Abstract
IL-22 is a cytokine that acts mainly on epithelial cells. In the skin, it mediates keratinocyte proliferation and epidermal hyperplasia and is thought to play a central role in inflammatory diseases with marked epidermal acanthosis, such as psoriasis. Although IL-22 was initially considered a Th17 cytokine, increasing evidence suggests that T helper cells can produce IL-22 even without IL-17 expression. In addition, we have shown the existence of this unique IL-22-producing T cell in normal skin and in the skin of psoriasis and atopic dermatitis patients. In the present study, we investigated the ability of cutaneous resident dendritic cells (DCs) to differentiate IL-22-producing cells. Using FACS, we isolated Langerhans cells (LCs; HLA-DR+CD207+ cells) and dermal DCs (HLA-DRhiCD11c+BDCA-1+ cells) from normal human epidermis and dermis, respectively. Both LCs and dermal DCs significantly induced IL-22-producing CD4+ and CD8+ T cells from peripheral blood T cells and naive CD4+ T cells in mixed leukocyte reactions. LCs were more powerful in the induction of IL-22-producing cells than dermal DCs. Moreover, in vitro-generated LC-type DCs induced IL-22-producing cells more efficiently than monocyte-derived DCs. The induced IL-22 production was more correlated with IFN-γ than IL-17. Surprisingly, the majority of IL-22-producing cells induced by LCs and dermal DCs lacked the expression of IL-17, IFN-γ, and IL-4. Thus, LCs and dermal DCs preferentially induced helper T cells to produce only IL-22, possibly “Th22” cells. Our data indicate that cutaneous DCs, especially LCs, may control the generation of distinct IL-22 producing Th22 cells infiltrating into the skin.
Keywords: dermal dendritic cells, skin, Th22
IL-22, a member of the IL-10 family, is preferentially produced by terminally differentiated Th17 cells, and thus it has been considered an effector cytokine of Th17 cells (1–3). IL-22 is also expressed by other immune cell subsets, including for example, Th1 cells (4, 5), CD8+ T cells (6, 7), NK-22 subset of natural killer cells (8, 9), and CD11c+ dendritic cells (DCs) (7, 10). IL-22 receptor is highly expressed on nonhematopoietic tissue cells at outer body barriers, such as epithelial cells of gastrointestinal tract and keratinocytes of the skin; in contrast, it is not detectable on immune cells (11). In those epithelial cells, IL-22 induces the production of antimicrobial proteins such as human β-defensin, indicating the involvement of this cytokine in early host defense against microbial pathogens (12–14). In the skin, IL-22 also mediates keratinocyte proliferation and epidermal hyperplasia by downmodulating terminal keratinocyte differentiation genes (12, 15, 16). Hence, IL-22 is thought to play a central role in inflammatory diseases with marked epidermal acanthosis, such as psoriasis (7, 15–18).
Although IL-22 production was initially linked with IL-17 expression in Th17 cells, IL-22 production can also occur in an apparently unique subset of cells that does not cosynthesize IL-17 (6, 7, 15, 19, 20). In particular, we have found distinct IL-22-producing T cells among the resident T cells in normal skin (15). In addition, we have recently demonstrated that unique IL-22-producing CD4+ and CD8+ T cells are increased in the lesional skin of atopic dermatitis (AD) patients (6). Interestingly, the frequency of IL-22-producing CD8+ cells in the lesional skin correlates with AD disease severity. Furthermore, two independent groups have recently characterized a distinct IL-22-producing human helper T (“Th22”) cell population that coexpresses chemokine receptor CCR6 and skin homing receptors CCR4 and CCR10 and lacks the production of both IL-17 and IFN-γ (21, 22). Thus, this unique IL-22-producing T cell population is presumed to play an important role in skin homeostasis and in the pathogenesis of skin diseases.
The skin is equipped with a highly sophisticated system of immune surveillance. The skin immune system relies on a rich network of professional antigen-presenting DCs that populate the epidermis and the dermis (23). Epidermal DCs are known as Langerhans cells (LCs), whereas dermal DCs are heterogeneous and classified into several subsets. The major resident DC population in normal dermis is identified phenotypically with a single monoclonal antibody, CD1c, which is also known as blood dendritic cell antigen (BDCA)-1 (24, 25). These BDCA-1+ cells constitute ≈90% of all CD11c+ dermal DCs (24). LCs and dermal DCs are able to activate naive T cells to initiate Th1 and Th2 immune responses. Recent studies indicate LCs and dermal DCs exhibit differential capacity of polarizing naive T cells toward Th1 or Th2 cell phenotypes (26, 27). For instance, Klechevsky et al. showed that LCs preferentially induced the differentiation of CD4+ T cells secreting Th2 cytokines to a greater extent than dermal DCs (26). Additionally, Mathers et al. recently reported that LCs were the main cutaneous DC subset capable of inducing a Th17 response (28), although they did not study IL-22 expression. At this time, it is unknown whether tissue resident DCs, including cutaneous DCs, can induce the unique IL-22-producing CD4+ T cells, Th22, distinct from Th17 cells.
In this study, we evaluated whether resident cutaneous DCs can induce T cells producing IL-22, an important cytokine in the skin homeostasis and immune response. We used epidermal LCs and BDCA-1+ dermal DCs directly isolated from normal human epidermis and dermis, respectively. We found that LCs strongly induced distinct IL-22-producing CD4+ T cells without IL-17 production. BDCA-1+ dermal DCs were also capable of inducing this unique type of cell, although to a lesser extent than LCs. We demonstrated for the first time that resident LCs and dermal DCs can induce the differentiation of Th22 cells.
Results
Isolation of Human LCs and Dermal DCs.
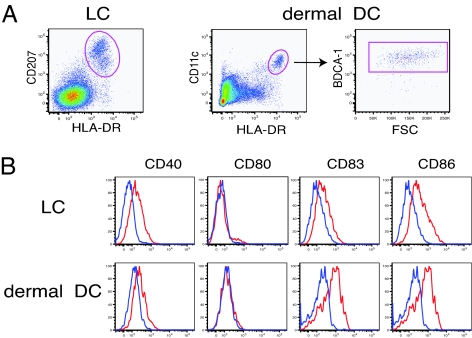
FACS sorting was used to purify LCs and dermal DCs in single cell suspensions from human epidermis and dermis, respectively. Single cell epidermal suspensions of normal skin were obtained by enzymatic splitting of the epidermis and dermis and subsequent incubation with trypsin followed by overnight culture. The dermis was cultured overnight to allow the leukocytes to migrate out of the dermal scaffold. Then we sorted the cells from these bulk epidermal and dermal single cell suspensions. As shown in Fig. 1A, we sorted LCs as an HLA-DR+CD207+ cell population from epidermal single cell suspensions. For purification of dermal DCs, cells were first gated on HLA-DRhiCD11c+, a classic definition of a myeloid DC, and further gated according to BDCA-1 expression (Fig. 1A). More than 90% of HLA-DRhiCD11c+ cells were positive for BDCA-1, confirming our previous observations (24, 29). We isolated HLA-DRhiCD11c+BDCA-1+ cells as a major dermal DC subset and used these cells in the following experiments. Examination of cell surface molecule expression illustrated that LCs and dermal DCs expressed significant levels of CD40, CD83, and CD86. CD80 expression was almost undetectable in both LCs and dermal DCs. Data from dermal DCs were consistent with our previous findings (29).
Fig. 1.
Sorting strategy and surface molecule expression of LCs and dermal DCs. (A) LCs were sorted as HLA-DR+CD207+ cells from epidermal single cell suspensions, whereas dermal DCs were sorted as HLA-DRhiCD11c+BDCA-1+ cells from single cell suspension of dermal emigres. (B) Flow cytometric analysis of surface molecules on LCs and dermal DCs. HLA-DR+CD207+ cells from epidermis and HLA-DRhiCD11c+BDCA-1+ cells from dermis were subjected to the analysis of cell surface expression of CD40, CD80, CD83, and CD86. Blue lines indicate isotype controls. Data are representative of four independent experiments.
LCs and Dermal DCs Activate CD4+ and CD8+ T Cells to Produce IL-22.
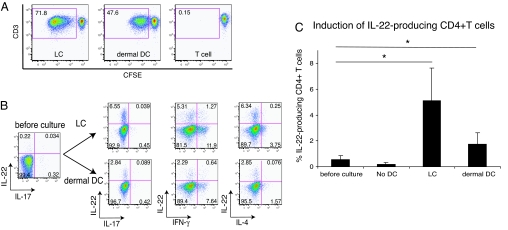
DCs exhibit great capacity to induce allogeneic T cell proliferation. To test the immunostimulatory ability of isolated LCs and dermal DCs, we cocultured carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled allogeneic whole peripheral T cells and sorted LCs or dermal DCs for 7 days and assessed T cell proliferation by flow cytometry. We first set a gate on live, CD4+ cells during analysis. Both LCs and dermal DCs induced the proliferation of allogeneic peripheral CD4+ T cells, as determined by CFSE dilution (Fig. 2A). In the mixed leukocyte reaction (MLR) induced by LCs, 71.8% of the surviving T cells had undergone extensive proliferation at a stimulator/responder ratio of 1:50 on day 7 of culture. In parallel cultures stimulated by dermal DCs, 47.6% of the T cells had proliferated, compared with 0.15% background T cell proliferation. Thus, LCs were stronger stimulators of allogeneic CD4+ T cells than dermal DCs.
Fig. 2.
LCs and dermal DCs activate CD4+ T cells, leading to cell proliferation and IL-22 production. (A) Allogeneic whole peripheral T cells were labeled with CFSE and cultured for 7 days with sorted LCs or dermal DCs. Proliferation was determined by the dilution of CFSE analyzed by flow cytometry. Live, CD4+ cells were first gated and then plotted as CFSE vs. CD3. Percentage of live, proliferating CD4+ T cells is indicated in gate. Data are representative of four independent experiments. (B) Intracellular IL-22 vs. IL-17, IFN-γ, and IL-4 detected in proliferating CD4+ T cell population following 7-day coculture of whole peripheral T cells and sorted allogeneic LCs or dermal DCs. T cells stimulated by LCs or dermal DCs were restimulated with PMA and ionomycin in the presence of BFA for 4 h and subsequently analyzed for the production of indicated cytokines by flow cytometry. Live, CD4+ proliferating T cells were first gated and then cytokine production profile was analyzed. Numbers in quadrants indicate percentage of gated cells in each. Data are representative of six independent experiments. (C) Frequency of the cells producing IL-22 among CD4+ T cells before culture and that among proliferating CD4+ T cells after 7-day MLR assay. Horizontal axis indicates stimulators of T cells, and “No DC” group represents T cells cultured for 7 days without DCs. Asterisks (*) indicate statistical significance (P < 0.05). Data represent the mean (±SD) of six independent experiments.
We next examined whether proliferating T cells produced IL-22, using intracellular cytokine staining. T cells cocultured with sorted LCs or dermal DCs for 7 days were subsequently activated by phorbol 12-myristate 13-acetate (PMA) and ionomycin for 4 h in the presence of brefeldin A (BFA), and then intracellular cytokine staining was conducted to evaluate the production of IL-22 together with IFN-γ, IL-4, and IL-17. As shown in Fig. 2B, both LCs and dermal DCs clearly induced CD4+ T cells producing IL-22. At the beginning of MLR, the frequency of IL-22-producing cells among CD4+ T cells was 0.58 ± 0.29%. When T cells were cultured without stimulating DCs, the frequency of IL-22-producing cells was unchanged (0.22 ± 0.10%). In contrast, after T cells were stimulated by LCs or dermal DCs, significantly higher frequencies of IL-22-producing cells were observed: 5.14 ± 2.5% (P = 0.008) and 1.77 ± 0.85% (P = 0.013) of proliferating CD4+ T cells were positive for intracellular IL-22, respectively (n = 6; Fig. 2C). The proportions of IL-22-producing cells among nonproliferating CD4+ T cells cultured with LCs or dermal DCs were not increased: 0.37 ± 0.27% and 0.30 ± 0.18%, respectively. Interestingly, these IL-22-producing T cells almost completely lacked IL-17 production. Further, LCs and dermal DCs did not induce IL-17 production at all. The percentage of IL-17-producing cells among peripheral CD4+ T cells was 0.75 ± 0.54%. However, even after coculture with LCs or dermal DCs, only 0.77 ± 0.43% and 0.21 ± 0.15% of responding CD4+ T cells produced IL-17, respectively. In other words, while LCs and dermal DCs induced IL-22 production in CD4+ T cells, production of IL-17 was not induced by these cutaneous DCs.
We also analyzed the frequency of IL-22-producing CD8+ cells. Similar to the case of CD4+ cells, LCs were stronger stimulators of allogeneic CD8+ T cells than dermal DCs [supporting information (SI) Fig. S1a]. Once T cells were cultured with LCs or dermal DCs for 7 days, significant induction of IL-22 production was observed in comparison to baseline IL-22 production (0.25 ± 0.23%): 3.00 ± 2.13% (P = 0.041) and 1.47 ± 0.64% (P = 0.018) of proliferating CD8+ T cells were positive for intracellular IL-22, respectively (n = 5; Fig. S1 b and c). IL-22 production was not induced in CD8+ T cells cultured without DCs (0.16 ± 0.17%) or nonproliferating CD8+ T cells cultured with LCs or dermal DCs (0.36 ± 0.18% and 0.28 ± 0.15%, respectively).
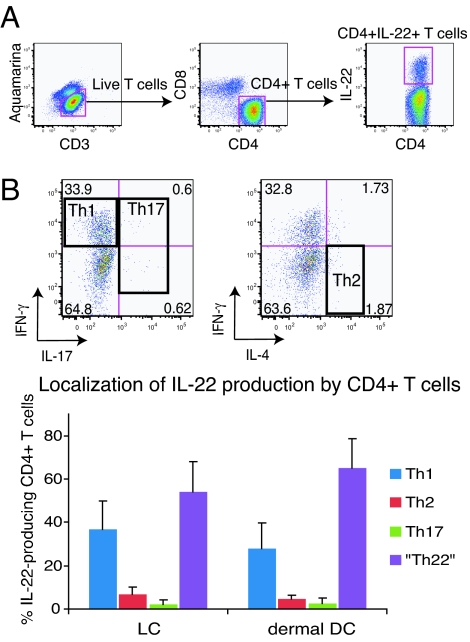
In reference to coproduction of Th1 and Th2 cytokines with IL-22, a portion of IL-22-positive cells also produced IFN-γ, but cells producing both IL-22 and IL-4 were scarce (Fig. 2B). To further characterize the IL-22-producing CD4+ T cells, we localized IL-22 production within the different CD4+ T cell subsets. FACS gates were first set on all CD3+CD4+ cells synthesizing IL-22 (Fig. 3A), and then cosynthesis of IFN-γ, IL-4, and IL-17 was determined within CD4+IL-22+ T cells (Fig. 3B). We defined IFN-γ-producing, IL-4-producing, and IL-17-producing helper T cells as Th1, Th2, and Th17 cells, respectively (Fig. 3B). As shown in the lower panel of Fig. 3B, there were very limited Th2 (<10%) and Th17 (<5%) cells among CD4+IL-22+ T cells, whereas an appreciable portion of CD4+IL-22+ T cells belong to Th1 (LC-stimulated, 36.9 ± 12.8%; dermal DC-stimulated, 28.1 ± 11.7%). However, the largest subset of CD4+IL-22+ T cells (LC stimulated, 54.1 ± 13.7%; dermal DC stimulated, 64.9 ± 13.5%) was not Th1, Th2, or Th17 cells and appeared to represent a unique subset of IL-22-producing helper T cells, Th22.
Fig. 3.
Unique IL-22-producing cells represent a major subset among IL-22-producing CD4+ T cells. (A) FACS gating used in the analysis of IL-22-producing T cells stimulated by LCs or dermal DCs. (B) Frequency of Th1, Th2, and Th17 cells among IL-22-producing CD4+ T cells. Horizontal axis indicates stimulators of T cells. Data represent the mean (±SD) of six independent experiments.
LCs and Dermal DCs Induce the Differentiation of Th22 Cells from Naive CD4+ T Cells.
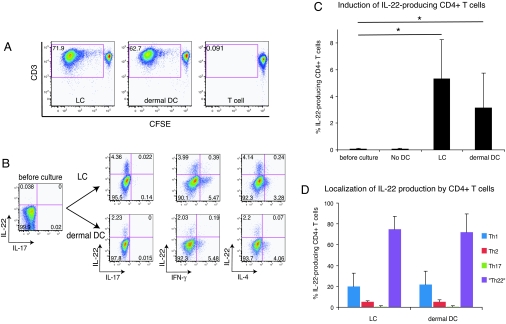
Peripheral T cells are mostly composed of memory type cells. A function unique to DCs is their ability to induce naive T cell proliferation. Therefore, we secondly investigated whether cutaneous DCs can induce IL-22 production from allogeneic naive CD4+ T cells. Both LCs and dermal DCs were powerful stimulators of allogeneic naive CD4+ T cells as determined by CFSE dilution assay (Fig. 4A). Totals of 71.9% and 62.7% of living, CD4+ T cells stimulated with LCs and dermal DCs at a stimulator/responder ratio of 1:50 had undergone extensive proliferation, respectively, on day 7 of culture. MLR without a stimulator population had 0.091% background proliferation. Thus, again, LCs were stronger stimulators of naive CD4+ T cells than dermal DCs, which was consistent with previous observations by Klechevsky et al. (26).
Fig. 4.
LCs and dermal DCs induce Th22 differentiation from naive CD4+ T cells. (A) Allogeneic naive CD4+ T cells were labeled with CFSE and cultured for 7 days with sorted LCs or dermal DCs. Proliferation was determined by the dilution of CFSE analyzed by flow cytometry. Live, CD4+ cells were first gated and then plotted as CFSE vs. CD3. Percentage of live, proliferating CD4+ T cells is indicated in gate. Data are representative of four independent experiments. (B) Intracellular IL-22 vs. IL-17, IFN-γ, and IL-4 detected in proliferating, CD4+ T cell population following 7-day coculture of naive CD4+ T cells and sorted allogeneic LCs or dermal DCs. Naive CD4+ T cells stimulated by LCs or dermal DCs were restimulated with PMA and ionomycin in the presence of BFA for 4 h and subsequently analyzed for the production of indicated cytokines by flow cytometry. Live, CD4+ proliferating T cells were first gated and then cytokine production profile was analyzed. Numbers in quadrants indicate percentage of gated cells in each. Data are representative of six independent experiments. (C) Frequency of the cells producing IL-22 among CD4+ T cells before culture and that among proliferating CD4+ T cells after 7-day MLR assay. Horizontal axis indicates stimulators of T cells, and “No DC” group represents T cells cultured for 7 days without DC. Asterisks (*) indicate statistical significance (P < 0.05). Data represent the mean (±SD) of six independent experiments. (D) Frequency of Th1, Th2, and Th17 cells among IL-22-producing CD4+ T cells. Analysis was performed as described in Fig. 3 A and B. Horizontal axis indicates stimulators of T cells. Data represent the mean (±SD) of six independent experiments.
Next, we investigated whether cutaneous DCs were able to induce IL-22 production from naive CD4+ T cells. We assessed intracellular cytokine production profile of proliferating naive T cells stimulated by cutaneous DCs in the same way as described above. As shown in Fig. 4B, both LCs and dermal DCs induced CD4+ T cells to produce IL-22. At the beginning of the MLRs, almost no IL-22-producing CD4+ T cells were observed (0.09 ± 0.04%). Naive T cells did not produce IL-22 when cultured without DCs (0.10 ± 0.06%). On the other hand, when naive T cells were stimulated by LCs and dermal DCs, significantly higher frequencies of IL-22-producing cells were observed: 5.3 ± 2.9% (P = 0.007) and 3.2 ± 2.6% (P = 0.034) of proliferating CD4+ T cells were positive for intracellular IL-22, respectively (n = 6; Fig. 4C). The proportions of IL-22-producing cells among nonproliferating CD4+ T cells cultured with LCs and dermal DCs were not increased: 0.33 ± 0.19% and 0.39 ± 0.29%, respectively. Interestingly, once again, these IL-22-producing T cells almost completely lacked IL-17 production. In addition, the induction of IL-17-producing cells was hardly detectable. T cells producing IL-17 consistently accounted for <0.5% of responding cells.
The analysis of cosynthesis of IFN-γ and IL-4 by responding CD4+ T cells demonstrated that most of the cells producing IL-22 did not cosynthesize either IFN-γ or IL-4. To further determine the nature of IL-22-producing cells, we localized IL-22 production within the different CD4+ T cell subsets as described above. As shown in Fig. 4D, there were very limited (<10%) Th2 cells and almost no (<0.5%) Th17 cells among CD4+IL-22+ T cells, whereas a higher frequency of CD4+IL-22+ T cells belongs to Th1 (LC stimulated, 19.9 ± 12.8%; dermal DC stimulated, 21.7 ± 16.6%). However, again, the overwhelming majority of CD4+IL-22+ T cells (LC stimulated, 74.8 ± 12.4%; dermal DC stimulated, 72.1 ± 17.4%) was neither Th1, Th2, nor Th17 cell, and may represent a unique subset of IL-22-producing helper T cells, Th22. These data indicate LCs and dermal DCs induce the differentiation of Th22 cells from naive CD4+ T cells.
LCs Induce IL-22-Producing T Cells More Efficiently Than Dermal DCs.
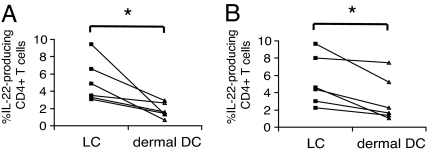
As shown in Figs. 2 and 4, both LCs and dermal DCs significantly induced IL-22-producing CD4+ T cells. We compared the ability of LCs and dermal DCs to induce IL-22-producing cells. We found that LC-activated CD4+ T cells always bore higher percentages of IL-22-producing cells than dermal DC-activated CD4+ T cells, irrespective of the usage of whole peripheral T cells or naive CD4+ T cells as responder cells (Fig. 5). These data indicate that LCs are more efficient in activating CD4+ T cells to produce IL-22.
Fig. 5.
LCs are more efficient than dermal DCs at inducing IL-22-producing CD4+ T cells. (A) Whole peripheral T cells were cultured with LCs or dermal DCs for 7 days and subsequently restimulated with PMA and ionomycin in the presence of BFA for 4 h. The frequency of IL-22-producing cells among proliferating CD4+ T cells stimulated by LCs or dermal DCs was examined by intracellular cytokine staining. (B) Naive CD4+ T cells were cultured with LCs or dermal DCs for 7 days and subsequently restimulated with PMA and ionomycin in the presence of BFA for 4 h. The frequency of IL-22-producing cells among proliferating CD4+ T cells was examined by intracellular cytokine staining. Asterisks (*) indicate statistical significance (P < 0.05).
In Vitro-Generated LC-Type DCs Induce IL-22-Producing CD4+ T Cells More Powerfully Than Monocyte-Derived Conventional DCs.
It has been established that culturing CD34+ hematopoietic progenitor cells (HPCs) with GM-CSF and TNF-α gives rise to both CD1a+CD14− LC-type DCs and CD1a−CD14+ DCs related to dermal DCs (26, 30, 31). We generated these DC subsets in vitro to verify that LCs were superior in the induction of IL-22-producing CD4+ T cells (Fig. S2a). Unfortunately, because the ability of CD1a−CD14+ DCs to induce proliferation of allogeneic T cells was much poorer than LC-type DCs, as previously reported (26), we could not compare intracellular cytokine profiles of activated T cells. Thus, instead of the CD1a−CD14+ DCs, we used monocyte-derived conventional DCs from the same donor for comparison. Comparing the capacity of LC-type DCs and monoctye-derived DCs to induce IL-22-producing CD4+ T cells from whole peripheral T cells, we found that LC-type DCs were more powerful inducers of IL-22-producing CD4+ T cells than monocyte-derived DCs, which was consistent with the data from in vivo-derived LCs and dermal DCs (Fig. S2b). In addition, Th22 cells appeared to be induced more efficiently by LC-type DCs than monocyte-derived DCs (Fig. S2c).
Discussion
Our study has demonstrated that cutaneous resident DCs, LCs, and dermal DCs are capable of inducing distinct IL-22-producing CD4+ T cells, possibly Th22, from both whole peripheral T cells and naive T cells. Until very recently, IL-22 had been considered a Th17-associated cytokine (1–3). Indeed, in previous studies, although Th1 cells produced more IL-22 in comparison to Th2 cells or unpolarized T cells, Th17 cells were clearly the dominant IL-22 producers at both mRNA and protein levels (7, 13, 32). These data established that IL-22 was an effector cytokine of Th17 cells (1). Indeed, psoriasis skin lesions contain a population of T cells that cosynthesize IL-17 and IL-22, but the majority of IL-22-producing T cells are neither Th17, Th1, nor Th2 (6). In AD, there is an even larger population of T cells that uniquely synthesize IL-22 (6). Moreover, two independent groups have recently identified a human helper T cell population producing abundant IL-22 that is distinct from Th17 and Th1 (21, 22). In their paper, Trifari et al. named this unique population Th22 (22). The unique characteristic of DCs is their ability to polarize naive helper T cells into Th1, Th2, and Th17 cells. DCs reside in antigen-capture areas related to epithelial surface and secondary lymphoid organs (33). Indeed, using activated conventional DCs and plasmacytoid DCs, Trifari et al. have also demonstrated that human DCs actually induce the development of Th22 cells from naive T cells (22). According to their results, plasmacytoid DCs are more powerful than conventional DCs in the expansion of Th22 cells. However, it was unknown whether tissue resident DCs, including cutaneous DCs, were actually able to induce this unique IL-22-producing helper T cell population. Thus, our present study is unique evidence illustrating that tissue resident DCs can induce the differentiation of Th22 cells.
Our data show that the majority of IL-22-producing CD4+ T cells induced by cutaneous DCs did not cosynthesize IFN-γ, IL-4, or IL-17. Although a significant proportion of IL-22-producing cells were Th1 cells, surprisingly, very few CD4+IL-22+ T cells cosynthsized IL-17 (Figs. 2 and 4). The observation that IL-22 production was much more linked to Th1 cells than Th17 cells would be an important finding as well (Figs. 3 and 4). In fact, a very recent study has shown that IL-22 production among CD4+ T cells is more closely related to IFN-γ than IL-17, indicating that IL-17 and IL-22 are differentially regulated during T helper cell differentiation (34). In accordance with this, we have previously elucidated that the majority of IL-22-producing CD4+ T cells in normal human dermis do not produce IL-17, irrespective of IFN-γ expression (15). Moreover, in sharp contrast to significant induction of IL-22-producing CD4+ T cells, LCs and dermal DCs isolated from normal skin induced almost no IL-17-producing CD4+ T cells (Figs. 2 and 4). A previous study, however, reported that human skin-migratory LCs from normal skin were capable of inducing Th17 response, although they did not explore IL-22 expression (28). Although the precise reasons for this discrepancy remain unclear, it may be due to the methodological differences. The previous study used skin explants composed of epidermis and a thin layer of dermis to obtain cutaneous DCs (28), whereas we separated epidermis and dermis using dispase. The culture conditions were different as well. The previous study performed MLRs under serum-free conditions (28). In any case, we can safely state that LCs and dermal DCs are able to induce IL-22-producing T cells lacking IL-17 production at least conditionally.
In the skin, the primary target of IL-22 is epidermal keratinocytes. Stimulation of keratinocytes with IL-22 leads to the induction of antimicrobial peptides, such as S100A7 and β-defensin (12, 14). In addition, this cytokine has much stronger effects in regulating hyperplasia and differentiation in keratinocytes (7, 12, 13, 15). In vitro, IL-22 induces marked acanthosis and hypogranulosis and suppression of terminal differentiation of cultured reconstructed epidermis (12, 16). In vivo overexpression of IL-22 induces marked hyperplasia and inflammation in murine skin, bearing similarities to both psoriatic and AD skin (18). Indeed, IL-22 expression is upregulated in both psoriatic and AD skin (6, 14, 35). Importantly, ablation or deficiency of IL-22 alone reversed the skin pathology in a murine model of psoriasis (7, 18). In addition, we have recently demonstrated that unique IL-22-producing CD4+ and CD8+ T cells are increased in the lesional skin of AD patients (6). Interestingly, the frequency of IL-22-producing CD8+ cells in the lesional skin correlates with AD disease severity (6). In line with this, we have also demonstrated that LCs and dermal DCs can induce IL-22-producing CD8+ T cells (Fig. S1). Thus, IL-22 may play a pivotal role in skin homeostasis and in the pathogenesis of skin diseases. In this study, we discovered that skin-derived LCs and dermal DCs induced Th22 polarization from naive CD4+ T cells. Therefore, LCs and dermal DCs may be involved in IL-22-mediated skin homeostasis and inflammation by inducing the differentiation of naive CD4+ T cells into skin-homing Th22 cells in the cutaneous draining lymph nodes. In addition, given that these cutaneous DCs induced Th22 cells from whole peripheral T cells mostly composed of cells of a memory phenotype, it seems plausible that in certain conditions of antigenic stimulation, they may induce the expansion of IL-22-producing CD4+ T cells through local antigen presentation to resident memory T cells in the skin as well.
We also found that LCs induced significantly higher frequency of IL-22-producing CD4+ T cells from both whole peripheral T cells and naive CD4+ T cells than dermal DCs (Fig. 5). This relatively higher ability of LCs in comparison to dermal DCs could have stemmed from the differential isolation process: the use of trypsin for LC isolation and the difference of mAbs used for cell sorting. However, results from the comparison of IL-22 induction ability between in vitro-generated LC-type DCs and monocyte-derived DCs strongly support the concept that LCs are superior in the induction of IL-22-producing CD4+ T cells (Fig. S2). Although we should be cautious, it is possible to hypothesize that LCs are the main antigen-presenting DCs that control the expansion of Th22 cells infiltrating into the skin. Considering that IL-22 secreted by T cells mainly acts on epidermal keratinocytes, this hypothesis seems reasonable, because the information of the epidermal environment should be much more accessible to LCs than dermal DCs.
Recent studies have shown that a population of skin-homing memory T cells expressing CCR4, CCR6, and CCR10 constitutes Th22 cells (21, 22). However, we still do not know chemokine receptor expression profile of T cells stimulated by cutaneous DCs. Hence, further investigation into the expression of these chemokine receptors on cutaneous DC-expanded Th22 cells is required. It has also been reported that polarization of Th22 cells is largely, but not completely, dependent on IL-6 and TNF-α (22), both of which are generally produced by DCs during their maturation. Therefore, future experiments must explore other key factors, in combination with IL-6 and TNF-α, involved in the differentiation of Th22 cells by resident cutaneous DCs.
In summary, we demonstrated that LCs and dermal DCs from normal human skin induced the differentiation of distinct IL-22-producing Th22 cells. In addition, LCs were more efficient in the induction of IL-22-producing CD4+ T cells than dermal DCs. Our findings indicate that cutaneous DCs, especially LCs, control the generation of Th22 cells infiltrating into the skin. In inflamed skin, there is an additional DC population, inflammatory DCs (25, 36). For example, the inflammatory infiltrate in psoriasis and AD lesions contains a considerable number of myeloid CD11c+ DCs (35, 37). Thus, further elucidation of the roles of inflammatory DCs and LCs in diseased skin in the generation of Th22 cells would be helpful to understand the whole picture of IL-22-mediated skin inflammation and contribute to the development of IL-22-related therapeutics for combating inflammatory skin disease.
Materials and Methods
The study was approved by The Rockefeller University Institutional Review Board. Informed consent was obtained and the study was performed in adherence with the Declaration of Helsinki principles.
Skin Sample Preparation for Flow Cytometry.
Epidermis and dermis of normal human skin were separated by dispase digestion. Epidermal cell suspensions were obtained following trypsin treatment. To obtain dermal cell suspensions, dermal emigres were collected. The protocols are described in detail in SI Methods.
Flow Cytometry.
Flow cytometry was performed to examine the expressions of CD40, CD80, CD83, and CD86 on HLA-DR+CD207+ cells (LCs) and HLA-DRhiCD11c+BDCA-1+ cells (dermal DCs). The protocols are described in detail in SI Methods.
Generation of LC-Type DCs in Vitro.
LC-type DCs were generated in vitro from CD34+ HPCs as described previously (26, 30, 31). The protocols are described in detail in SI Methods.
Generation of Monocyte-Derived Conventional DCs.
Conventional DCs were generated from blood monocyte precursors as previously described (38). The protocols are described in detail in SI Methods.
MLR.
Using HLA-DR+CD207+ LCs, HLA-DRhiCD11c+BDCA-1+ dermal DCs, in vitro-generated LC-type DCs, and monocyte-derived DCs, we performed a modified allo-MLR previously described by Zaba et al. (24). The protocols are described in detail in SI Methods.
Intracellular Cytokine Staining.
T cells were first stimulated with PMA and ionomycin in the presence of BFA and subsequently subjected to intracellular cytokine staining as described previously (6). The protocols are described in detail in SI Methods.
Statistical Analysis.
A two-tailed paired Student's t test was used to analyze the results, and a P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We appreciate the assistance and advice of the Flow Cytometry Core Facility (S. Mazel, X. Fan, and C. Bare) at The Rockefeller University. We thank L. M. Johnson-Huang for careful editing of the manuscript. This study was supported in part by the Empire State Stem Cell Fund through New York State Department of Health (NYSDOH) contract C023046. Opinions expressed here are solely those of the authors and do not necessarily reflect those of the Empire State Stem Cell Fund, the NYSDOH, or the state of New York.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911472106/DCSupplemental.
References
- 1.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spolski R, Leonard WJ. Cytokine mediators of Th17 function. Eur J Immunol. 2009;39:658–661. doi: 10.1002/eji.200839066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zenewicz LA, Flavell RA. IL-22 and inflammation: Leukin' through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 4.Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–677. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Asadullah K, Sabat R. Immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 6.Nograles KE, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Sabat R. Interleukin-22: A novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367–380. doi: 10.1016/j.cytogfr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Boniface K, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 13.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolk K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 15.Nograles KE, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sa SM, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 17.Boniface K, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, et al. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol. 2009;39:1472–1479. doi: 10.1002/eji.200838811. [DOI] [PubMed] [Google Scholar]
- 20.Eyerich K, et al. IL-17 in atopic eczema: Linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 22.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 23.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 24.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morelli AE, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 28.Mathers AR, et al. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 29.Zaba LC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 32.Chung Y, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Volpe E, et al. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 35.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson-Huang LM, McNutt NS, Krueger JG, Lowes MA. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. J Clin Immunol. 2009;29:247–256. doi: 10.1007/s10875-009-9278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guttman-Yassky E, et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.