Abstract
Biological oscillation occurs at various levels, from cellular signaling to organismal behaviors. Mathematical modeling has allowed a quantitative understanding of slow oscillators requiring changes in gene expression (e.g., circadian rhythms), but few theoretical studies have focused on the rapid oscillation of cellular signaling. The tobacco pollen tube, which exhibits growth bursts every 80 s or so, is an excellent system for investigating signaling oscillation. Pollen tube growth is controlled by a tip-localized ROP1 GTPase, whose activity oscillates in a phase about 90 degrees ahead of growth. We constructed a mathematical model of ROP1 activity oscillation consisting of interlinking positive and negative feedback loops involving F-actin and calcium, ROP1-signaling targets that oscillate in a phase about 20 degrees and 110 degrees behind ROP1 activity, respectively. The model simulates the observed changes in ROP1 activity caused by F-actin disruption and predicts a role for calcium in the negative feedback regulation of the ROP1 activity. Our experimental data strongly support this role of calcium in tip growth. Thus, our findings provide insight into the mechanism of pollen tube growth and the oscillation of cellular signaling.
Keywords: model, ROP1 GTPase, tip growth
Oscillation is a fundamental mechanism underlying the homeostasis, efficiency, and robustness of many biological processes (1–4). An interesting question is whether there exists a common design principle for highly diverse biological oscillation phenomena, ranging from circadian rhythms in various organisms to rapid cyclic changes in signaling events, such as cellular calcium. It has been shown that oscillations with relatively long periods, such as circadian clocks, involve feedback-mediated oscillatory changes in the transcription of key transcription factors (3–5); however, some oscillatory changes are independent of gene expression, such as biochemical metabolism (6), cellular signaling (7), calcium fluxes (8), and cytoskeletal dynamics–mediated cell movement (9, 10).
In plants, to deliver sperm to the ovule for fertilization, pollen tubes rapidly elongate by tip growth in an oscillatory manner, with a period ranging from 10 seconds to a few minutes (2). Several ions (e.g., Ca2+, H+, K+) and signaling events in the apical region oscillate with the same period of growth oscillation in pollen tubes (2). Cytosolic calcium forms a steep tip-focused gradient, the elimination of which leads to growth arrest (11, 12). Tip-localized calcium has been proposed to regulate tip growth by regulating local exocytosis (13, 14); however, the peaks of the cytoplasmic calcium level lag behind those of growth rates in the oscillating pollen tubes (15), suggesting that the apical calcium may have other roles.
Another key oscillator in pollen tubes is tip-localized ROP1 GTPase (1), a central regulator of pollen tube growth (16–18). Interestingly, apical ROP1 activity oscillates in a phase ≈90 degrees ahead of growth and 120 degrees ahead of the apical calcium (1). ROP1 activates 2 downstream pathways that check and balance each other: RIC4-dependent assembly of tip-localized actin microfilaments (F-actin) and RIC3-mediated accumulation of apical calcium (19).
In this study, we developed 2 mathematical models to simulate the interaction between the RIC4–F-actin and the RIC3–calcium pathways and experimentally tested these models. Our findings strongly support a role for the RIC4–F-actin pathway in the positive feedback regulation of ROP1 signaling and suggest a new role for calcium in the negative feedback regulation of ROP1 signaling. We also establish a molecular framework underlying the oscillation of Rho GTPase signaling in the control of polarized cell growth.
Results
Model Structure.
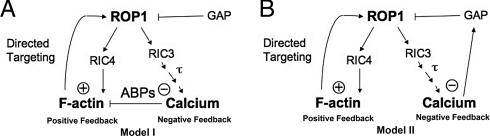
To model the oscillation of the apical ROP1 activity, we proposed a general structure of ROP1 oscillation consisting of F-actin–mediated positive and calcium-mediated negative feedback loops (Fig. 1). Some previous observations support a role for apical F-actin in the positive feedback loop. First, F-actin oscillates in a phase only slightly behind ROP1 activity (1). Second, overexpression of the ROP1 effector RIC4, which promotes accumulation of apical F-actin, induces a significant increase in ROP1 activity (1, 19). Third, RIC4-induced ROP1 activation is suppressed by latrunculin B, an actin-depolymerizing drug (1, 19). Apical F-actin promotes the apical targeting of exocytic vesicles (13, 20), which may carry positive regulators of ROP1 to the apical plasma membrane (PM). We assumed that the positive feedback loop could reach saturation, due in part to the limited amount of ROP1 available for its activation. The cellular concentrations of Rho-family proteins (e.g., Rho, Rac, Cdc42) have an estimated range of 2.4–7.5 μM (21, 22). Given the conserved functions of Rho proteins (including ROPs), we reasoned that the concentration of ROPs in pollen tubes would fall within this range. Accordingly, the upper limit of ROP1 concentration in the PM was assumed to be 5 μM.
Fig. 1.
Structure of the ROP1 oscillation models. The oscillation models consist of 2 major feedback loops: the F-actin–mediated positive feedback loop (indicated by “+”) and the calcium-dependent negative feedback loop (indicated by “−”). Compared with an increase in ROP1 activation rate, an increase in the rate of calcium accumulation in the tip is delayed, which is described as a time delay, τ, in the models. Calcium is assumed to participate in the negative feedback regulation of ROP1 activity by 1 of 2 mechanisms. In model I, calcium signaling inhibits ROP1 activation by promoting F-actin depolymerization through the activation of actin-binding proteins (ABPs). In model II, calcium signaling promotes ROP1 deactivation by activating ROP1-negative regulators, such as RhoGAP.
Another key assumption is an essential role for calcium in the negative feedback regulation of ROP1 activity. Increases in apical calcium level depend on ROP1 activation of the RIC3 downstream pathway (17, 19) and lag behind those in the apical ROP1 by ≈120 degrees (1). This suggests a time delay between ROP1 activation and the calcium increase. This delay can be explained by multiple biochemical and physical steps between ROP1 activation and calcium accumulation, because the ROP1 effector RIC3 is not a calcium channel, but acts as a scaffolding protein (19, 23). Once activated, RIC3 may trigger extracellular calcium influxes, which in turn may activate calcium release from the internal calcium pool. We assumed that the rising calcium level leads to ROP1 inactivation, which is consistent with several previous observations. First, RIC3 overexpression suppresses the overaccumulation of apical active ROP1 induced by RIC4 overexpression (1, 19). RIC3 suppression of the RIC4 overexpression phenotype is calcium-dependent and is not due to competition between these 2 ROP1 effectors (1, 19). Second, ROP1-dependent pollen tube elongation is suppressed when extracellular calcium exceeds a threshold level (17, 19). Third, the loss-of-function mutant of ACA9, which encodes a PM-localized calcium pump, is impaired in pollen tube growth (24) and has decreased localization of ROP1 to the apical PM [supporting information (SI) Fig. S1].
We consider 2 possible modes of calcium action in the negative feedback loop (Fig. 1). Calcium might promote the disassembly of the apical F-actin, leading to the down-regulation of ROP1 activity by countering the F-actin–mediated positive feedback (Fig. 1A). Calcium is known to regulate several actin-binding proteins (25–27). Alternatively, calcium signaling might down-regulate ROP1 activity by activating negative regulators of ROP1 (Fig. 1B). In Arabidopsis pollen tubes, apically localized REN1 RhoGAP participates in the negative feedback regulation of ROP1 and may be subject to regulation by calcium (28, 29).
Modeling ROP1 Activity Oscillation and Its Phase Relationship With Calcium Oscillation.
We formulated equations for the 2 different possible modes of calcium-dependent negative feedback. The biological contexts and values of the parameters considered are listed in Tables 1 and 2.
Table 1.
Parameter setting for standard oscillation of ROP activity in model I
| Parameter | Biological meaning (unit) | Value |
|---|---|---|
| βx | Parameter for ROP1 activation rate (s−1) | 0.1 |
| βy | Parameter for calcium accumulation rate (s−1) | 0.25 |
| αx | ROP1 inactivation rate (s−1) | 0.25 |
| αy | Calcium depletion rate (s−1) | 0.05 |
| x0 | Initial value for active ROP1 concentration (μM) | 0.1 |
| y0 | Initial value for calcium concentration (μM) | 0.8 |
| R0 | Parameter for saturated ROP1 concentration in cell (μM) | 5 |
| C0 | Parameter for high calcium threshold to decrease F-actin (μM) | 3 |
| τ | Time delay between ROP1 and calcium accumulation (s) | 8 |
| k | Parameter for ROP1 and calcium-dependent F-actin level (1) | 5 |
| b | Parameter for calcium accumulation (μM) | 0.05 |
Table 2.
Parameter setting for standard oscillation of ROP activity in model II
| Parameter | Biological meaning (unit) | Value |
|---|---|---|
| βx | Parameter for ROP1 activation rate (s−1) | 0.1 |
| βy | Parameter for calcium accumulation rate (s−1) | 0.25 |
| αx | ROP1 inactivation rate (s−1) | 0.8 |
| αy | Calcium depletion rate (s−1) | 0.05 |
| x0 | Initial value for active ROP1 concentration (μM) | 0.1 |
| y0 | Initial value for calcium concentration (μM) | 0.8 |
| R0 | Parameter for saturated ROP1 concentration in cell (μM) | 5 |
| k | Parameter for ROP1-dependent F-actin level (1) | 2.2 |
| b | Parameter for basal calcium increase (μM) | 0.05 |
| τ | Time delay between ROP1 and calcium accumulation (s) | 8 |
| M | Parameter for piecewise activation of GAP by calcium (1) | 10 |
| ymin | Parameter for piecewise activation of GAP by calcium (μM) | 0.5 |
| ymax | Parameter for piecewise activation of GAP by calcium (μM) | 5 |
Equations for model I (calcium promotion of actin disassembly):
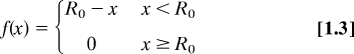 |
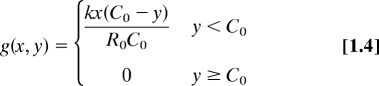 |
Equations for model II (calcium modulation of ROP regulators):
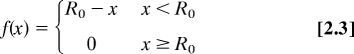 |
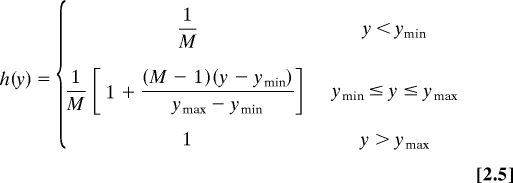 |
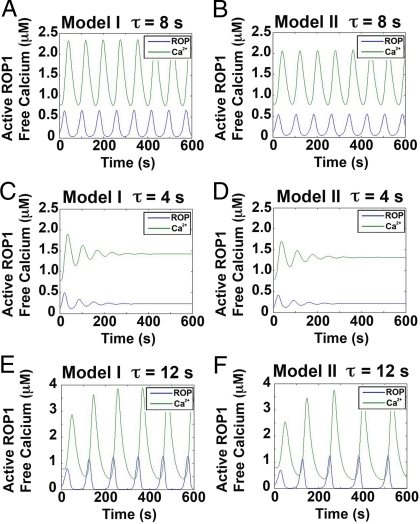
We found that both models could reproduce observed oscillations of ROP1 activity and apical calcium concentration (Fig. 2 A and B). Both ROP1 activity and calcium level show sustained oscillation with periods around 80 s. The amplitudes for ROP1 activity and apical calcium level are 0–0.6 μM and 0.8–2.2 μM, respectively. Calcium level oscillation lags behind ROP1 activity oscillation by ≈120 degrees; thus, these models simulate typical ROP1 activity and calcium oscillations observed in tobacco pollen tubes (1, 15). The interlinked positive and negative feedback loops of ROP1 activity do not automatically lead to the oscillations of ROP1 activity and calcium level. An important factor in these oscillation patterns is the time delay, τ, between ROP1 activity and increased calcium level. At a τ of 8 s, our model reproduced the observed oscillation pattern for tobacco pollen tubes with an oscillation period of ≈80 s. A short time delay (τ ≤ 4 s) will destabilize the oscillation of ROP1 activity and calcium level (Fig. 2 C and D). An overly long time delay (τ ≥ 12 s) will significantly prolong the oscillation period (Fig. 2 E and F).
Fig. 2.
Simulating the effect of time delay on the oscillation of apical ROP1 activity based on models I and II. (A and B) Given a proper time delay (τ = 8 s), model I (A) and model II (B) can reproduce the observed oscillation pattern for both active ROP1 and calcium. The simulation generates oscillation periods of ≈80 s and amplitudes of 0–0.6 μM for active ROP1 and 0.8–2.2 μM for calcium. Calcium oscillation lags behind ROP1 activity oscillation by ≈120 degrees. Parameter settings are given in Tables 1 and 2. (C and D) With short time delays (τ = 4 s), ROP1 activity and calcium oscillations are unstable. (E and F) With long time delays, the oscillation period is increased significantly, reaching ≈120 s at τ = 12 s.
As a negative regulator of ROPs, RhoGAP is critical for polarized pollen tube growth (28). The simulation of our models predicted that ROP1 activity oscillation is highly sensitive to increasing amounts of RhoGAP (Fig. S2). To experimentally test this prediction, we overexpressed different dosages of RopGAP1. We found that increasing amounts of RopGAP1 significantly suppressed ROP1 activity oscillation, as predicted from our models (Fig. S2).
Simulation and Experimental Validation of the Effect of F-Actin on ROP1 Activity Oscillation.
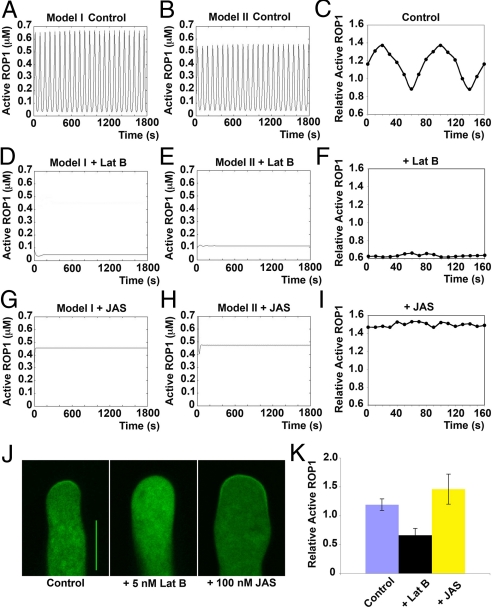
To test our models in terms of the role of F-actin–mediated positive feedback, we simulated the effects of depleting and stabilizing apical F-actin. Our simulations predicted that severe depletion of apical F-actin would lead to a dramatic reduction of ROP1 activity to a basal level (< 0.1 μM) and the termination of its oscillation (Fig. 3 D and E), and that moderate depletion of apical F-actin would dampen ROP1 activity oscillation by reducing the oscillation amplitude (Fig. S3 C and D). We tested these predictions by experimentally manipulating the apical F-actin level. By sequestering G-actin (monomeric actin), latrunculin B promotes F-actin depolymerization (1, 30). Treatment of tobacco pollen tubes with 5 nM latrunculin B caused a significant decrease in the amount of apical F-actin and subsequent inhibition of tip growth (31). None of the 30 observed pollen tubes treated with 5 nM latrunculin B exhibited a detectable active ROP1 cap and oscillation of apical ROP1 activity (Fig. 3 F, J, and K), whereas 6 of 10 control tubes exhibited normal oscillation (Fig. 3 C, J, and K). We previously showed that the oscillation amplitude of ROP1 activity is consistently reduced by treatment with 0.5 nM latrunculin B (1).
Fig. 3.
Simulation and experimental validation of the effect of F-actin on ROP1 activity oscillation. (A and B) Simulation of ROP1 activity oscillation in control pollen tubes using model I (A) and model II (B). (C) Experimental measurement of ROP1 activity oscillation in control pollen tubes. The distribution of GFP-RIC4ΔC to the apical PM indicates the apical ROP1 activity (1). Relative ROP1 activity was determined as the ratio of mean PM GFP intensity to mean cytosolic GFP intensity (1). Shown is a typical oscillating pollen tube with an amplitude of 0.5 (from 0.9 to 1.4) and a period of ≈80 s. Six out of 10 pollen tubes exhibited this oscillation pattern. (D and E) Simulations predicted that the apical ROP1 activity would be maintained at very low levels and would not oscillate in pollen tubes treated with 5 nM latrunculin B (Lat B). For model I, k = 3. For model II, k = 1.3. (F) Experimental validation of the predictions in (D) and (E). In tubes treated with 5 nM Lat B, GFP-RIC4ΔC was depleted from the apical PM, and the relative ROP1 activity was consistently below 0.8. Shown is a representative of 30 tubes examined, all of which exhibited low and nonoscillating ROP1 activity. (G and H) Simulations predicted that the relative ROP1 activity would remain high and lose oscillation in pollen tubes treated with 100 nM jasplakinolide (JAS). Under this condition, F-actin is maintained at a high level. For model I, g(x,y) = 0.25. For model II, g(x) = 0.44. (I) Experimental validation of the predictions in (G) and (H). The relative ROP1 activity remained above 1.3. Shown is a representative of 20 tubes examined, all of which demonstrated high and nonoscillating ROP1 activity. (J) Representative snapshot images of GFP-RIC4ΔC localization in control, Lat B–treated, and JAS-treated pollen tubes. (Scale bar: 10 μm.) (K) Quantitative analysis of the relative ROP1 activity in control, Lat B–treated, and JAS-treated pollen tubes. Shown are mean relative ROP1 activity measured from snapshot images (n = 20; P < .05, Student t-test). Error bars indicate SD.
We next investigated the effect of the F-actin–stabilizing drug jasplakinolide. Our models predicted that the stabilization of apical F-actin would increase nonoscillating ROP1 activity to high levels (Fig. 3 G and H). Indeed, we found that tobacco pollen tubes treated with 100 nM jasplakinolide exhibited sustained high levels of apical ROP1 activity that did not oscillate (n = 20) (Fig. 3 I, J, and K). Based on our data, we conclude that apical F-actin provides positive feedback regulation of apical ROP1 activity and thus is crucial to the oscillation of ROP1 activity.
Simulation and Experimental Validation of the Effect of Calcium on ROP1 Activity.
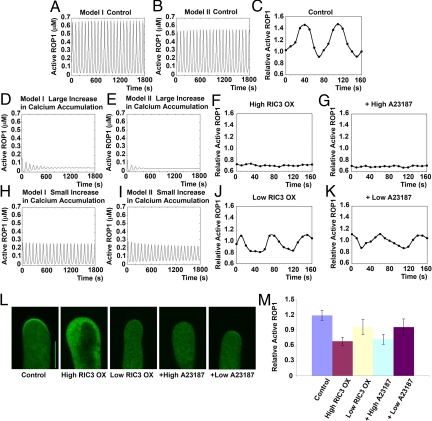
In our models, apical cytosolic calcium plays a critical role in the negative feedback loop of ROP1 activity control. To validate this, we simulated and experimentally tested the effect of calcium on ROP1 activity using several means of perturbing apical calcium. The simulation predicted that a significantly increased rate of calcium accumulation would persistently suppress ROP1 activity to a minimal level (about 0.05 μM) and rapidly lose its oscillation (Fig. 4 D and E). With a moderate increase in rate of calcium accumulation, ROP1 activity would be moderately reduced and oscillate with smaller amplitudes (Fig. 4 H and I). To test these predictions, we first examined the effect of RIC3 overexpression on ROP1 activity. RIC3 is a ROP1 effector responsible for the ROP1-dependent calcium accumulation, and RIC3 overexpression increases calcium accumulation in pollen tube tips (19). Appropriately, 60% of control tubes exhibited a typical oscillation pattern. When pollen was transformed with 0.1 μg of RIC3 plasmid construct, apical active ROP1 was depleted (n = 30), and no ROP1 activity oscillation was seen in these pollen tubes (Fig. 4 F, L, and M). When 0.02 μg of RIC3 construct was used, 5 of the 20 observed pollen tubes exhibited oscillation in ROP1 activity with reduced amplitudes (Fig. 4 J, L, and M), while the remaining tubes did not oscillate.
Fig. 4.
Simulation and experimental validation of the effect of calcium on ROP1 activity oscillation. (A and B) Simulation of ROP1 activity oscillation in control pollen tubes using model I (A) and model II (B). (C) Experimental measurement of ROP1 activity oscillation in control pollen tubes. Relative ROP1 activity was measured as described in Fig. 3. Shown is a typical oscillating pollen tube with an amplitude of 0.5 (from 0.9 to 1.4) and a period of ≈80 s. (D and E) Simulations from both models I and II predicted that apical ROP1 activity would remain low and lose oscillation in pollen tubes, in which the calcium accumulation rate is greatly increased. For both models, βy = 0.75 s−1. (F) Time-course analysis of the relative ROP1 activity in a pollen tube expressing a high level of RIC3. Here 0.1 μg of RIC3 plasmid DNA was used for transformation. Shown is a representative of 30 tubes examined, all of which exhibited low and nonoscillating relative ROP1 activity (< 0.8). (G) Time-course analysis of relative ROP1 activity in a pollen tube treated with 1 μM A23187 ionophore (high A23187). Shown is a representative of 20 tubes examined, all of which exhibited low and nonoscillating relative ROP1 activity (< 0.8). (H and I) Simulations from both models I and II predicted that apical ROP1 activity could maintain oscillation but with much smaller amplitudes in pollen tubes with a moderately increased calcium accumulation rate. For model I, βy = 0.5 s−1. For model II, βy = 0.4 s−1. (J) Time-course analysis of relative ROP1 activity in a pollen tube expressing a low level of RIC3. Here 0.02 μg of RIC3 was used for transformation. Relative ROP1 activity oscillates with similar periods but smaller amplitudes (0.3, from 0.8 to 1.1) compared with control tubes. Shown is a representative of 5 tubes examined that exhibited a similar pattern. (K) Time-course analysis of relative ROP1 activity in a pollen tube treated with 10 nM A23187 ionophore (low A23187). Relative ROP1 activity oscillated with similar period but smaller amplitude (0.2, from 0.9 to 1.1). Shown is a representative of 3 tubes examined that all exhibited a similar pattern. (L) Representative snapshot images of GFP-RIC4ΔC localization in various treated tubes. (Scale bar: 10 μm.) (M) Quantitative analysis of relative ROP1 activity in various treated pollen tubes. Shown is mean relative ROP1 activity measured from snapshot images (n = 20; P < .05, Student t-test). Error bars indicate SD.
We next manipulated an increase in cytosolic calcium level by treating tobacco pollen tubes with A23187, a calcium ionophore that causes leakage of extracellular calcium into cells. We treated pollen tubes with different levels of A23187 to examine their effects on ROP1 activity in tobacco pollen tubes. With 1 μM A23187 treatment, the active ROP1 cap was depleted (n = 20), and no ROP1 activity oscillation was seen in these pollen tubes (Fig. 4 G, L, and M). With 10 nM A23187 treatment, 3 out of 20 observed pollen tubes exhibited oscillation in ROP1 activity, with lower levels and reduced amplitudes (Fig. 4 K, L, and M). Consistent with these results, we found that pollen tubes from an aca9–2 knockout mutant had greatly reduced protein in the apical PM (Fig. S1). These results lend further support to our model's prediction that high calcium levels in pollen tubes will inhibit ROP1 activity and dampen its oscillation.
Finally, we predicted that a reduction in cytosolic calcium levels would significantly increase apical ROP1 activity and eliminate its oscillation (Fig. S4 D and E). We tested this prediction by treating pollen tubes with LaCl3, a calcium channel blocker that has been shown to lower cytosolic calcium levels in pollen tubes (32). When treated with 10 μM LaCl3, the pollen tubes maintained a high level of apical ROP1 activity (> 1.5) but lost its oscillation (Fig. S4 F–H; n = 20). Thus, all of our experimental data agree with our model predictions and support an important role for calcium in the negative feedback regulation of the apical ROP1 activity and its oscillation in pollen tubes.
Discussion
Our mathematical modeling provides insight into the mechanism underlying oscillatory pollen tube growth and Rho GTPase–dependent signaling circuitry. Together, our theoretical and experimental results suggest that ROP1 activity oscillation depends on downstream ROP1 signaling events, F-actin assembly and calcium accumulation, which provide positive and negative feedback regulation of ROP1, respectively. Our results strongly support the existence of oscillatory signaling circuitries that depend on the highly conserved Rho GTPase family. This may have implications for the understanding of Rho GTPase-mediated processes in other systems. Rho-family GTPases control cellular processes involving cell polarization and/or oscillatory behaviors in various eukaryotic cells, such as oscillatory cell movement (9, 10). Similar oscillatory Rho GTPase signaling, as we show here for polarized pollen tube growth, might regulate the oscillation of other Rho-dependent processes as well. Furthermore, the ROP1 signaling circuit provides a mechanism for calcium oscillation, which serves as an important temporal calcium signal. Given the conservation of both Rho GTPases and calcium oscillation, it is tempting to speculate that Rho GTPase–mediated calcium oscillation also might exist in other systems.
Our findings suggest a key role for calcium in the negative feedback regulation of ROP1 activity, which may explain the long-standing dilemma in the field of pollen tip growth of the oscillatory accumulation of calcium at the cell tip lagging behind the increase in tip growth rate, given calcium's essential role in tip growth. Our modeling predicts that either mode of calcium-dependent negative feedback—calcium promotion of F-actin depolymerization to counter F-actin–mediated positive feedback or calcium activation of ROP1 inactivators, such as RopGAPs—is sufficient for the generation of oscillating ROP1 activity. Calcium sensors, such as calcium-dependent protein kinases, are important for pollen tube growth (33, 34) and may play a role in negative feedback regulation. Future studies of such calcium sensors should help elucidate the mechanisms by which calcium feedback regulates ROP1 signaling in pollen tubes. Future research also should focus on modeling the linkage between calcium- and F-actin–mediated feedback regulation and the polarization of ROP1 activity.
Materials and Methods
Plant Materials and Growth Conditions.
Seeds of the aca9–2 homozygote mutant were kindly provided by Dr. Jeffrey Harper (24). Wild-type Arabidopsis plants of the WS ecotype were used as control plants. Arabidopsis plants were grown under a 16 h:8 h (light:dark) period at 20 °C and were used as the source for pollen for immunostaining experiments. Wild-type tobacco (Nicotiana tabacum) plants were grown under a 12 h:12 h (light:dark) period at 25 °C.
Detection of ROP1 Localization Using Indirect Immunofluorescence Microscopy.
Pollen grains of Arabidopsis flowers were germinated in solid germination medium for 4 h at room temperature and then immunostained with Anti-Rop1Ps polyclonal antibody and FITC-conjugated goat anti-rabbit IgG antibody (Sigma), as described previously (28). Mounted pollen tubes from the WS wild-type background and aca9–2 mutant background were observed under a Leica SP2 confocal microscope. Median planes of pollen tubes were scanned to reveal the subcellular localization pattern of ROP1 protein.
Bollistics-Mediated Transient Expression in Tobacco Pollen.
Three DNA constructs described previously (1, 23) were used for bollistics-mediated transient expression: pLAT52:RhoGAP1, pLAT52:RIC3 and the GFP-based ROP1 activity marker pLAT52:GFP-RIC4ΔC. DNA constructs were amplified in the Escherichia coli strain DH5α and purified using the QiagenPlasmid Mini Kit according to the manufacturer's instructions.
Particle bombardment–mediated transient expression in tobacco pollen was performed as described previously (1). Here 0.1 μg of LAT52:GFP-RIC4ΔC construct was transformed to show the oscillation of ROP1 activity. To study the effect of RIC3 or RhoGAP1 overexpression on ROP1 activity, 0.1 μg or 0.02 μg of pLAT52:RIC3 or pLAT52:RhoGAP1 was cotransformed with 0.1 μg of GFP-RIC4ΔC. Pollen tubes were observed under a Leica SP2 confocal microscope 3–6 h after germination.
Drug Treatments.
To explore the role of F-actin, at 3 h after pollen germination, 5 μM latrunculin B and 100 μM jasplakinolide stock solutions were added to pollen germination medium to a final concentration of 5 nM and 100 nM, respectively. For A23187 treatment, 1 mM A23187 stock solution was added to pollen germination medium to final concentrations of 1 μM (for high-concentration treatment) and 10 nM (for low-concentration treatment). For LaCl3 treatment, 10 mM LaCl3 stock solution was added to pollen germination medium to a final concentration of 10 μM.
Confocal Microscopy.
For confocal laser scanning microscopy, the laser was focused on the median plane, where the apical PM-localized GFP-RIC4ΔC was clearest. Imaging of GFP-RIC4ΔC was done with a Leica SP2 confocal microscope with 488-nm laser excitation and 500- to 570-nm emission for GFP. For time course analysis, images of pollen tube tips were collected using a 63× water immersion lens, zoomed with 4× with a 512 × 512 frame and 400-Hz scanning speed at 10-s intervals.
Measurement of Relative ROP1 Activity.
Relative ROP1 activity is defined as the ratio between the mean value of the GFP-RIC4ΔC signal localized to the apical PM and that localized to the cytosol. The mean intensity of GFP-RIC4ΔC signals on the apical PM and in the cytosol was measured using MetaMorph 4.5 (Universal Imaging) for each median scan of GFP-RIC4ΔC–expressing pollen tubes. The mean intensity of the cytosolic part was obtained from an area 15 μm in circumference in the middle of pollen tube, 2 μm away from the tip of the pollen tube.
Numerical Simulation of the ODEs Model.
Numerical simulation of the ODEs model was done using dde23 solver in Matlab 7.0 software.
Supplementary Material
Acknowledgments.
We thank Dr Jeffrey Harper for his kind gift of the aca9–2 mutant seeds. This work was supported by grants from the National Institute of General Medical Sciences (R01 GM081451–01), National Science Foundation, and U.S. Department of Energy (to Z.Y.).
Footnotes
The authors declare no conflicts of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910811106/DCSupplemental.
References
- 1.Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feijo JA, et al. Cellular oscillations and the regulation of growth: The pollen tube paradigm. Bioessays. 2001;23:86–94. doi: 10.1002/1521-1878(200101)23:1<86::AID-BIES1011>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Forger DB, Peskin CS. A detailed predictive model of the mammalian circadian clock. Proc Natl Acad Sci USA. 2003;100:14806–14811. doi: 10.1073/pnas.2036281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heintzen C, Liu Y. The Neurospora crassa circadian clock. Adv Genet. 2007;58:25–66. doi: 10.1016/S0065-2660(06)58002-2. [DOI] [PubMed] [Google Scholar]
- 5.Geva-Zatorsky N, et al. Oscillations and variability in the p53 system. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100068. 2006 0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patnaik PR. Oscillatory metabolism of Saccharomyces cerevisiae: An overview of mechanisms and models. Biotechnol Adv. 2003;21:183–192. doi: 10.1016/s0734-9750(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, et al. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304:875–878. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- 8.Sneyd J, Tsaneva-Atanasova K, Yule DI, Thompson JL, Shuttleworth TJ. Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci USA. 2004;101:1392–1396. doi: 10.1073/pnas.0303472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannone G, et al. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 10.Wolgemuth CW. Lamellipodial contractions during crawling and spreading. Biophys J. 2005;89:1643–1649. doi: 10.1529/biophysj.105.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierson ES, et al. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 12.Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase–dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol. 2008;181:1155–1168. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy SJ, et al. Uncoupling secretion and tip growth in lily pollen tubes: Evidence for the role of calcium in exocytosis. Plant J. 1999;19:379–386. doi: 10.1046/j.1365-313x.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 15.Messerli MA, Creton R, Jaffe LF, Robinson KR. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol. 2000;222:84–98. doi: 10.1006/dbio.2000.9709. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Wang Y, Zhu JK, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a ROP GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kost B, et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Y, et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol. 2005;169:127–138. doi: 10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stites EC, Trampont PC, Ma Z, Ravichandran KS. Network analysis of oncogenic Ras activation in cancer. Science. 2007;318:463–467. doi: 10.1126/science.1144642. [DOI] [PubMed] [Google Scholar]
- 22.Dawes AT, Edelstein-Keshet L. Phosphoinositides and Rho proteins spatially regulate actin polymerization to initiate and maintain directed movement in a one-dimensional model of a motile cell. Biophys J. 2007;92:744–768. doi: 10.1529/biophysj.106.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Gu Y, Li S, Yang Z. A genome-wide analysis of Arabidopsis ROP-interactive CRIB motif–containing proteins that act as ROP GTPase targets. Plant Cell. 2001;13:2841–2856. doi: 10.1105/tpc.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiott M, et al. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA. 2004;101:9502–9507. doi: 10.1073/pnas.0401542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovar DR, Drobak BK, Staiger CJ. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokota E, Muto S, Shimmen T. Calcium-calmodulin suppresses the filamentous actin-binding activity of a 135-kilodalton actin-bundling protein isolated from lily pollen tubes. Plant Physiol. 2000;123:645–654. doi: 10.1104/pp.123.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HJ, Wan AR, Jauh GY. An actin-binding protein, LlLIM1, mediates calcium and hydrogen regulation of actin dynamics in pollen tubes. Plant Physiol. 2008;147:1619–1636. doi: 10.1104/pp.108.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JU, Vernoud V, Szumlanski A, Nielsen E, Yang Z. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr Biol. 2008;18:1907–1916. doi: 10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh JS, Manzerra P, Kennedy MB. Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- 30.Gibbon BC, Kovar DR, Staiger CJ. latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11:2349–2363. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, Wu G, Yang Z. ROP GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malho R, Read ND, Trewavas AJ, Pais MS. Calcium channel activity during pollen tube growth and reorientation. Plant Cell. 1995;7:1173–1184. doi: 10.1105/tpc.7.8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers C, et al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoon GM, Dowd PE, Gilroy S, McCubbin AG. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell. 2006;18:867–878. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.