Abstract
Most myosins have a positively charged loop 2 with a cluster of lysine residues that bind to the negatively charged N-terminal segment of actin. However, the net charge of loop 2 of very fast Chara myosin is zero and there is no lysine cluster in it. In contrast, Chara myosin has a highly positively charged loop 3. To elucidate the role of these unique surface loops of Chara myosin in its high velocity and high actin-activated ATPase activity, we have undertaken mutational analysis using recombinant Chara myosin motor domain. It was found that net positive charge in loop 3 affected Vmax and Kapp of actin activated ATPase activity, while it affected the velocity only slightly. The net positive charge in loop 2 affected Kapp and the velocity, although it did not affect Vmax. Our results suggested that Chara myosin has evolved to have highly positively charged loop 3 for its high ATPase activity and have less positively charged loop 2 for its high velocity. Since high positive charge in loop 3 and low positive charge in loop 2 seem to be one of the reasons for Chara myosin's high velocity, we manipulated charge contents in loops 2 and 3 of Dictyostelium myosin (class II). Removing positive charge from loop 2 and adding positive charge to loop 3 of Dictyostelium myosin made its velocity higher than that of the wild type, suggesting that the charge strategy in loops 2 and 3 is widely applicable.
Keywords: actin, ATPase, motility, cytoplasmic streaming, molecular engineering
Cytoplasmic streaming in characean algal cells is extremely fast, and this streaming is brought about by the movement of myosin-coated organelles along actin filament bundles fixed inside the cell (1). Myosin purified from Chara corallina could translocate actin filaments in the in vitro motility assay at a velocity comparable to that of the cytoplasmic streaming (approximately 50 μm s−1) (2, 3). This velocity is about 10 times faster than that of the fast skeletal muscle myosin and the Chara myosin is the fastest motor protein known so far. We have cloned cDNA of the Chara myosin heavy chain (4) and succeeded in expressing functional motor domain (5, 6). The velocity of the expressed motor domain measured by in vitro motility assay was comparable to that of the native Chara myosin if we consider the difference in the lever arm length. Relevant steps in actomyosin ATPase cycle to the sliding velocity are ADP release from actomyosin and ATP reassociation resulting in actin-myosin dissociation. Kinetic analyses of Chara myosin motor domain revealed that both the ADP release and the ATP-induced dissociation from actin were very fast (6). Time spent in the strongly bound state with actin, which was estimated from these rates, was less than 1 ms. This very short strongly bound state, together with a large step size (7), are probably the reason for the very high velocity of Chara myosin. Actin-activated ATPase activity of expressed Chara motor domain was approximately 500 s−1head−1, which was also the highest of all myosins so far measured (5). Since the ADP release and ATP-induced dissociation was very fast as mentioned above, the rate-limiting step of acto-Chara myosin ATPase is suggested to be the transition from the weekly to the strongly bound state with actin with concomitant release of Pi (6).
It is known that mutations in various surface loops that interact with actin affect these steps (8). In this study, we focused our attention on loops 2 and 3 of Chara myosin. Loops 2 and 3 are part of actin binding sites (9, 10). Cross-linking studies suggested that positively charged residues in loops 2 and 3 interact with negatively charged residues in subdomain 1 of actin at the initial weakly bound state (11–21).
Since these surface loops are highly variable in sequence and length among various classes of myosins (Tables 1 and 2), it is suggested that diverse enzymatic and motile activities of the myosins are achieved in part through the variations in the sequences of these loops (22, 23). Many studies have revealed the relationship between positive charge of loop 2 and actin-activated ATPase activities (24–29).
Table 1.
Loop 2 sequences of various myosins
| Source* | Length | Net charge | Sequence† |
|---|---|---|---|
| Chara | 17 | 0 | FP ADEGTKAPSKFMSIG |
| Chick Sk | 28 | +3 | FATYG GEAEGGGGKKGGKKKGSSFQTVS |
| Chick Sm | 36 | +3 | WKDVDRIV GLDQM AKMTESSLPSASKTKKGMFRTVG |
| Chick Va | 45 | +6 | FQDEEKAISPTSATPSGRVPLSRTPVKPAKARPGQTSKEHKKTVG |
*Chara (Chara corallina myosin XI), Chick Sk (Chicken fast skeletal myosin II), Chick Sm (Chicken smooth muscle myosin II), Chick Va (Chicken myosin Va).
†Positively and negatively charged amino acids are shown in bold and italic, respectively.
Table 2.
Loop 3 sequences of various myosins
| Source* | Length | Net charge | Sequence† |
|---|---|---|---|
| Chara | 6 | +5 | KHKFKR |
| Chick Sk | 10 | +3 | KPKPAKGKAE |
| Chick Sm | 8 | +3 | KSKQLKDK |
| Chick Va | 6 | +2 | KPRLSN |
*Chara (Chara corallina myosin XI), Chick Sk (Chicken fast skeletal myosin II), Chick Sm (Chicken smooth muscle myosin II), Chick Va (Chicken myosin Va).
†Positively and negatively charged amino acids are shown in bold and italic, respectively.
Chara myosin has unique feature in these surface loops: the net charge of loop 2 is zero and that of loop 3 is the largest among all myosins so far known (Tables 1 and 2). To elucidate the role of these unique surface loops of Chara myosin in its high velocity and high actin-activated ATPase activity, we studied the activity of recombinant Chara myosin motor domains in which charge and length of these loops were altered. We found that the actin-activated ATPase activity of Chara myosin was highly dependent on the net positive charge in loop 3 while the velocity was not. We also found that addition of positive charge to loop 2 had only small effect on the actin-activated ATPase activity but greatly reduced the ADP release rate and thus the velocity of Chara myosin.
Results
Expression and Purification of Mutants.
Because recombinant Chara myosin motor domains used in this study contain only motor domain but not IQ motifs while native Chara myosin has 6 IQ motifs, lever arm length, and thus the velocity of the recombinant Chara myosin motor domains, are about one-eighth of those of the native Chara myosin (5, 6). Loops 2 and 3 of the motor domain were mutated by PCR-based mutagenesis. After expression in insect cells, mutant motor domains were purified by coprecipitation with actin and by nickel-affinity resin. However, we could not use the coprecipitation with actin for mutant motor domains whose affinity for actin was very low. These mutant motor domains were further fused with FLAG tag and purified by Ni-affinity resin and anti-FLAG antibody resin.
Loop 3 Mutants.
Chara myosin has very short loop 2 with no net charge (Table 1), but its apparent affinity for actin in the presence of ATP (estimated from Kapp of actin-activated ATPase activity) was much higher than that of skeletal muscle myosin that has a long and positively charged loop 2 (Table 1 and Fig. 1). Chara myosin, on the other hand, has much more positive net charge in loop 3 than any other myosins (Table 2). Since this loop of skeletal (11) and cardiac muscle (21) myosins is also cross-linked to actin, we first altered net charge in loop 3 systemically by substituting positively charged amino acids to alanine or by adding lysine residues and examined the effect on the functional characteristics of Chara myosin.
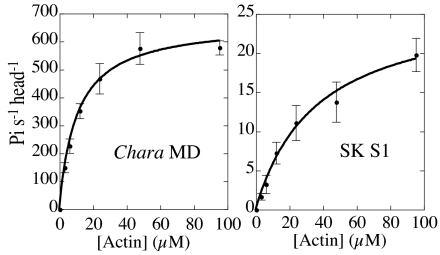
Fig. 1.
Actin-activated ATPase activities of Chara myosin motor domain and skeletal myosin subfragment-1. Values are averages of 3 measurements. Data were fitted to the Michaelis-Menten equation. Vmax of Chara myosin motor domain and skeletal myosin subfragment-1 (S1) were 670 ± 20 and 27 ± 2 Pi/s/head, respectively. Kapp values of Chara myosin motor domain and skeletal myosin S1 were 11 ± 1 and 27 ± 2 μM, respectively. Note that the ATPase assays shown in this figure were done at low salt (10 mM KCl, 4 mM MgCl2, 20 mM Hepes-KOH, pH 7.4) because the ATPase activity of skeletal myosin S1 in standard ATPase assay solution increased linearly with the increase in actin concentration and both Vmax and Kapp values could not be determined.
We made 4 loop 3 mutant motor domains (Table 3). A mutant designated L3 (-5) has substitutions at all 5 positively charged amino acids of loop 3. L3 (N-3) has substitutions at 3 positively charged amino acids at the N-terminal side of loop 3. L3 (C-2) has substitutions at 2 positively charged amino acids at the C-terminal side of loop 3. L3 (+2) has 2 additional lysine residues at the N-terminal side of loop 3.
Table 3.
Sequences, net charge and activity of wild type and loop 3 mutants of Chara myosin motor domain
| Sequence* | Net charge | Vmax (Pi/head/s)† | Kapp (μM)† | Velocity (μm/s)§ | |
|---|---|---|---|---|---|
| L3 (−5) | AAAFAA | 0 | ND‡ | ND‡ | 3.3 ± 0.2¶ |
| L3 (N-3) | AAAFKR | +2 | 260 | 81 | 4.3 ± 0.3 |
| L3 (C-2) | KHKFAA | +3 | 540 | 44 | 4.5 ± 0.2 |
| WT | KHKFKR | +5 | 580 | 23 | 4.8 ± 0.2 |
| L3 (+2) | KKKHKFKR | +7 | 390 | 12 | 4.3 ± 0.2 |
*Positively charged amino acid and altered amino acid are shown in bold and italic, respectively.
†Values are averages of two measurements on two independent preparations.
‡We could not determine Vmax and Kapp of L3 (−5) because activity increased almost linearly (see Fig. 2).
§Values are averages ± SD of 40–50 actin filaments on two independent preparations.
¶Velocity of L3 (−5) was done in the presence of 0.8% methylcellulose.
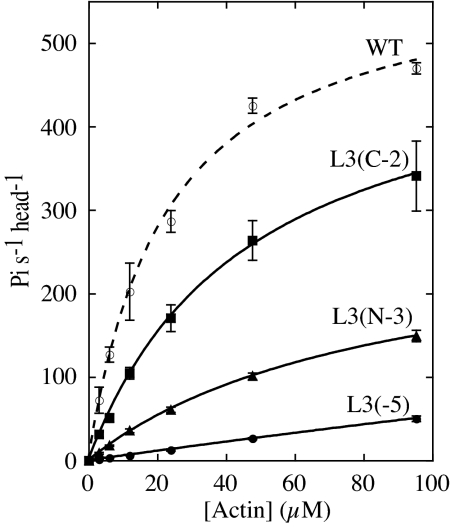
Fig. 2 shows actin concentration dependence of the ATPase activities of loop 3 mutants. Data were fitted to the Michaelis-Menten equation and Vmax and Kapp values for each mutant were determined. Both Vmax and Kapp were affected by the net positive charge in loop 3 (Table 3). When net positive charge in loop 3 was reduced from + 5 to + 2 [L3 (N-3) mutant], Vmax decreased to 45% and Kapp increased (apparent affinity for actin decreased) from 23 μM to 81 μM. We could not determine both Vmax and Kapp of L3 (-5) mutant because the activity of L3 (-5) increased almost linearly with actin concentration (Fig. 2). Increase in the net positive charge in loop 3 by adding 2 lysine residues [L3 (+2)] lowered Kapp but Vmax also became lower than that of the wild type (Table 3), suggesting that there might be an optimal amount of positive charge and/or optimal length for loop 3. Actin cosedimentation assays using wild-type and L3 (-5) mutant also supported the effect of the positive charge of loop 3 on the actin affinity. Because Chara myosin has very high actin-activated ATPase activity and most ATP would be depleted during the standard actin cosedimentation assay, we used nonhydrolyzable ATP analog AMP-PNP in our cosedimentation assays. Kd (dissociation constant for actin) of wild type and L3 (-5) were 7.3 μM and 190 μM, respectively. These results showed that the affinity of Chara myosin for actin was largely dependent on the positive charge of loop 3.
Fig. 2.
Actin-activated ATPase activities of wild-type and loop 3 mutants of Chara myosin motor domain. Values are averages of 3 measurements for each mutant preparation. Vmax and Kapp were determined by fitting data to the Michaelis-Menten equation. Averaged Vmax and Kapp values from 2 independent preparations were shown in Table 3. Because the activity of L3 (-5) increased almost linearly with the increase in actin concentration, both Vmax and Kapp values of L3 (-5) could not be determined.
In contrast to the ATPase activity, charge alteration of loop 3 did not affect the velocity so much. For example, Vmax of L3(N-3) was only 45% that of the wild type but its velocity was 90% that of the wild type (Table 3). ATPase activity of L3(-5) was less than 10% that of the wild type at all actin concentrations measured (Fig. 2). Due to very low affinity for actin, L3(-5) could not move actin filaments in the standard buffer for in vitro motility assay. However, in the presence of 0.8% methylcellulose that prevent detachment of actin filaments from the myosin-coated surface (30), the velocity of L3(-5) could be observed, and it was 70% that of the wild type (Table 3).
Loop 2 Mutants.
Loop 2 of Chara myosin is very short and has only 2 positively charged amino acids. Unlike other myosins, these 2 lysine residues are not in a cluster but separated by 3 aa residues (Table 1). We designated a lysine residue at the N-terminal side as “lysine-N” and one at the C-terminal side as “lysine-C”. To estimate the contribution of loop 2 to the actin-activated ATPase activity and velocity of Chara myosin, we made 7 loop 2 mutants (Table 4). L2 (-2) has substitutions to alanine at both lysine-N and lysine-C. L2 (N-1) and L2 (C-1) have substitution at the lysine-N and at the lysine-C, respectively. L2 (N+2) has additional GGKKGG sequence at the N-terminal side of lysine-N. Skeletal and smooth muscle myosins have 2 to 3 successive lysine residues near the C-terminal end of loop 2 (Table 1) and the importance of these lysine residues in the interaction with actin is reported (17, 31). Thus, we intended to generate such a lysine cluster in loop 2 by adding 2 lysine residues (KK) to the C-terminal side of lysine-C and designated this as L2 (C+2). L2 (+4) has GGKKGG at the N-terminal side of lysine-N and KK at the C-terminal side of lysine-C. This mutant has loop 2 that is very similar in charge composition and length to that of skeletal muscle myosin among mutants used in this study. We also made a mutant L2 (N+0) that has GGAAGG at the N-terminal side of lysine-N to see the effect of length change in loop 2 without charge alteration.
Table 4.
Sequences, net charge, and activity of wild type and loop 2 mutants of Chara myosin motor domain
| Sequence* | Net charge | Vmax† (Pi/head/s) | Kapp† (μM) | Velocity‡ (μm/s) | |
|---|---|---|---|---|---|
| L2 (−2) | FPADEG TA APSAFMSIG | −2 | 570 | 103 | 6.2 ± 0.3 |
| L2 (C-1) | FPADEG TK APSAFMSIG | −1 | 690 | 69 | 5.9 ± 0.3 |
| L2 (N-1) | FPADEG TA APSKFMSIG | −1 | 540 | 21 | 4.4 ± 0.3 |
| WT | FPADEG TK APSKFMSIG | 0 | 580 | 23 | 4.8 ± 0.2 |
| L2 (N+0) | FPADEGGGAAGG TK APSKFMSIG | 0 | 520 | 25 | 4.2 ± 0.2 |
| L2 (N+2) | FPADEGGGKKGG TK APSKFMSIG | +2 | 630 | 8.2 | 2.4 ± 0.2 |
| L2 (C+2) | FPADEG TKAPSKKKFMSIG | +2 | 500 | 8.3 | 1.7 ± 0.2 |
| L2 (+4) | FPADEGGGKKGGTKAPSKKKFMSIG | +4 | 470 | 2.8 | 1.1 ± 0.1 |
*Positively charged amino acid and altered amino acid are shown in bold and italic, respectively.
†Values are averages of two measurements on two independent preparations.
‡Values are average ± SD of 40–50 actin filaments on two independent preparations.
Unlike loop 3 mutants, net charge in loop 2 had only limited effect on Vmax (Table 4). However, it dramatically affected the velocity with a concomitant change in Kapp (Table 4). The velocity of L2 (C-1) and L2 (-2) increased by 20 to 30% with the increase in Kapp (a decrease in the apparent affinity for actin), but those of L2 (N-1) were almost the same as those of the wild type (Table 4), suggesting large contribution of lysine-C to the interaction with actin. In contrast, the velocity of L2 (N+2) and L2 (C+2) dropped to 35 to 50% that of the wild type with the decrease in Kapp (Table 4). Addition of 4 lysine residues to Chara loop 2 [L2 (+4)], further lowered the velocity (23% that of the wild type, Table 4) with further decrease in Kapp. Change in the length of loop 2 had little effect on the functional characteristics of Chara myosin [L2 (N+0) mutant, Table 4]. Chara myosin motor domain seems to have allowance for long loop 2.
Kinetics.
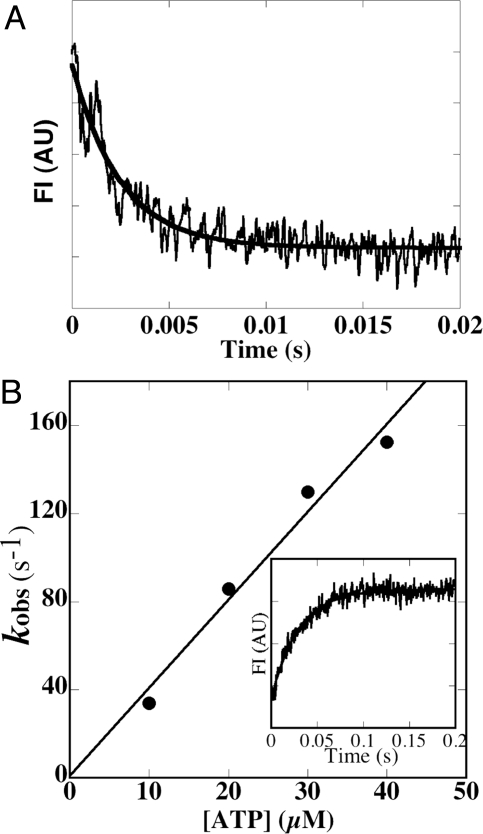
Although charge alteration in loop 2 did not affect the Vmax of the actin-activated ATPase activity so much, we observed obvious effect on the velocity. In particular, the velocity of L2 (+4) was considerably lower than that of the wild type as mentioned above. In general, velocity depends on the time of strongly bound state with actin, which is determined by ADP dissociation rate from acto-myosin·ADP complex and acto-myosin dissociation rate by ATP binding (32, 33). Thus, it is anticipated that the slow movement of L2 (+4) is due to reduction in either of these 2 rates or in both. So we measured the rates of mantADP release from acto-L2 (+4)·mantADP and ATP-induced dissociation of acto-L2 (+4) using stopped flow technique. As shown in Fig. 3A, mantADP release rate from acto-L2 (+4)·mantADP (480 ± 70 s−1) was less than 20% that of the wild type (2800 s−1) (6). In contrast, ATP-induced acto-L2 (+4) dissociation rate (4.0 μM−1 s−1, Fig. 3B) was almost the same as that of the wild type (4.1 μM−1 s−1) (6). These results suggest that low velocity of L2 (+4) is due to slow ADP release from acto-L2 (+4)·ADP.
Fig. 3.
Kinetic analysis of acto-L2 (+4). (A) MantADP dissociation from acto-L2 (+4). A solution containing 1 μM L2 (+4), 20 μM actin and 100 μM mantADP was mixed with 5 mM ATP (final concentration) at 25 °C. MantADP dissociation from acto-MD was monitored using fluorescence energy transfer between tryptophan of motor domain and mantADP. The transient is an average of 5 to 7 separate recordings and the solid line is a single exponential fit, which gave a rate constant (k-ADP) of 406 s−1 in the example shown. Averaged value of 5 independent assays for 2 independent preparations was 480 ± 70 s−1 (n = 5). AU = arbitrary units. (B) ATP-induced dissociation of the pyrene-actin- L2 (+4) complex. Observed rate of ATP-induced dissociation (kobs) at 25 °C was plotted against ATP concentrations (10 to 40 μM). Slope of the plot gave a value for K1k+2 of 4.0 μM−1s−1. (Inset) Representative data when 0.5 μM pyrene-acto- L2 (+4) complex was mixed with 10 μM ATP (final concentration). Single exponential fit to the observed fluorescence change gave a rate constant of 34 s−1 (solid line).
Molecular Design for High Velocity.
It was of our surprise that 2 of loop 2 mutants [L2 (-2) and L2 (C-1)] moved faster than the wild type (Table 4). Because we thought that Chara myosin has evolutionary established elaborated structure to move very fast, we expected that any manipulation of its amino acids sequence would perturb this structure and impair the velocity. Our results that even the fastest myosin could be made to move faster than the wild type suggested that we might be able to make various myosins move faster than their original by removing the positive charge from loop 2 and/or by adding positive charge to loop 3. To test this hypothesis, we manipulated loops 2 and 3 of Dictyostelium myosin. Loop 2 and loop 3 sequences of Dictyostelium myosin are as follows.
Loop 2 612 FNDPNIASRAKKGANFITVA 631
Loop 3 560 EPRFSK 565.
We made 3 mutant myosins. S564K mutant myosin had one additional positive charge in loop 3 and R620A-K622A mutant myosin lost 2 positive charges from loop 2. S564K/R620A-K622A mutant myosin has these both of 2 mutations. Since actin-activated ATPase activity of these mutants and wild-type Dictyostelium myosin increased linearly with actin concentration due to low affinity for actin under our assay condition (34), we compared their ATPase activity at 95 μM (4 mg/ml) actin. Addition of one positive charge to loop 3 (S564K) enhanced the ATPase activity 1.7-fold higher than that of the wild type while leaving the velocity the same (Table 5, S564K). Removing 2 positive charges from loop 2 dramatically decreased the ATPase activity (Table 5, R620A-K622A). The R620A-K622A myosin could not translocate actin filaments probably due to low affinity for actin even in the presence of methylcellulose. While actin filaments attached to the R620A-K622A myosin-coated surface in the absence of ATP, the majority of the actin filaments dissociated on addition of ATP. Some stayed near the surface and exhibited random, lateral motion without making noticeable unidirectional axial movement. Adding one positive charge to loop 3 (S564K) in the R620A-K622A mutant myosin increased ATPase activity 3.8-fold higher than that of the R620A-K622A mutant and could move actin filaments (Table 5, S564K/R620A-K622A). The actin sliding velocity of the S564K/R620A-K622A myosin is 1.3-fold higher than that of wild type.
Table 5.
ATPase activity and velocity of WT and mutant Dictyostelium myosin II
| ATP ase activity* (Pi/head/s) | Velocity (μm/s)† | |
|---|---|---|
| WT | 1.4 ± 0.2 | 2.4 ± 0.2 |
| S564K | 2.4 ± 0.3 | 2.4 ± 0.2 |
| R620A K622A | 0.12 ± 0.01 | 0 |
| S564K/R620A K622A | 0.45 ± 0.06 | 3.1 ± 0.4‡ |
Actin-activated ATPase activity and velocity of wild type and mutant Dictyostelium myosins
*Values are averages ± SD of at least three measurements on two independent preparations. Each value was obtained by subtracted activities in the absence of actin from the measured values in the presence of 95 μM actin.
†Values are averages ± SD of 20–30 actin filaments on two independent preparations.
‡Velocity of S564K/R620A K622A myosin was done in the presence of 0.8% methylcellulose.
Discussion
Role of Loop 3 in the Actin Activation.
Most myosins have positively charged a loop 2 with a cluster of lysine residues (Table 1). Many studies using various myosins suggested that the net charge of loop 2 controls the affinity of myosin for actin (25–29). Unlike other myosins, loop 2 of Chara myosin is very short and the number of positively charged amino acids in it is only 2, which are not in a cluster but separated (Table 1). In contrast, Chara myosin has much more positive net charge in loop 3 than any other myosins (Table 2). In agreement with this sequence feature, our results clearly showed a large contribution of loop 3 of Chara myosin to the Vmax of the actin-activated ATPase activity (Fig. 2 and Table 3). In addition, the affinity of Chara myosin for actin was largely dependent on the positive charge of loop 3, which was shown by both the Kapp of the actin-activated ATPase activity (Fig. 2 and Table 3) and actin-cosedimentation assays (see Results).
As mentioned earlier, the rate-limiting step of acto-Chara myosin ATPase is suggested to be the transition from a weekly bound state with actin to a strongly bound state with concomitant release of Pi (6). Because substitution of positively charged amino acids in loop 3 to alanine lowered Vmax, the interaction with actin through loop 3 seems to play a critical role in triggering the transition from the weakly to the strongly bound state. Results using Dictyostelium mutant myosins also supported the relationship between loop 3 and actin-activated ATPase activity (Table 5). Combination of x-ray crystallographic results with information from electron microscopy revealed that loop 2 interacts with a subdomain 1 of actin molecule at which strong binding through hydrophobic interaction occurs and loop 3 interacts with a subdomain 1 of the next actin molecule (on the barbed-end side) in the same strand of double helical actin filament (35). This simultaneous interaction will make myosin head bind stably with 2 neighboring actin molecules in a filament. This interaction may, at the same time, alter the conformation of myosin the motor domain, which somehow deforms the active site and accelerates phosphate release. Variation in extent and manner of acto-myosin interactions would change the extent of the deformation of the active site. Among myosins so far measured, actin-activated ATPase activity of Chara myosin is the highest and this may be relevant to the fact that the net positive charge in loop 3 of Chara myosin is the largest among all myosins (Table 2).
Effect of Loop 2 Charge on the Velocity.
When the net charge in loop 2 increased, Kapp decreased (the apparent affinity for actin increased) but Vmax was less affected. In the loop 2 mutants, however, the velocity decreased with the decrease in Kapp (Table 4). Decrease in the velocity was caused by the reduction in ADP dissociation rate from acto-Chara myosin motor domain (Fig. 3). Similar results for the effect of positive charge in loop 2 on the velocity were reported for smooth muscle myosin II (25). The inverse correlation between the net charge of loop 2 and the velocity may be a general rule for myosin velocity although an exceptional result was reported for slow myosin with very long loop 2. Addition of positive charges to loop 2 of myosin V did not alter the velocity (29). Since α-helix after loop 2 is connected to a β-strand that surrounds the nucleotide binding site of myosin (36), strong interaction with actin through loop 2 somehow reduces the ADP release rate and thus velocity. It surprised us that 2 of loop 2 mutants moved faster than wild type (Table 4).
Increase in the positive charge in both loop 2 and loop 3 lowered the Kapp similarly, but their electrostatic interaction with actin affected the ATPase activity and the velocity differently. It is probably due to the difference in the stress exerted on the motor domain by their interaction with actin as mentioned above. The affinity for actin can be maintained by positive charge in either loop 2 or loop 3, but affinity increment through loop 2 accompanies reduction in the ADP release rate and thus lowers the velocity (Figs. 3 and 4). It is speculated that Chara myosin has evolved to keep the high affinity for actin through loop 3 and reduce the positive charge in loop 2 to enhance the ADP release rate for its high velocity.
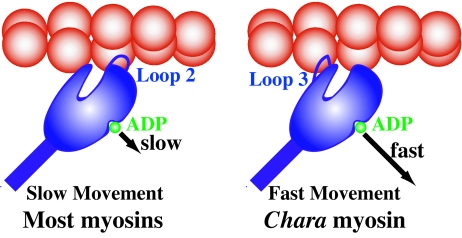
Fig. 4.
Schematic diagram of strategy of Chara myosin for fast movement. For most myosins, the hydrophilic interaction with actin is mainly through loop 2, which slows ADP release and thus velocity (Left). In contrast for Chara myosin, hydrophilic interaction with actin is mainly through loop 3, which does not slow (Right).
Our results suggest that we can make various myosins move faster than the original one by reducing the positive charge from loop 2. To maintain actin affinity of the mutant myosin of which positive charge in loop 2 is decreased, the positive charge in loop 3 should be increased because charge alteration in loop 3 has little effect on velocity. This was also supported by the mutation experiments using Dictyostelium myosin (Table 5). Although the velocity of the Dictyostelium mutant myosin was far lower than that of Chara myosin, suggesting that there is intrinsic difference between Dictyostelium myosin and Chara myosin, the results obtained from different class myosin (class II) proved our hypothesis and supported our view that one of the reasons for the high velocity of Chara myosin is low positive charge in loop 2 and high positive charge in loop 3.
Materials and Methods
Protein Engineering, Expression, and Purification.
A baculovirus transfer vector for Chara myosin motor domain (pFastBac MD) was made as mentioned previously (6). Chara myosin motor domain contains residues 4–746 of the Chara myosin heavy chain, a flexible linker (GGG), a Myc-epitope sequence (EQKLISEEDL), and a (His)8-tag (Molecular weight: 86,676). A baculovirus transfer vector for Chara myosin motor domain with FLAG-tag (pFastBac MD with FLAG-tag) was generated as follows. pFastBac MD was cut with XhoI and SacI (a XhoI-SacI fragment of Chara myosin motor domain). pFastBac MD-Vneck (6) was cut with XhoI and SacI and legated with a XhoI-SacI fragment of pFastBac MD. The resultant protein, Chara myosin motor domain with FLAG-tag, encodes the N-terminal sequence (MSYYHHHHHHDYKDDDDKNIPTTENLYFQGA) containing the sequence of (His)6-tag and FLAG-tag (DYKDDDDK), residues 4–746 of Chara myosin heavy chain, a flexible linker (GGG), a Myc-epitope sequence (EQKLISEEDL) and a (His)8-tag (Molecular weight: 91,761). Chara myosin motor domains with FLAG-tag were L3 (-5), L3 (N-3), L3 (C-2), L2 (-2) and L2 (C-1). Mutations of loop 2, loop 3 of Chara motor domain and Dictyostelium myosins were made by site-directed mutagenesis using ExSite PCR-Based Site-Directed Mutagenesis Kit (Stratagene). Mutant and wild-type motor domains were expressed in insect cells and purified as mentioned previously (6). Mutant motor domains with FLAG-tag were purified as mentioned previously (6), except that Ni-affinity-purification was also done before FLAG-tag affinity purification. Wild-type and mutant Dictyostelium myosins were purified as described (37) and phosphorylated as described (38) using bacterially expressed myosin light chain kinase that carried a T166E mutation (39).
ATPase and in Vitro Velocity Assays.
Steady-state ATPase activities were measured as described (5). The reactions were done in 25 mM KCl, 4 mM MgCl2, 25 mM Hepes-KOH (pH 7.4), 1 mM ATP, 1 mM DTT, and 1 mg/ml BSA and at 30 °C, except for those in Fig. 1, which were done in 10 mM KCl, 4 mM MgCl2, 20 mM Hepes-KOH (pH 7.4), 1 mM ATP, 1 mM DTT, and 1 mg/ml BSA and at 30 °C. The reactions were started by the addition of ATP, except for those of Dictyostelium full length myosin in Table 5, which were started by the addition of myosin as described (34). The velocity of MD was measured using an anti-myc antibody-based version of the in vitro actin filament gliding assay as described (6). The velocity of Dictyostelium myosin was measured as described (37). The velocity of actin filaments was measured in 25 mM KCl, 4 mM MgCl2, 25 mM Hepes-KOH (pH 7.4), 3 mM ATP, 10 mM DTT and oxygen scavenger system (120 μg/ml glucose oxidase, 12.8 mM glucose, and 20 μg/ml catalase) at 25 °C. Methyl cellulose (0.8%) was added to the assay buffer to measure the velocity of L3(-4) and Dictyostelium myosin S564K/R620A-K622A.
Cosedimentation Assays.
Cosedimentation assays were done as described (37). The solution used in the transient kinetic experiments was the same as that used in the steady-state ATPase measurements and in vitro velocity assays, except that nonhydrolyzable ATP analog AMP-PNP was used, instead of ATP.
Transient Kinetic Measurements.
All kinetic experiments were done at 25 °C using an Applied Photophysics SX18MV stopped-flow spectrophotometer (dead time: 1.15 ms). The solution used in the transient kinetic experiments was the same as that used in the steady-state ATPase measurements and in vitro velocity assays. Dissociation of acto-motor domain by ATP was monitored through the change in fluorescence intensity of pyrene-labeled actin stabilized by phalloidin excited at 365 nm and detected after passing through a 389 nm cutoff filter. Dissociation of mantADP from acto-motor domain was monitored by the decrease in its fluorescence. MantADP were excited at 290 nm via fluorescence resonance energy transfer from tryptophan of motor domain and emission was observed after passing through a 389 nm cutoff filter.
Acknowledgments.
We thank Drs. Kazuo Sutoh and Takahide Kon of University of Tokyo, Tokyo, Japan, for allowing us to use a stopped-flow apparatus. This work was supported in part by Grants-in-Aid from the Ministry of Science, Culture and Education of Japan (to K. I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kachar B, Reese TS. The mechanism of cytoplasmic streaming in characean algal cells: Sliding of endoplasmic reticulum along actin filaments. J Cell Biol. 1988;106:1545–1552. doi: 10.1083/jcb.106.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto K, Kikuyama M, Sutoh-Yamamoto N, Kamitsubo E. Purification of actin based motor protein from Chara corallina. Proc Jpn Acad. 1994;70:175–180. [Google Scholar]
- 3.Higashi-Fujime S, et al. The fastest actin-based motor protein from the green algae, Chara, and its distinct mode of interaction with actin. FEBS Lett. 1995;375:151–154. doi: 10.1016/0014-5793(95)01208-v. [DOI] [PubMed] [Google Scholar]
- 4.Kashiyama T, Kimura N, Mimura T, Yamamoto K. Cloning and characterization of a myosin from characean alga, the fastest motor protein in the world. J Biochem. 2000;127:1065–1070. doi: 10.1093/oxfordjournals.jbchem.a022699. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, et al. Recombinant motor domain constructs of Chara corallina myosin display fast velocity and high ATPase activity. Biochem Biophys Res Commun. 2003;312:958–964. doi: 10.1016/j.bbrc.2003.10.202. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, et al. Kinetic mechanism of the fastest motor protein, Chara myosin. J Biol Chem. 2007;282:19534–19545. doi: 10.1074/jbc.M611802200. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Toyoshima N, Hirakawa N, Okamoto K, Ishijima A. A kinetic mechanism for the fast movement of Chara myosin. J Mol Biol. 2003;328:939–950. doi: 10.1016/s0022-2836(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 8.Onishi H, Mikhailenko SV, Morales MF. Toward understanding actin activation of myosin ATPase: The role of myosin surface loops. Proc Natl Acad Sci USA. 2006;103:6136–6141. doi: 10.1073/pnas.0601595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto K, Sekine T. Interaction of myosin subfragment-1 with actin. I. Effects of actin binding on the susceptibility of subfragment-1 to trypsin. J Biochem. 1979;86:1855–1862. doi: 10.1093/oxfordjournals.jbchem.a132708. [DOI] [PubMed] [Google Scholar]
- 10.Mornet D, Pantel P, Audemard E, Kassab R. The limited tryptic cleavage of chymotryptic S-1: An approach to the characterization of the actin site in myosin heads. Biochem Biophys Res Commun. 1979;89:925–932. doi: 10.1016/0006-291x(79)91867-9. [DOI] [PubMed] [Google Scholar]
- 11.Sutoh K. An actin-binding site on the 20K fragment of myosin subfragment 1. Biochemistry. 1982;21:4800–4804. doi: 10.1021/bi00262a043. [DOI] [PubMed] [Google Scholar]
- 12.Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982;21:3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- 13.Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983;22:1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Sekine T. Difference between subfragment-1 and heavy meromyosin in their interaction with F-actin. J Biochem. 1986;99:199–206. doi: 10.1093/oxfordjournals.jbchem.a135460. [DOI] [PubMed] [Google Scholar]
- 15.Chaussepied P. Interaction between stretch of residues 633–642 (actin binding site) and nucleotide binding site on skeletal myosin subfragment 1 heavy chain. Biochemistry. 1989;28:9123–9128. doi: 10.1021/bi00449a025. [DOI] [PubMed] [Google Scholar]
- 16.Mornet D, Ue K, Chaussepied P, Morales MF. Modification of the actin interface of skeletal myosin subfragment-1 by treatment with dibromobimane. Eur J Biochem. 1986;159:555–561. doi: 10.1111/j.1432-1033.1986.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K. Binding manner of actin to the lysine-rich sequence of myosin subfragment 1 in the presence and absence of ATP. Biochemistry. 1989;28:5573–5577. doi: 10.1021/bi00439a035. [DOI] [PubMed] [Google Scholar]
- 18.Cheung P, Reisler E. Synthetic peptide of the sequence 632–642 on myosin subfragment 1 inhibits actomyosin ATPase activity. Biochem Biophys Res Comm. 1992;189:1143–1149. doi: 10.1016/0006-291x(92)92323-p. [DOI] [PubMed] [Google Scholar]
- 19.Bonafe N, Chaussepied P. A single myosin head can be cross-linked to the N termini of two adjacent actin monomers. Biophys J. 1995;68(Suppl 4):35S–43S. [PMC free article] [PubMed] [Google Scholar]
- 20.Andreev OA, Takashi R, Borejdo J. Fluorescence polarization study of the rigor complexes formed at different degrees of saturation of actin filaments with myosin subfragment-1. J Muscle Res Cell Motil. 1995;16:353–367. doi: 10.1007/BF00114501. [DOI] [PubMed] [Google Scholar]
- 21.Andreev OA, Borejdo J. Interaction of the heavy and light chains of cardiac myosin subfragment-1 with F-actin. Circ Res. 1997;81:688–693. doi: 10.1161/01.res.81.5.688. [DOI] [PubMed] [Google Scholar]
- 22.Spudich JA. How molecular motors work. Nature. 1994;372:515–518. doi: 10.1038/372515a0. [DOI] [PubMed] [Google Scholar]
- 23.Goodson HV, Warrick HM, Spudich JA. Specialized conservation of surface loops of myosin: Evidence that loops are involved in determining functional characteristics. J Mol Biol. 1999;287:173–185. doi: 10.1006/jmbi.1999.2565. [DOI] [PubMed] [Google Scholar]
- 24.Uyeda TQP, Ruppel KM, Spudich JA. Enzymatic activities correlate with chimaeric substitutions at the actin-binding face of myosin. Nature. 1994;368:567–569. doi: 10.1038/368567a0. [DOI] [PubMed] [Google Scholar]
- 25.Joel PB, Sweeney HL, Trybus KM. Addition of lysines to the 50/20 kDa junction of myosin strengthens weak binding to actin without affecting the maximum ATPase activity. Biochemistry. 2003;42:9160–9166. doi: 10.1021/bi034415j. [DOI] [PubMed] [Google Scholar]
- 26.Furch M, Geeves MA, Manstein DJ. Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry. 1998;37:6317–6326. doi: 10.1021/bi972851y. [DOI] [PubMed] [Google Scholar]
- 27.Knetsch ML, Uyeda TQ, Manstein DJ. Disturbed communication between actin- and nucleotide-binding sites in a myosin II with truncated 50/20-kDa junction. J Biol Chem. 1999;274:20133–20138. doi: 10.1074/jbc.274.29.20133. [DOI] [PubMed] [Google Scholar]
- 28.Yengo CM, Sweeney HL. Functional role of loop 2 in myosin V. Biochemistry. 2004;43:2605–2612. doi: 10.1021/bi035510v. [DOI] [PubMed] [Google Scholar]
- 29.Hodges AR, Krementsova EB, Trybus KM. Engineering the processive run length of Myosin V. J Biol Chem. 2007;282:27192–27197. doi: 10.1074/jbc.M703968200. [DOI] [PubMed] [Google Scholar]
- 30.Uyeda TQ, Warrick HM, Kron SJ, Spudich JA. Quantized velocities at low myosin densities in an in vitro velocity assay. Nature. 1991;352:307–311. doi: 10.1038/352307a0. [DOI] [PubMed] [Google Scholar]
- 31.Joel PB, Trybus KM, Sweeney HL. Two conserved lysines at the 50/20-kDa junction of myosin are necessary for triggering actin activation. J Biol Chem. 2001;276:2998–3003. doi: 10.1074/jbc.M006930200. [DOI] [PubMed] [Google Scholar]
- 32.Millar NC, Geeves MA. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Letters. 1983;160:141–148. doi: 10.1016/0014-5793(83)80954-5. [DOI] [PubMed] [Google Scholar]
- 33.Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, et al. Filament structure as an essential factor for regulation of Dictyostelium myosin by regulatory light chain phosphorylation. Proc Natl Acad Sci USA. 1998;95:14124–14129. doi: 10.1073/pnas.95.24.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rayment I, et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 36.Rayment I, et al. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Uyeda TQ, Suzuki Y, Sutoh K, Yamamoto K. Requirement of domain–domain interaction for conformational change and functional ATP hydrolysis in myosin. J Biol Chem. 2003;278:31049–31057. doi: 10.1074/jbc.M304138200. [DOI] [PubMed] [Google Scholar]
- 38.Ruppel KM, Uyeda TQ, Spudich JA. Role of highly conserved lysine 130 of myosin motor domain. In vivo and in vitro characterization of site specifically mutated myosin. J Biol Chem. 1994;269:18773–18780. [PubMed] [Google Scholar]
- 39.Smith JL, Silveira LA, Spudich JA. Myosin light chain kinase (MLCK) gene disruption in Dictyostelium: A role for MLCK-A in cytokinesis and evidence for multiple MLCKs. Proc Natl Acad Sci USA. 1996;93:12321–12326. doi: 10.1073/pnas.93.22.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]