A significant Gh indicates that data are not homogeneous and deviations from our expectation may be in different directions (e.g., two subjects are well above chance whereas one is below chance). If Gp is significant, it indicates that the data are significantly different from the indicated chance level (25%).
Supplementary Material
Acknowledgments.
We thank Christine Webb, Kristin Leimgruber, Amanda Greenberg, Eva Kennedy, Charine Tabbah, Daniel Brubaker, Karianne Chung, and Tara McKenney for technical assistance; William Hopkins, Robert Hampton, Philippe Rochat, and Kim Wallen for helpful discussions; and the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) for the maintenance and care of our research subjects. Research was supported by Grant IOS-0718010 from the National Science Foundation (to F.B.M.d.W.) and by a base grant (RR-00165) from the National Institutes of Health to YNPRC. The YNPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912174106/DCSupplemental.
References
- 1.Dufour V, Pascalis O, Petit O. Face processing limitation to own species in primates: A comparative study in brown capuchins, Tonkean macaques and humans. Behav Process. 2006;73:107–113. doi: 10.1016/j.beproc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Bruce C. Face recognition by monkeys: Absence of an inversion effect. Neuropsychologia. 1982;20:515–521. doi: 10.1016/0028-3932(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 3.Pokorny JJ, de Waal FBM. Face recognition in capuchin monkeys (Cebus apella) J Comp Psychol. 2009;123:151–160. doi: 10.1037/a0014073. [DOI] [PubMed] [Google Scholar]
- 4.Parr LA, Winslow JT, Hopkins WD, de Waal FBM. Recognizing facial cues: Individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl CD, Logothetis NK, Hoffman KL. Individuation and holistic processing of faces in rhesus monkeys. Proc R Soc Lond B Biol Sci. 2007;274:2069–2076. doi: 10.1098/rspb.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascalis O, Bachevalier J. Face recognition in primates: A cross-species study. Behav Process. 1998;43:87–96. doi: 10.1016/s0376-6357(97)00090-9. [DOI] [PubMed] [Google Scholar]
- 7.Parr LA, Dove T, Hopkins WD. Why faces may be special: Evidence of the inversion effect in chimpanzees. J Cogn Neurosci. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- 8.Overman WH, Doty RW. Hemispheric specialization displayed by man but not macaques for analysis of faces. Neuropsychologia. 1982;20:113–128. doi: 10.1016/0028-3932(82)90002-1. [DOI] [PubMed] [Google Scholar]
- 9.Wright AA, Roberts WA. Monkey and human face perception: Inversion effects for human faces but not for monkey faces or scenes. J Cogn Neurosci. 1996;8:278–290. doi: 10.1162/jocn.1996.8.3.278. [DOI] [PubMed] [Google Scholar]
- 10.Perrett DI, et al. Specialized face processing and hemispheric asymmetry in man and monkey: Evidence from single unit and reaction time studies. Behav Brain Res. 1988;29:245–258. doi: 10.1016/0166-4328(88)90029-0. [DOI] [PubMed] [Google Scholar]
- 11.Tomonaga M. How laboratory-raised Japanese monkeys (Macaca fuscata) perceive rotated photographs of monkeys: Evidence for an inversion effect in face perception. Primates. 1994;35:155–165. [Google Scholar]
- 12.Tomonaga M. Inversion effect in perception of human faces in a chimpanzee (Pan troglodytes) Primates. 1999;40:417–438. [Google Scholar]
- 13.Tomonaga M. Visual search for orientation of faces by a chimpanzee (Pan troglodytes): Face-specific upright superiority and the role of facial configural properties. Primates. 2007;48:1–12. doi: 10.1007/s10329-006-0011-4. [DOI] [PubMed] [Google Scholar]
- 14.Pokorny JJ, Webb CE, de Waal FB. Expertise and the inversion effect in capuchin (Cebus apella) face processing. Am J Primatol. 2009;71:99–100. [Google Scholar]
- 15.Adachi I, Chou D, Hampton RR. Thatcher effect in monkeys demonstrates conservation of face perception across primates. Curr Biol. 2009;19:1–4. doi: 10.1016/j.cub.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: A functional MRI study. Proc Natl Acad Sci USA. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parr LA, Hecht E, Barks SK, Preuss TM, Votaw JR. Face processing in the chimpanzee brain. Curr Biol. 2009;19:50–53. doi: 10.1016/j.cub.2008.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the macaque. J Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- 21.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parr LA, de Waal FBM. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- 23.de Waal FBM, Pokorny JJ. Faces and behinds: Chimpanzee sex perception. Adv Sci Lett. 2008;1:9–13. [Google Scholar]
- 24.Adachi I, Hampton RR. Cross-modal representations of familiar conspecifics in rhesus monkeys. International Conference on Comparative Cognition; Melbourne, FL. 2008. [Google Scholar]
- 25.Kojima S, Izumi A, Ceugniet M. Identification of vocalizers by pant hoots, pant grunts and screams in a chimpanzee. Primates. 2003;44:225–230. doi: 10.1007/s10329-002-0014-8. [DOI] [PubMed] [Google Scholar]
- 26.Bauer HR, Philip M. Facial and vocal individual recognition in the common chimpanzee. Psychol Rec. 1983;33:161–170. [Google Scholar]
- 27.Bovet D, Deputte BL. Matching vocalizations to faces of familiar conspecifics in grey-cheeked mangabeys (Lophocebus albigena) Folia Primatol. 2009;80:220–232. doi: 10.1159/000235688. [DOI] [PubMed] [Google Scholar]
- 28.Ittelson WH. Visual perception of markings. Psychonomic Bull Rev. 1996;3:171–187. doi: 10.3758/BF03212416. [DOI] [PubMed] [Google Scholar]
- 29.Fagot J. Picture Perception in Animals. East Sussex, UK: Psychology Press; 2000. [Google Scholar]
- 30.Bovet D, Vauclair J. Picture recognition in animals and humans. Behav Brain Res. 2000;109:143–165. doi: 10.1016/s0166-4328(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 31.Keating CF, Keating EG. Visual scan patterns of rhesus monkeys viewing faces. Perception. 1982;11:211–219. doi: 10.1068/p110211. [DOI] [PubMed] [Google Scholar]
- 32.Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- 33.Guo K, Robertson RG, Mahmoodi S, Tadmor Y, Young MP. How do monkeys view faces?—A study of eye movements. Exp Brain Res. 2003;150:363–374. doi: 10.1007/s00221-003-1429-1. [DOI] [PubMed] [Google Scholar]
- 34.Sackett GP. Responses of rhesus monkeys to social stimulation presented by means of colored slides. Percept Mot Skills. 1965;20:1027–1028. doi: 10.2466/pms.1965.20.3c.1027. [DOI] [PubMed] [Google Scholar]
- 35.Boysen ST, Berntson GG. Conspecific recognition in the chimpanzee (Pan troglodytes): Cardiac responses to significant others. J Comp Psychol. 1989;103:215–220. doi: 10.1037/0735-7036.103.3.215. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzawa T. Form perception and visual acuity in a chimpanzee. Folia Primatol. 1990;55:24–32. doi: 10.1159/000156494. [DOI] [PubMed] [Google Scholar]
- 37.Pascalis O, Petit O, Kim JH, Campbell R. In: Picture Perception in Animals. Fagot J, editor. East Sussex, UK: Psychology Press; 2000. pp. 263–294. [Google Scholar]
- 38.Fagot J, Martin-Malivel J, Depy D. In: Picture Perception in Animals. Fagot J, editor. Philadelphia, PA: Psychology Press; 2001. pp. 295–320. [Google Scholar]
- 39.Itakura S. Recognition of line-drawing representation by a chimpanzee (Pan troglodytes) J Gen Psychol. 1994;121:189–197. doi: 10.1080/00221309.1994.9921195. [DOI] [PubMed] [Google Scholar]
- 40.Truppa V, Spinozzi G, Stegagno T, Fagot J. Picture processing in tufted capuchin monkeys (Cebus apella) Behav Process. 2009;82:140–152. doi: 10.1016/j.beproc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Parron C, Call J, Fagot J. Behavioural responses to photographs by pictorially naïve baboons (Papio anubis), gorillas (Gorilla gorilla) and chimpanzees (Pan troglodytes) Behav Process. 2008;78:351–357. doi: 10.1016/j.beproc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Bovet D, Vauclair J. Functional categorization of objects and of their pictures in baboons (Papio anubis) Learn Motiv. 1998;29:309–322. [Google Scholar]
- 43.Spironello WR. In: Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest. Bierregaard R, Gascon C, Lovejoy TE, Mesquita R, editors. New Haven, CT: Yale Univ Press; 2001. pp. 271–284. [Google Scholar]
- 44.Fragaszy DM, Baer J, Adams-Curtis LE. Introduction and integration of strangers into captive groups of tufted capuchins (Cebus apella) Int J Primatol. 1994;15:399–420. [Google Scholar]
- 45.Crofoot MC. Mating and feeding competition in white-faced capuchins (Cebus capucinus): The importance of short- and long-term strategies. Behaviour. 2007;144:1473–1495. [Google Scholar]
- 46.Cooper MA, Bernstein IS, Fragaszy DM, de Waal FBM. Integration of new males into four social groups of tufted capuchins (Cebus apella) Int J Primatol. 2001;22:663–683. [Google Scholar]
- 47.Defler TR. A comparison of intergroup behavior in Cebus albifrons and C. apella. Primates. 1982;23:385–392. [Google Scholar]
- 48.Darwin C. The Expression of the Emotions in Man and Animals. London: Murray; 1872. reprinted (2009) (Oxford Univ Press, New York) [Google Scholar]
- 49.Andrew RJ. Evolution of facial expression. Science. 1963;142:1034–1041. doi: 10.1126/science.142.3595.1034. [DOI] [PubMed] [Google Scholar]
- 50.van Hooff JARAM. Facial expressions in higher primates. Symp Zool Soc London. 1962;8:97–125. [Google Scholar]
- 51.van Hooff JARAM. In: Primate Ethology. Morris D, editor. London: Weidenfeld & Nicolson; 1967. pp. 7–68. [Google Scholar]
- 52.Burt DM, Perrett DI. Perception of age in adult Caucasian male faces: Computer graphic manipulation of shape and colour information. Proc R Soc London Ser B. 1995;259:137–143. doi: 10.1098/rspb.1995.0021. [DOI] [PubMed] [Google Scholar]
- 53.Brown E, Perrett DI. What gives a face its gender? Perception. 1993;22:829–840. doi: 10.1068/p220829. [DOI] [PubMed] [Google Scholar]
- 54.Burton AM, Bruce V, Dench N. What's the difference between men and women? Evidence from facial measurement. Perception. 1993;22:153–176. doi: 10.1068/p220153. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi MK, Hirukawa T, Kanazawa S. Judgment of gender through facial parts. Perception. 1995;24:563–575. doi: 10.1068/p240563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.