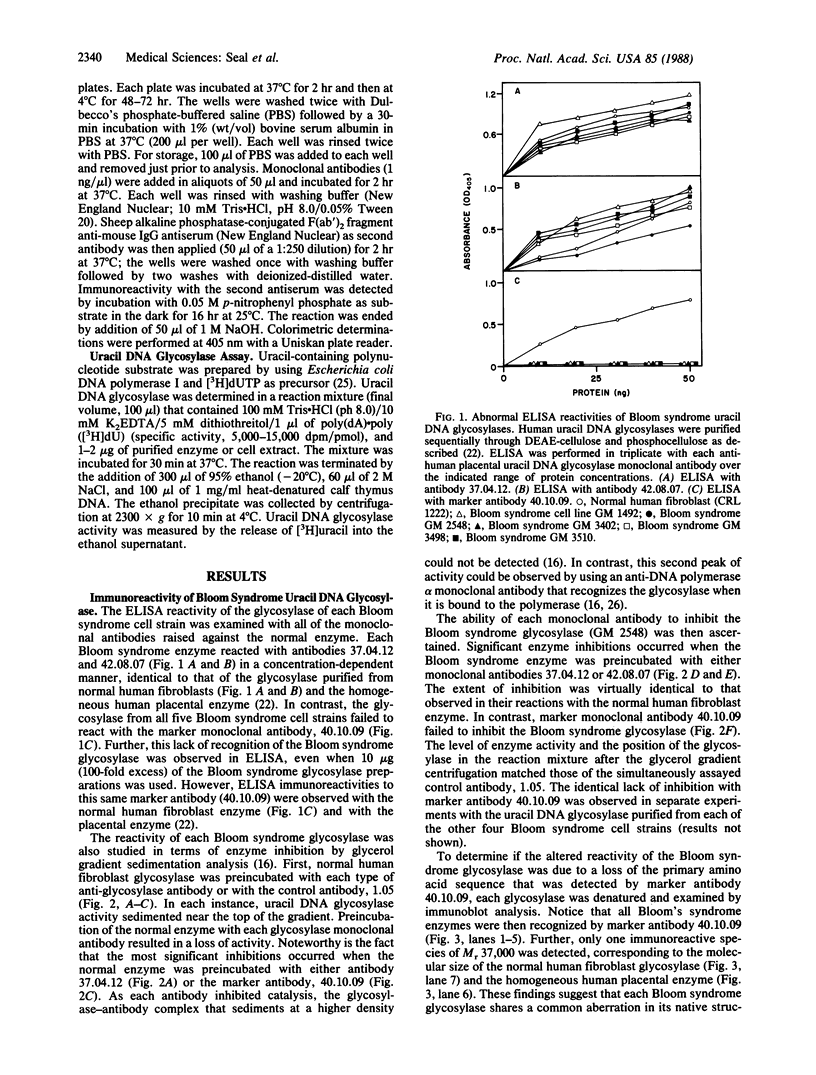
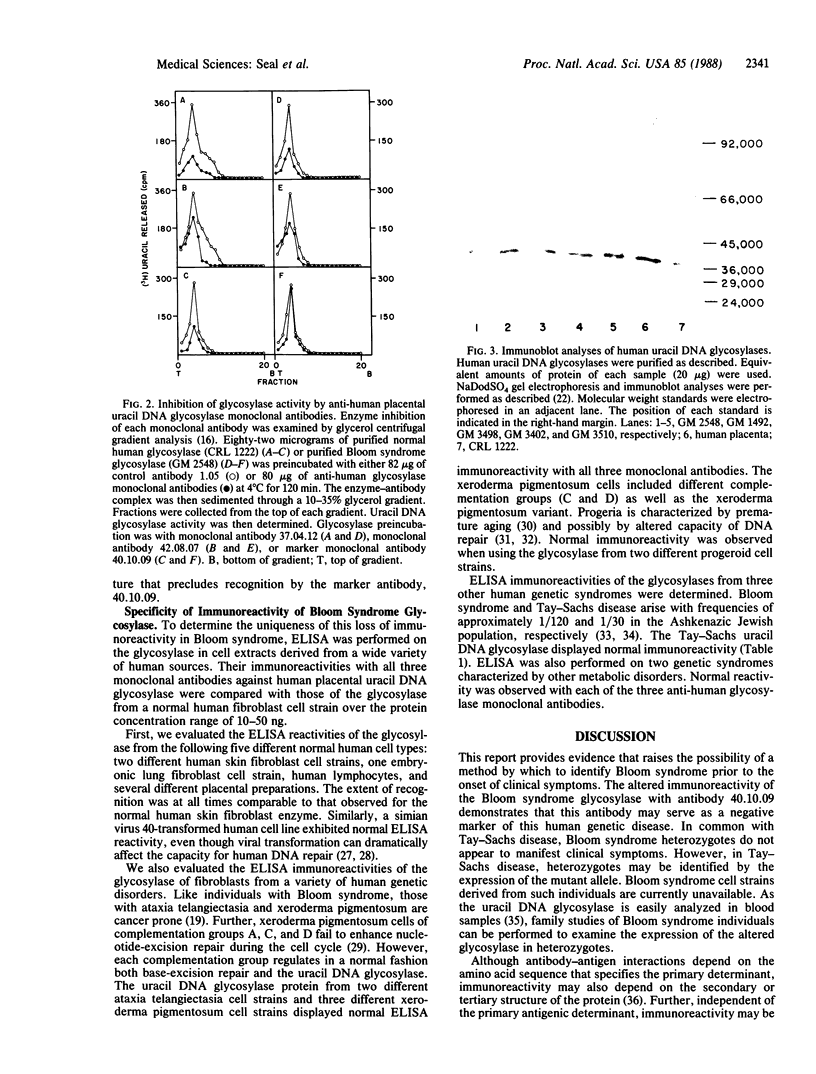
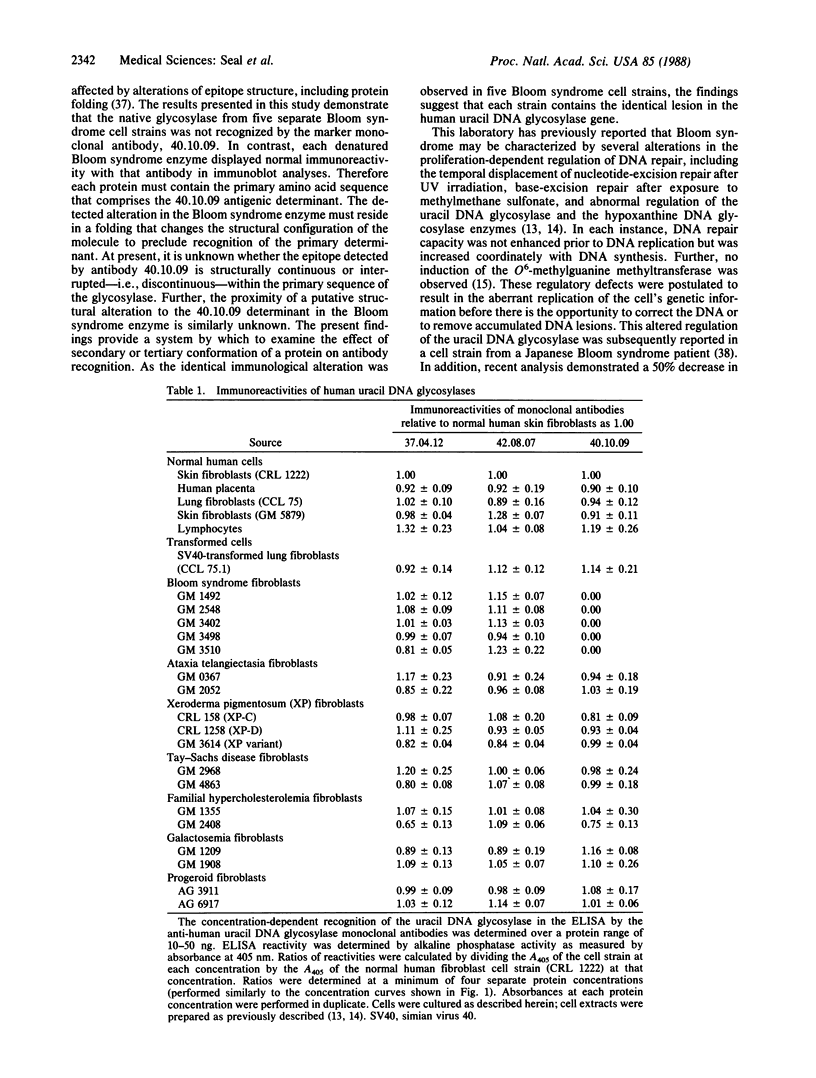
Abstract
Three monoclonal antibodies that react with uracil DNA glycosylase of normal human placenta were tested to determine whether one of the antibodies could be used as a negative marker for Bloom syndrome. As defined by enzyme-linked immunosorbent assay, monoclonal antibody 40.10.09, which reacts with normal human glycosylase, neither recognized nor inhibited native uracil DNA glycosylase from any of five separate Bloom syndrome cell strains. Immunoblot analyses demonstrated that the denatured glycosylase protein from all five Bloom syndrome cell strains was immunoreactive with the 40.10.09 antibody. Further, each native enzyme was immunoreactive with two other anti-human placental uracil DNA glycosylase monoclonal antibodies. In contrast, ELISA reactivity was observed with all three monoclonal antibodies in reactions of glycosylases from 5 normal human cell types and 13 abnormal human cell strains. These results experimentally verify the specificity of the aberrant reactivity of the Bloom syndrome uracil DNA glycosylase. The possibility arises that determination of the lack of immunoreactivity with antibody 40.10.09 may have value in the early diagnosis of Bloom syndrome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenaz P., Sirover M. A. Isolation and characterization of monoclonal antibodies directed against the DNA repair enzyme uracil DNA glycosylase from human placenta. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5822–5826. doi: 10.1073/pnas.80.19.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman M. J., Lehman I. R., Adler J., Zimmerman S. B., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. III. THE INCORPORATION OF PYRIMIDINE AND PURINE ANALOGUES INTO DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond S., Goldberg M. Partly native epitopes are already present on early intermediates in the folding of tryptophan synthase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1147–1151. doi: 10.1073/pnas.84.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D. The syndrome of congenital telangiectatic erythema and stunted growth. J Pediatr. 1966 Jan;68(1):103–113. doi: 10.1016/s0022-3476(66)80426-2. [DOI] [PubMed] [Google Scholar]
- Bryant E. M., Hoehn H., Martin G. M. Normalisation of sister chromatid exchange frequencies in Bloom's syndrome by euploid cell hybridisation. Nature. 1979 Jun 28;279(5716):795–796. doi: 10.1038/279795a0. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- DeBusk F. L. The Hutchinson-Gilford progeria syndrome. Report of 4 cases and review of the literature. J Pediatr. 1972 Apr;80(4):697–724. doi: 10.1016/s0022-3476(72)80229-4. [DOI] [PubMed] [Google Scholar]
- Dehazya P., Sirover M. A. Regulation of hypoxanthine DNA glycosylase in normal human and Bloom's syndrome fibroblasts. Cancer Res. 1986 Aug;46(8):3756–3761. [PubMed] [Google Scholar]
- Epstein J., Williams J. R., Little J. B. Deficient DNA repair in human progeroid cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):977–981. doi: 10.1073/pnas.70.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J. Bloom's syndrome. I. Genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet. 1969 Mar;21(2):196–227. [PMC free article] [PubMed] [Google Scholar]
- German J., Bloom D., Passarge E. Bloom's syndrome XI. Progress report for 1983. Clin Genet. 1984 Feb;25(2):166–174. doi: 10.1111/j.1399-0004.1984.tb00480.x. [DOI] [PubMed] [Google Scholar]
- German J., Bloom D., Passarge E. Bloom's syndrome. V. Surveillance for cancer in affected families. Clin Genet. 1977 Sep;12(3):162–168. doi: 10.1111/j.1399-0004.1977.tb00919.x. [DOI] [PubMed] [Google Scholar]
- German J., Bloom D., Passarge E., Fried K., Goodman R. M., Katzenellenbogen I., Laron Z., Legum C., Levin S., Wahrman Bloom's syndrome. VI. The disorder in Israel and an estimation of the gene frequency in the Ashkenazim. Am J Hum Genet. 1977 Nov;29(6):553–562. [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Altered temporal expression of DNA repair in hypermutable Bloom's syndrome cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):757–761. doi: 10.1073/pnas.81.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Regulation of DNA repair in serum-stimulated xeroderma pigmentosum cells. J Cell Biol. 1984 Oct;99(4 Pt 1):1275–1281. doi: 10.1083/jcb.99.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Goldstein S. Diphtheria toxin resistance in human fibroblast cell strains from normal and cancer-prone individuals. Mutat Res. 1980 Dec;73(2):331–338. doi: 10.1016/0027-5107(80)90198-0. [DOI] [PubMed] [Google Scholar]
- Heddle J. A., Arlett C. F. Untransformed xeroderma pigmentosum cells are not hypersensitive to sister-chromatid exchange production by ethyl methanesulphonate--implications for the use of transformed cell lines and for the mechanism by which SCE arise. Mutat Res. 1980 Aug;72(1):119–125. doi: 10.1016/0027-5107(80)90227-4. [DOI] [PubMed] [Google Scholar]
- Hook G. J., Kwok E., Heddle J. A. Sensitivity of Bloom syndrome fibroblasts to mitomycin C. Mutat Res. 1984 May-Jun;131(5-6):223–230. doi: 10.1016/0167-8817(84)90029-4. [DOI] [PubMed] [Google Scholar]
- Kim S., Vollberg T. M., Ro J. Y., Kim M., Sirover M. A. O6-methylguanine methyltransferase increases before S phase in normal human cells but does not increase in hypermutable Bloom's syndrome cells. Mutat Res. 1986 Feb;173(2):141–145. doi: 10.1016/0165-7992(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Koistinen P., Vilpo J. A. Uracil-DNA glycosylase activity in human blood cells. Mutat Res. 1986 Jan-Feb;159(1-2):99–102. doi: 10.1016/0027-5107(86)90117-x. [DOI] [PubMed] [Google Scholar]
- Krepinsky A. B., Rainbow A. J., Heddle J. A. Studies on the ultraviolet light sensitivity of Bloom's syndrome fibroblasts. Mutat Res. 1980 Feb;69(2):357–368. doi: 10.1016/0027-5107(80)90100-1. [DOI] [PubMed] [Google Scholar]
- Lambert W. C., Lambert M. W. Co-recessive inheritance: a model for DNA repair, genetic disease and carcinogenesis. Mutat Res. 1985 May;145(3):227–234. doi: 10.1016/0167-8817(85)90031-8. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. DNA repair in human progeroid cells. Biochem Biophys Res Commun. 1974 Aug 5;59(3):858–864. doi: 10.1016/s0006-291x(74)80058-6. [DOI] [PubMed] [Google Scholar]
- Seal G., Arenaz P., Sirover M. A. Purification and properties of the human placental uracil DNA glycosylase. Biochim Biophys Acta. 1987 Aug 13;925(2):226–233. doi: 10.1016/0304-4165(87)90113-9. [DOI] [PubMed] [Google Scholar]
- Seal G., Sirover M. A. Physical association of the human base-excision repair enzyme uracil DNA glycosylase with the 70,000-dalton catalytic subunit of DNA polymerase alpha. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7608–7612. doi: 10.1073/pnas.83.20.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsky C. A., Henson P., Weichselbaum R. R., Little J. B. Defective reactivation of ultraviolet light-irradiated herpesvirus by a Bloom's syndrome fibroblast strain. Cancer Res. 1979 Sep;39(9):3392–3396. [PubMed] [Google Scholar]
- Shapiro R., Klein R. S. The deamination of cytidine and cytosine by acidic buffer solutions. Mutagenic implications. Biochemistry. 1966 Jul;5(7):2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]
- Teebor G. W., Frenkel K. The initiation of DNA excision-repair. Adv Cancer Res. 1983;38:23–59. doi: 10.1016/s0065-230x(08)60186-4. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Lehmann A. R., Müller R., Rajewsky M. F. Similar rate of O6-ethylguanine elimination from DNA in normal human fibroblast and xeroderma pigmentosum cell strains not transformed by SV40. Carcinogenesis. 1983 Aug;4(8):1075–1077. doi: 10.1093/carcin/4.8.1075. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Evans H. J., Ray J. H., German J. Bloom's syndrome: evidence for an increased mutation frequency in vivo. Science. 1983 Aug 26;221(4613):851–853. doi: 10.1126/science.6879180. [DOI] [PubMed] [Google Scholar]
- Vollberg T. M., Seal G., Sirover M. A. Monoclonal antibodies detect conformational abnormality of uracil DNA glycosylase in Bloom's syndrome cells. Carcinogenesis. 1987 Nov;8(11):1725–1729. doi: 10.1093/carcin/8.11.1725. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Fujiwara Y. Abnormal regulation of uracil-DNA glycosylase induction during cell cycle and cell passage in Bloom's syndrome fibroblasts. Carcinogenesis. 1986 Feb;7(2):305–310. doi: 10.1093/carcin/7.2.305. [DOI] [PubMed] [Google Scholar]