Abstract
Increased female reproductive rates usually result in accelerated senescence. This correlation provides a link between the evolutionary conflict of the sexes and aging when ejaculate components elevate female reproductive rates at the cost of future reproduction. It is not clear whether this female cost is manifest as shorter lifespan or an earlier onset or a steeper rate of reproductive senescence. It also is unclear whether beneficial ejaculates release females from reproductive trade-offs and, if so, which senescence parameters are affected. We examined these issues in the bedbug, Cimex lectularius, a long-lived insect that shows reduced female lifespan as well as female reproductive senescence at the male-determined mating frequency. We demonstrate experimentally that, independently of the mating frequency, females receiving more ejaculate show increased reproductive rates and enter reproductive senescence later than females receiving less ejaculate. The rate of reproductive senescence did not differ between treatments, and reproductive rates did not predict mortality. The ejaculate effects were consistent in inter- and intra-population crosses, suggesting they have not evolved recently and are not caused by inbreeding. Our results suggest that ejaculate components compensate for the costs of elevated female reproductive rates in bedbugs by delaying the onset of reproductive senescence. Ejaculate components that are beneficial to polyandrous females could have arisen because male traits that protect the ejaculate have positive pleiotropic effects and/or because female counteradaptations to antagonistic male traits exceed the neutralization of those traits. That males influence female reproductive senescence has important consequences for trade-offs between reproduction and longevity and for studies of somatic senescence.
Keywords: accessory gland proteins, disposable soma, interacting phenotype, life-history, mating rate
Physiological and genetic trade-offs cause individuals with increased current reproduction to show either accelerated future mortality or accelerated reproductive senescence (1–12). Female reproductive senescence, defined as a decline in reproductive function with age (7, 8, 13, 14), follows a similar pattern across animal taxa (8, 15) and is characterized by 3 main parameters (defined in ref. 8): (i) the onset of reproductive senescence at the critical age, after which (ii) female reproductive rates decline (iii) at the rate of senescence. Adjustment of any or a combination of these parameters will alter the dynamic of female reproductive senescence and therefore potential lifetime fecundity.
When both the male and the female determine the expression of a trait, such a shared trait can be described as an “interacting phenotype” (16). In polyandrous species, the male optimum for an interacting phenotype is likely to be different from the female's (15, 17), potentially resulting in an “evolutionary conflict between the individuals of the two sexes” (sexual conflict) (18, 19). During reproduction many interacting phenotypes occur, and sexual conflict may arise from any of these interactions, e.g, the timing or duration of mating, the re-mating rate, the female egg-laying rate, and/or offspring performance (4, 6, 20–22). Whenever an interacting phenotype results in net mating costs to one sex, the conflict can be resolved by trade-offs in resource allocations; these trade-offs, in turn, will disturb the existing equilibrium formed by the correlations between other interacting phenotypes. Thus, even if there are no current mating costs to females, there still may be a mosaic of coexisting interacting phenotypes that are, when measured in isolation, costly, neutral, or beneficial to one sex. Consequently, if males influence female reproductive rates and, therefore, the existing trade-offs, the 3 fundamental parameters of reproductive senescence (8) are interacting phenotypes. This relationship provides an important but largely unexplored link between sexual conflict and aging theories.
Ejaculate components are central to understanding aspects of this dynamic (23–27) because their effects on females are both strong and diverse, especially in insects (5, 21, 23). Most important is their well-documented effect on elevating female reproductive rates (1–6, 11). Although life-history theory and physiological models of aging predict that elevated current female reproductive rates result in reduced future reproduction (2, 3, 7–11), it is not clear whether the reduced future reproduction is the result of a faster rate of reproduction or an earlier onset of reproductive senescence. Correlational data in Drosophila suggest the latter possibility (8), but experimental evidence using ejaculate-induced elevation of reproductive rates is lacking. This lack of information is unfortunate, because several studies have found ejaculate components to be beneficial in several aspects of female reproduction (4, 5, 18, 23–31), but it is not known whether such benefits evolved via sexual conflict (e.g., 4, 6, 22) or by traditional female choice (25–27, 32). For example, females may have evolved the ability to metabolize manipulative ejaculate substances (4), or they may choose males that provide the largest direct benefits (e.g., nuptial gifts). In the former case, the metabolism of manipulative substances may be costly initially (33) and so result in trade-offs in females; in the latter case, one would not necessarily expect trade-offs. This uncertainty makes it difficult to predict whether, and which, female reproductive parameters are affected by ejaculate components. In particular, there seem to be few theoretical or empirical precedents that point to how females should allocate any ejaculate benefits to the core elements of age-specific fecundity (see ref. 8), i.e., toward higher reproductive rates, postponing the onset of reproductive senescence, reducing the rate of reproductive senescence, or any combination thereof.
We examined the effect(s) of ejaculate-mediated elevated reproductive rates on females in the common bedbug, Cimex lectularius. Bedbugs are suitable for this study for several reasons. First, males control the rate of mating and therefore ejaculate delivery (34). The realized mating rate is 12–20 times higher than the rate required for maximum fecundity (20) and reduces female lifespan and fecundity (20). Unlike in Drosophila (35), female bedbugs do not appear to obtain indirect benefits via their offspring (20), so there may be sexual conflict over the mating rate. Second, the ejaculates of C. lectularius have been found to contain antioxidant (29) and antibacterial activity (30) as well as essential amino acids (31). These substances all have potential fitness benefits for females. Third, bedbugs are long-lived (13, 20, 36) and show female reproductive senescence in the laboratory (13). The laboratory environment closely mimics natural conditions (13, 20, 36): Bedbugs found in human dwellings usually occur at high densities (36, 37) and show few signs of food limitation (37). Bedbugs also have few natural enemies (36) and so likely show the low extrinsic mortality that allows reproductive senescence to evolve (1, 2, 7). In this study we isolate the effect of ejaculate components from that of other mating traits by experimentally altering the ejaculate-mediated egg-laying rate without altering the traits that cause harm to females.
Results
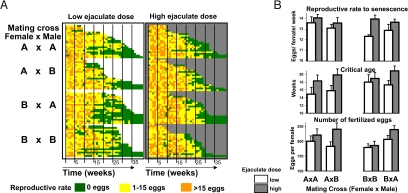
Females in this study either received 1 ejaculate unit (low exposure rate, n = 79) or 3 units (high exposure rate, n = 80) every week of their adult life. Even our low treatment rate exceeds the ejaculate provision rate required to maintain female fecundity, because 1 ejaculate unit sustains female fecundity and fertility for 4–8 weeks (20, 36). Females in both treatments received 3 copulatory intromissions per week, thus experimentally controlling for the 2 processes that have been implicated in reducing female fitness: the wounding of the female (38), which occurs at every mating in bedbugs (traumatic insemination; ref. 20), and infection from male genital contamination with microbes (39, 40). Although all females were fully fed and did not experience macronutrient limitation, females receiving the high ejaculate dose showed a significant increase in reproductive rate compared with females receiving the low ejaculate dose (Fig. 1 A and B). This pattern was found across 2 populations, and female reproductive rates were similar to those in our previous studies (20, 39). There was no difference in reproductive rate between females that received ejaculates from males of their own population and that received ejaculates from males of the other population (Fig. 1B). The substances responsible for elevating reproductive rate, therefore, appear to be shared by males of both populations rather than having evolved recently or resulting from inbreeding. These results were not affected by variation in egg size, because variation in egg size could not be explained by treatment (t = 0.630, P = 0.532), female population (t = 0.286, P = 0.776), or mating type (t = 1.007, P = 0.320).
Fig. 1.
Reproductive senescence in female bedbugs in relation to female population origin (A or B), inter- or intra-population cross, and ejaculate exposure rate (low: white background and columns; high: gray background and columns). (A) Event-history plots of individual bedbug females: Within treatment groups, females are ordered by lifespan (46). The color of the squares codes for the weekly reproductive output. The larger number of orange squares in the early weeks in the right-hand plot shows the ejaculate-mediated increased reproductive rate. The longer persistence of orange in the right hand plot and the lower number of green squares illustrates the later onset of reproductive senescence in this treatment group. (B) Parameters of reproductive senescence in female bedbugs. (Top) Egg-laying rate before senescence was higher at the high ejaculate exposure rate than a the low ejaculate exposure rate (t = 2.908, P = 0.004) but did not differ with inter- or intra-population cross (t = 0.697, P = 0.45). The effect of female population did not reach significance (t = −1.923, P = 0.06). No interaction was significant. (Middle) The onset of senescence was delayed by 11.4% in females with a high ejaculate exposure rate compared with females with a low ejaculate exposure rate (t = 2.459, P = 0.015), but there was no effect of female population (t = 1.261, P = 0.3), or inter- or intra-population cross (t = 0.145, P > 0.5). (Bottom) The total number of fertile eggs per female, a close fitness measurement (21), was significantly higher in females in the high ejaculate exposure rate arm (t = 2.963, P = 0.004) and also was influenced by the interaction of female population and by inter- versus intra-population cross (t = 2.115, P = 0.04).
Counter to the predictions from senescence and life-history theory, the observed ejaculate-mediated increase in female reproductive rate was not associated with an earlier onset of reproductive senescence. Instead, the females that were exposed to the high ejaculate rate and that had the highest reproductive rate showed an 11.4% delay in the onset of senescence: The critical age in these females occurred 0.9 to 3.6 weeks later than in females in the low ejaculate arm (Fig. 1B). This effect was consistent between intra- and inter-population crosses, suggesting it did not result from recent divergent evolution. The rate of reproductive senescence (the parameter τ; see ref. 8) did not differ between treatments (τHigh dose = −1.069, τLow dose = −1.206, t = 0.884, P = 0.378) or between intra- versus inter-population crosses (τintra = −1.099, τinter = −1.190, t = 0.693, P = 0.489). However, the rate of reproductive senescence was higher in females of population A (τA = −1.36 ± 0.95) than population B (τB = −0.93 ± 0.77) (t = 2.836, P = 0.005).
Therefore, ejaculate treatment (t = 2.258, P = 0.03) but not female population (t = 0.367, P > 0.5) or intra- versus inter-population cross (t = 1.237, P > 0.3) predicted lifetime egg production. Across populations and their crosses, females in the high ejaculate treatment showed a 15–17% fitness increase. The results remain unaltered when all females that died before reaching senescence (Table 1) were excluded (ejaculate treatment: t = 2.969, P = 0.004).
Table 1.
Reproductive senescence of female bedbugs from 2 populations A and B in relation to ejaculate origin (population A or B) and weekly low (L) or high (H) ejaculate exposure rate
| Ejaculate origin and dose | Female population A |
Female population B |
||||||
|---|---|---|---|---|---|---|---|---|
| A, L | A, H | B, L | B, H | B, L | B, H | A, L | A, H | |
| Number of females | 20 | 20 | 19 | 20 | 20 | 20 | 20 | 20 |
| Median lifespan ± SE (LD50), days | 257 ± 4 | 257 ± 19 | 210 ± 16 | 257 ± 4 | 294 ± 4 | 288 ± 17 | 288 ± 12 | 264 ± 27 |
| Lifetime egg production | 218.3 ± 20.2 | 250.6 ± 22.2 | 221.1 ± 18.3 | 269.5 ± 23.9 | 234.2 ± 22.0 | 240.6 ± 21.4 | 240.2 ± 17.2 | 281.1 ± 16.7 |
| Egg size, mm2 (females/eggs per female) | 1.08 ± 0.004 | 1.12 ± 0.01 | 1.10 ± 0.008 | 1.07 ± 0.007 | 1.06 ± 0.024 | 1.04 ± 0.026 | 1.05 ± 0.019 | 1.04 ± 0.020 |
| (5/4.8) | (4/5) | (5/5) | (7/5) | (8/4.6) | (3/3.7) | (10/4.6) | (6/4.8) | |
| Reproductive senescence | ||||||||
| Females dead before senescent | 2 | 3 | 1 | 1 | 2 | 5 | 1 | 4 |
| Onset of senescence (Critical age T), weeks | 14.9 ± 1.8 | 18.2 ± 1.5 | 15.8 ± 1.4 | 18.9 ± 1.7 | 18.0 ± 1.9 | 18.9 ± 1.6 | 17.3 ± 1.2 | 20.9 ± 1.3 |
| Laying rate before T, eggs/week | 13.6 ± 0.6 | 14.1 ± 0.3 | 13.1 ± 0.4 | 13.6 ± 0.5 | 12.0 ± 0.5 | 14.0 ± 0.4 | 12.9 ± 0.6 | 13.7 ± 0.3 |
| % of eggs laid before senescence | 83 ± 2.6 | 86 ± 2.4 | 78 ± 4.3 | 85 ± 3.6 | 73 ± 4.6 | 75 ± 4.2 | 77 ± 3.1 | 82 ± 3.2 |
| Rate of reproductive senescence, τ | −1.443 | −1.286 | −1.262 | −1.480 | −0.820 | −0.839 | −1.315 | −0.728 |
| Fertility senescence | ||||||||
| Lifetime number of fertile eggs | 199.4 ± 17.8 | 220.4 ± 20.6 | 182.2 ± 15.3 | 238.9 ± 21.3 | 176.2 ± 14.3 | 184.9 ± 14.3 | 205.8 ± 13.7 | 238.5 ± 12.9 |
| Proportion of fertilized eggs | 92.8 ± 1.7 | 86.3 ± 4.8 | 82.1 ± 36.4 | 90.0 ± 2.7 | 78.5 ± 2.8 | 81.1 ± 3.5 | 86.9 ± 1.3 | 86.8 ± 2.5 |
| Critical age T, weeks | 15.40 ± 1.25 | 12.88 ± 0.93 | 10.94 ± 0.85 | 14.65 ± 1.37 | 11.94 ± 0.79 | 12.43 ± 0.63 | 13.63 ± 0.88 | 14.62 ± 1.22 |
| % fertile eggs before senescence | 81.2 ± 3.0 | 82.13 ± 5.0 | 77.2 ± 4.1 | 83.6 ± 3.8 | 66.08 ± 5.43 | 73.78 ± 4.98 | 75.0 ± 3.21 | 78.88 ± 3.50 |
Males of C. lectularius produce sperm continuously; nonetheless, the males used to provide ejaculate units (see Materials and Methods) in this experiment were replaced regularly. Therefore, females that produced infertile eggs had been mating with non-depleted males. The results for reproductive senescence still held when infertility differences between females (Fig. 1B and Table 1) were taken into account. Overall, infertility varied between 0 and 100% (overall mean 14.5 ± 14.0% or 7.3 ± 10.7%) before the onset of reproductive senescence, but it did not affect reproductive senescence. First, reproductive senescence and fertility senescence were not correlated (partial r = −0.013, P = 0.882, df = 128, controlled for the number of eggs). Second, although more infertile eggs were laid by the females that laid more eggs overall (t = 10.575, P ≪ 0.0001), females in the high ejaculate exposure laid fewer infertile eggs than females in the low ejaculate exposure, relative to their total egg number (t = −2.296, P = 0.025). Relative infertility was decreased in inter-population crosses compared with intra-population crosses in population B females but not in population A females (interaction, t = −2.810, df = 149, P = 0.007). It is possible that this phenomenon is related to heterosis.
Females in population A had a higher mortality rate than females in population B [odds ratio, exp (b) = 0.340, P < 0.001] but the high ejaculate treatment increased mortality only in population B [odds ratio, exp (b) = 1.462, P = 0.004; no other significant interaction] (Fig. 1A). The reproductive rate before the onset of senescence did not predict female mortality (P = 0.809). In 5 of our 8 treatment groups only 1 or 2 females died before reaching senescence (Table 1). This low mortality provides little opportunity for our senescence calculations to be affected by mortality heterogeneity (41) (i.e., for high- or low-reproducing females that die before reaching reproductive senescence to generate a bias in the calculation of critical ages). Moreover, in the treatment groups in which 3 or more females died before reaching reproductive senescence (Table 1), the reproductive rate of those females was within the 95% confidence interval of the females who reached senescence.
Discussion
Here we provide experimental evidence to show that components of the ejaculate have the combined effect of delaying the onset of female reproductive senescence and simultaneously elevating female reproductive rate. Because of the low extrinsic mortality of bedbugs in nature, it is likely that the benefit of reproductive senescence also is found under natural conditions. Ejaculate-mediated increases in female reproductive rate are well documented (1–6, 11, 23–27) and, according to physiological aging models (7–9, 11), should result in reproductive senescence via the accumulation of metabolic damage (but see ref. 41). We did not find the trade-offs predicted by life-history theory and evolutionary models of aging (1–3, 7, 9, 10), because increased reproductive rates early in life did not result in earlier reproductive senescence. It is possible that ejaculate components either directly reduce aging damage in the female or release females from trade-offs. Candidate traits (9) for the reduction of somatic damage and/or trade-off release are immune effectors, antioxidants, stress-defense substances, and micronutrients in the seminal fluid. Representatives of all 4 types of compound have been found among the >100 components in the semen of bedbugs (29–31).
That male traits evolve which are beneficial to females may seem surprising in a species that has a high mating frequency (20, 36) that is controlled by males (34) and has last-male sperm precedence (20): Any newly evolving beneficial ejaculate substance favors competing males that mate subsequently (15). We envisage 2 possible routes by which such beneficial male effects may have arisen. First, ejaculate traits originally may have had a negative effect on females, as is currently observed for some Drosophila proteins (23). Consequently, female traits, such as the ability to metabolize ejaculate substances (4), may be selected for in females. Although the evolution of such female counteradaptations currently is believed merely to neutralize male harm (1, 2, 6, 15), it may have progressed in bedbugs to benefit females. Alternatively, it is possible that male–male competition does not always have negative side effects on females (pleiotropic sexual antagonism; see refs. 1, 2, 6, 15). Instead it may result in male traits that evolved to protect the ejaculate (ejaculate defense traits, ref. 15) having a positive pleiotropic effect on the female such as the antioxidant (29) and the antibacterial (30) activity found in bedbug ejaculates.
Ejaculate components increased the egg-laying rate in this study, whereas a previous study on the same population did not find a positive effect of mating on laying rate (20). This finding suggests that, in addition to the direct negative effect of mating on female lifespan (20, 38, 39), additional costs to females might remove the positive effect of ejaculate substances. In conclusion, our data show an important male effect on female fitness and aging patterns and demonstrate that beneficial male postcopulatory traits can evolve alongside harmful male traits. Our results have implications for reproduction–longevity trade-off models and thus for studies whose main focus is somatic senescence.
Materials and Methods
Study Species and General Culture.
Bedbugs (Cimex lectularius) were cultured at 26 °C, and 70% relative humidity as previously described (21, 38). We used 2 populations. Population A has been cultured in our laboratory for 7 years and originated from a stock of ca. 600 animals provided by the Medical Entomology Centre. Population B had been cultured for 2 years and originated from Insect Control & Research, Inc. Last-instar nymphs were separated from the stocks and kept in individual vials. Upon eclosion virgin individuals were sexed and isolated. Males and females were fed once a week as described earlier (20, 39).
Experimental Treatment and Culture.
Females, nourished ad libitum, were allocated randomly to 1 of the 2 treatment levels and to a mating partner that was either from the same population (shared evolutionary history, such as male–female co-evolution or similar natural selection) or a different population (different evolutionary history). If any of the predicted effects on female fitness resulted from a shared evolutionary history in senescence (e.g., co-evolution between sexes, see ref. 2), the slopes of the 2 treatment levels should differ for intra- and inter-population crosses. In our design, this prediction corresponds to a 2-way interaction effect of treatment × inter- or intra-population cross. However, populations may differ only in the shape of the ejaculate dose–female response curve but not in their absolute response values. This situation can produce spurious statistical interactions wrongly interpreted as co-evolution (43). However, in our design such different dose–response curves would be apparent in a 3-way interaction effect of population × treatment × inter- or intra-population cross.
All females entered the experiment 7–12 days after adult eclosion. Males transfer ejaculates at a constant rate during copulation (44). All copulations were interrupted after 60 s to standardize ejaculate units to either 1 or 3 weekly doses. Previously identified mating costs (20, 38–40) were controlled for experimentally: Females receiving the low ejaculate dose received 2 additional intromissions by the male. These intromissions were of <5 s duration and so did not result in sperm transfer (44).
In the experimental cultures all females were kept individually in vials with a strip of filter paper. Males also were sexually isolated between matings but were kept within population pools containing 20–50 males of the same age (>2 weeks old). Male pools were replaced every 4–6 weeks to avoid male sperm depletion. Females were kept in their individual vials until they died.
Age-Specific Fecundity and Senescence Parameters.
Under our laboratory conditions common bedbugs, Cimex lectularius, live and reproduce for a year (39). Mortality and the core 3 elements of age-specific fecundity [the reproductive rate (the weekly oviposition rate before senescence, RC), the critical age (T), and the rate of senescence (τ) (8)] can be measured easily (20, 39).
The experiment was staggered with 6 of 20 females per treatment starting in week 1, 4 starting in week 2, and 10 starting in week 3. Female survival was monitored once a week. When the first of the 3 cohorts reached age 52 weeks, the experimental trial was terminated. All females that had not died were recorded. In the survival analysis their respective ages were entered but censored from that day. There were 4 experimental periods of a total of 11 weeks in which females did not receive any treatment and were not fed. Because a lack of food stops reproduction, these weeks were discarded from the analysis.
Every week at the same time, the filter paper was removed from each vial, and all eggs per female were counted and their fertilization status assessed (see below). The curve of cumulative egg production was obtained for each female. The onset of reproductive senescence, i.e., the critical age (8), was obtained by 2 methods that were highly correlated to each other (Pearson r = 0.894, P < 0.001, without 1 outlier r = 0.919). In the first method, visual inspection revealed the relatively sudden decline from the cumulative egg-production curve. In the second method, the cumulative egg-production curve of each female was fitted by a cubic polynomial function (mean r2 = 0.987, range 0.797–0.999). The female age at which the first derivative of this curve was zero, i.e., the inflexion point of the curve, was used as a measurement of the critical age. Because the second method was found to be imprecise in the 23 cases in which the reproductive rate dropped sharply, the first method was used. Twenty-six females showed a single episode of recovery before reaching the critical age a second time. In such cases we used the first senescence point.
The rate of senescence (8) was measured as the exponential decline exponent.
Each week, when the eggs were counted, we scored the number of fertile eggs. Fertile eggs are taut and whitish with clear indications of the developing embryo (e.g., eye spots). Infertile eggs are gray or dark gray and usually collapse soon after being laid. The week in which infertile eggs were laid for the second time was taken as the time of the onset of fertility senescence.
Eggs were collected from 4–10 females per treatment at week 4 and were photographed. The egg size was analyzed on an image analysis system (QCapturePro, Media Cybernetics),and was not affected by our experimental treatments. The eggs of population A females (n = 104) were 3.5% smaller than those of population B females (n = 122) (ANOVA, F = 8.313, P = 0.006) (Table 1). The hatching success of eggs measured from 10 clutches per female group in 3 different weeks was 100%.
Statistical Analyses.
Using generalized linear modeling (quasi-likelihood option with a log link), we allowed the error variance of the model increase with the square of the mean. We entered the treatment level (1 versus 3 ejaculate units), the female population (A or B), the male origin relative to the female (intra- or inter-population cross), and their interactions into the full model. To obtain the minimum adequate model, we used a backwards mode of variable exclusion. The exclusion was based on whether the model would improve significantly if the variable was included, as shown by a significant improvement of the Akaike Information Criterion (45).
Female survival was analyzed with respect to treatment, female population, co-evolution status, and their interactions. Using Cox Regression in SPSS 12.0, non-significant predictor variables were excluded stepwise from the model based on F-values. We used indicator contrasts.
Acknowledgments.
We thank W. Foster and R. Todd for providing bedbugs. We thank R. Bonduriansky, P. Brakefield, A. Maklakov, J. Rolff, and 2 referees for comments that substantially improved the manuscript. This work was funded by grants from the Leverhulme Trust, the Wellcome Trust, and the Natural Environment Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. Sexual selection, sexual conflict and the evolution of ageing and life span. Functional Ecology. 2008;22:443–453. [Google Scholar]
- 2.Maklakov A, Kremer N, Arnqvist G. Adaptive male effects on female ageing in seed beetles. Proc R Soc Lond Ser B. 2005;272:2485–2489. doi: 10.1098/rspb.2005.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Promislow D. Mate choice, sexual conflict, and evolution of senescence. Behav Genet. 2003;33:191–201. doi: 10.1023/a:1022562103669. [DOI] [PubMed] [Google Scholar]
- 4.Arnqvist G, Nilsson T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. [DOI] [PubMed] [Google Scholar]
- 5.Poiani A. Complexity of seminal fluid: A review. Behav Ecol Sociobiol. 2006;60:289–310. [Google Scholar]
- 6.Arnqvist G, Rowe L. Sexual Conflict. Princeton: Princeton Univ. Press; 2005. [Google Scholar]
- 7.Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Novoseltsev VN, Novoseltseva JA, Boyko SI, Yashin AI. What fecundity patterns indicate about aging and longevity: Insights from Drosophila studies. J Gerontol. 2003;58A:484–494. doi: 10.1093/gerona/58.6.b484. [DOI] [PubMed] [Google Scholar]
- 9.Harshman LG, Zera AJ. The cost of reproduction: The devil in the details. Trends in Ecology and Evolution. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 11.Partridge L, Gems D, Withers DJ. Sex and death: What is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Charmantier A, Perrins C, McCleery RH, Sheldon BC. Quantitative genetics of age at reproduction in wild swans: Support for antagonistic pleiotropy models of senescence. Proc Natl Acad Sci USA. 2006;103:6587–6592. doi: 10.1073/pnas.0511123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janisch E. Beobachtungen bei der Aufzucht von Bettwanzen. I. Über das Verhalten von Populationen bei verschiedenen Zuchtbedingungen. Z Parasitenkunde. 1933;5:460–514. [Google Scholar]
- 14.Jones OR, et al. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol Lett. 2008;11:664–673. doi: 10.1111/j.1461-0248.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 15.Rice WR. Dangerous liaisons. Proc Natl Acad Sci USA. 2000;97:12953–12955. doi: 10.1073/pnas.97.24.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore AJ, Brodie ED, III, Wolf JB. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects on social interactions. Evolution (Lawrence, Kans) 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 17.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 18.Parker GA. Sexual selection and sexual conflict. In: Blum MS, Blum NA, editors. Sexual Selection and Reproductive Competition in Insects. London: Academic; 1979. pp. 123–166. [Google Scholar]
- 19.Parker GA. Sexual conflict over mating and fertilization: An overview. Philos Trans R Soc B. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stutt A, Siva-Jothy MT. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc Natl Acad Sci USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 22.Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc R Soc London Ser B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman T, Davies SJ. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides (Tarrytown, NY) 2004;25:1477–1490. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Gwynne DT. Sexual conflict over nuptial gifts in insects. Annu Rev Entomol. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- 25.Wiklund C, Kaitala A, Lindfors V, Abenius J. Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L.) Behav Ecol Sociobiol. 1993;33:25–33. [Google Scholar]
- 26.Fox CW. Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera, Bruchidae) Functional Ecology. 1993;7:203–208. [Google Scholar]
- 27.Schwartz SK, Peterson MA. Strong material benefits and no longevity costs of multiple mating in an extremely polyandrous leaf beetle, Chrysochus cobaltinus (Coleoptera: Chrysomelidae) Behav Ecol. 2006;17:1004–1010. [Google Scholar]
- 28.Lung O, Kuo L, Wolfner MF. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J Insect Physiol. 2001;47:617–622. doi: 10.1016/s0022-1910(00)00151-7. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt K, Wong CH, Georgiou SA. Seminal fluid proteins in the bed bug, Cimex lectularius, detected using two-dimensional gel electrophoresis and mass spectrometry. Parasitology. 2009;136:283–292. doi: 10.1017/S0031182008005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otti O, Naylor R, Siva-Jothy MT, Reinhardt K. Bacteriolytic activity in the ejaculate of an insect. Am Nat. 2009;174:292–295. doi: 10.1086/600099. [DOI] [PubMed] [Google Scholar]
- 31.Rao V. Free amino acids in the seminal fluid and haemolymph of Cimex lectularious [sic] L. and their possible role in sperm metabolism. Indian J Exp Biol. 1974;12:346–348. [PubMed] [Google Scholar]
- 32.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 33.Arnqvist G. Sensory exploitation and sexual conflict. Philos Trans R Soc London Ser B. 2006;361:375–386. doi: 10.1098/rstb.2005.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhardt K, Naylor RA, Siva-Jothy MT. Situation exploitation: Higher male mating success when female resistance is reduced by feeding. Evolution. 2009;63:29–39. doi: 10.1111/j.1558-5646.2008.00502.x. [DOI] [PubMed] [Google Scholar]
- 35.Priest NK, Galloway LF, Roach DA. Mating frequency and inclusive fitness in Drosophila melanogaster. Am Nat. 2008;171:10–21. doi: 10.1086/523944. [DOI] [PubMed] [Google Scholar]
- 36.Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annu Rev Entomol. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt K, Isaac D, Naylor R. Estimating the feeding rate of the bedbug Cimex lectularius in an infested room: an inexpensive method and a case study. Medical and Veterinary Entomology. 2009 doi: 10.1111/j.1365-2915.2009.00847.x. in press. [DOI] [PubMed] [Google Scholar]
- 38.Morrow EH, Arnqvist G. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc R Soc London Ser B. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhardt K, Naylor R, Siva-Jothy MT. Reducing a cost of traumatic insemination: Female bedbugs evolve a unique organ. Proc R Soc London Ser B. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinhardt K, Naylor R, Siva-Jothy MT. Potential sexual transmission of environmental microbes in a traumatically inseminating insect. Ecological Entomology. 2005;30:607–611. [Google Scholar]
- 41.Carey JR, Liedo P, Orozco D, Tatar M, Vaupel JW. A male–female longevity paradox in medfly cohorts. J Anim Ecol. 1995;64:107–116. [Google Scholar]
- 42.Speakman JR, et al. Uncoupled and surviving: Individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 43.Rowe L, Cameron E, Day T. Detecting sexually antagonistic coevolution with population crosses. Proc R Soc London Ser B. 2003;270:2009–2016. doi: 10.1098/rspb.2003.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siva-Jothy MT, Stutt A. A matter of taste: Direct detection of mating status in the bed bug. Proc R Soc London Ser B. 2003;270:649–652. doi: 10.1098/rspb.2002.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawley MJ. Statistical Computing Using S-Plus. Chichester, United Kingdom: John Wiley & Sons; 2002. [Google Scholar]
- 46.Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]