Abstract
The goal of the current study was to investigate the role of exogenous and endogenous hydrogen sulfide (H2S) on neovascularization and wound healing in vitro and in vivo. Incubation of endothelial cells (ECs) with H2S enhanced their angiogenic potential, evidenced by accelerated cell growth, migration, and capillary morphogenesis on Matrigel. Treatment of chicken chorioallantoic membranes (CAMS) with H2S increased vascular length. Exposure of ECs to H2S resulted in increased phosphorylation of Akt, ERK, and p38. The KATP channel blocker glibenclamide or the p38 inhibitor SB203580 abolished H2S-induced EC motility. Since glibenclamide inhibited H2S-triggered p38 phosphorylation, we propose that KATP channels lay upstream of p38 in this process. When CAMs were treated with H2S biosynthesis inhibitors dl-propylargylglycine or beta-cyano-L-alanine, a reduction in vessel length and branching was observed, indicating that H2S serves as an endogenous stimulator of the angiogenic response. Stimulation of ECs with vascular endothelial growth factor (VEGF) increased H2S release, while pharmacological inhibition of H2S production or KATP channels or silencing of cystathionine gamma-lyase (CSE) attenuated VEGF signaling and migration of ECs. These results implicate endothelial H2S synthesis in the pro-angiogenic action of VEGF. Aortic rings isolated from CSE knockout mice exhibited markedly reduced microvessel formation in response to VEGF when compared to wild-type littermates. Finally, in vivo, topical administration of H2S enhanced wound healing in a rat model, while wound healing was delayed in CSE−/− mice. We conclude that endogenous and exogenous H2S stimulates EC-related angiogenic properties through a KATP channel/MAPK pathway.
Keywords: blood vessels, endothelial cell, MAP kinases
The realization that mammalian cells are capable of producing hydrogen sulfide (H2S) has sparked interest in the biology and pharmacology of this molecule (1–4). H2S is now considered the third member of the gaseotransmitter family, along with nitric oxide (NO) and carbon monoxide (CO) (3–5). H2S is generated from L-cysteine in reactions catalyzed by cystathionine-β-synthase (CBS) or cystathionine-γ-lyase (CSE). CSE is primarily responsible for most of the H2S production in the vasculature (2, 3), although additional pathways (e.g., 3-mercaptopyruvate sulfurtransferase) contribute also (6). ATP-sensitive potassium channel (KATP channel) activation contributes to H2S-mediated vasodilation (7). In animal models of critical illness, H2S donors protect from lethal hypoxia and reperfusion injury (3, 8, 9) and exert anti-inflammatory effects (10).
Angiogenesis is a complex biological process characterized by extracellular matrix remodeling and changes in endothelial cell (EC) behavior that lead to increased growth, migration, and assembly into capillary structures (11, 12). Dysregulated angiogenesis contributes to tumor growth, psoriasis, arthritis, neurodegeneration, wound healing defects, and hair loss (13). ECs are both targets and sources of H2S. In the vasculature, H2S has been mostly studied in the context of vessel tone (2, 14). The aim of the present study was to test the role of exogenous and endogenous H2S in angiogenesis.
Results
H2S Promotes the Angiogenic Properties of ECs.
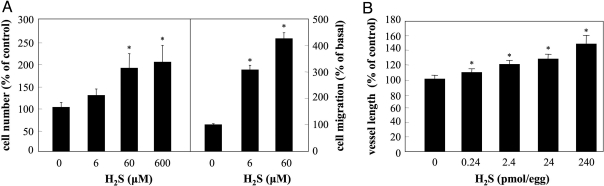
Exposure of human umbilical vein endothelial cells (HUVECs) to H2S promoted EC growth with a 2-fold increase in cell number observed at 60 μM (Fig. 1A, Left). Furthermore, H2S (60 μΜ) enhanced capillary-like structure formation of ECs cultured on reduced-growth factor Matrigel by 33.9 ± 3.3% (n = 6; P < 0.05). ECs also exhibited enhanced motility in the presence of H2S (Fig. 1A, Right). To test the ability of H2S to promote new blood vessel formation in vivo, we applied H2S in the chick chorioallantoic membrane (CAM) model. H2S increased the length of the vascular network in a dose-dependent manner (Fig. 1B and Fig. S1).
Fig. 1.
H2S promotes angiogenesis in vitro and in vivo. (A) ECs were incubated with vehicle or the indicated concentration of H2S for 48 h in complete growth medium. Cell number was determined using a hemocytometer. n = 4; *, P < 0.05 vs. vehicle (left axis). Cells were allowed to migrate for 4 h in the presence or absence of the indicated concentration of H2S. n = 5; *, P < 0.05 vs. vehicle (right axis). (B) CAMs were treated with the indicated doses of H2S for 48 h; total length of the vascular network was determined using image analysis software. n = 22–32; *, P < 0.05 vs. vehicle.
Signaling Pathways Mediating the Actions of H2S.
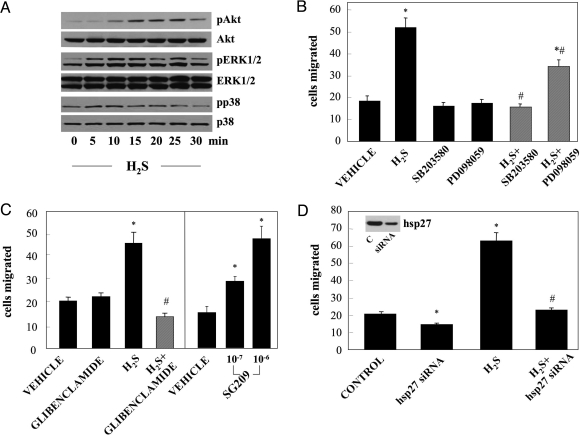
EC exposed to H2S (60 μΜ) exhibited a sustained increase in ERK1/2 phosphorylation that was evident as early as 5 min (Fig. 2A). Moreover, treatment with H2S led to p38 and Akt activation, but with different kinetics: phosphorylation of p38 was rapid and transient, while Akt phosphorylation showed a delayed and more sustained pattern. Inhibition of MEK by PD098059 significantly reduced H2S-induced EC migration, while inhibition of p38 with SB203580 completely blocked the migratory response (Fig. 2B). Similar results were obtained using two additional inhibitors of MEK (UO126) and p38 (SB239063) (Fig. S2C). In contrast, inhibition of the PI3/Akt pathway with LY-2924002 did not affect the migratory rate of ECs in response to H2S (Fig. S2A).
Fig. 2.
H2S enhances migration through an ATP-sensitive K+ channel/MAPK dependent pathway. (A) ECs were serum-starved for 5 h and then treated with H2S (60 μM) or vehicle for the indicated time. Cell lysates were prepared and immunoblotted with antibodies specific for the phosphorylated and total forms of ERK1/2, p38 and Akt kinases. Blots shown are representative from experiments performed at least twice. (B) ECs were serum-deprived overnight and then treated for 30 min with the MEK inhibitor PD098059 (10 μΜ) or the p38 inhibitor SB203580 (3 μΜ). Cells were then trypsinised, placed in Transwells, and allowed to migrate in response to H2S (60 μΜ) as described in Materials and Methods. n = 5; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. H2S. (C) ECs were pretreated with the KATP channel blocker glibenclamide (10 μM, 30 min) before being exposed to H2S (60 μΜ). After 4 h cells were stained and counted. Alternatively, ECs were treated with the KATP channel opener SG209 for 4 h. n = 5; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. H2S. (D) Cells were transfected with 50 nM hsp27 siRNA. After 48 h, cells were used in migration assays. Hsp27 siRNA-transfected or control cells were serum-starved overnight and then used in migration experiments in the presence or absence of H2S (60 μM). The inset shows a representative blot depicting a decrease in hsp27 protein after siRNA treatment. n = 5; *, P < 0.05 vs. control and #, P < 0.05 vs. H2S.
Blockade of KATP channels in ECs by glibenclamide blocked H2S-induced EC migration (Fig. 2C). Similar results were obtained with 5-hydroxydecanoate, a mitochondrial-KATP channel selective inhibitor (5-HD; Fig. S2B). Interestingly, incubation of ECs with the nitrate-free nicorandil analogue SG209 (a KATP channel opener) enhanced p38 phosphorylation (Fig. S2E) and EC motility (Fig. 2C), suggesting that KATP channel opening is sufficient to drive EC migration. Incubation of cells with the KATP channel modifying agents (5-HD and SG209) did not induce apoptosis (Fig. S3).
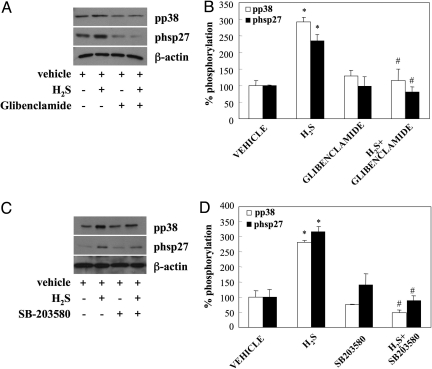
Hsp27 has been shown to be a downstream effector of p38 mediating its effects on cell migration (29). Transfection of HUVECs with hsp27 siRNA resulted in reduced hsp27 protein levels; this, in turn, led to a significantly lower migratory rate of cells in response of H2S (Fig. 2D). Inhibition of KATP channels by glibenclamide blocked H2S-stimulated p38 and hsp27 phosphorylation (Fig. 3 A and B), suggesting that KATP channels are upstream of MAPK pathways. In addition, cells pretreated with SB203580 (Fig. 3 C and D) or SB239063 (Fig. S2D) showed reduced hsp27 phosphorylation. The above data suggest that H2S enhances EC migration through a KATP channel/p38/hsp27 pathway.
Fig. 3.
KATP channels lay upstream of p38 and hsp27. (A and B) EC were serum-starved for 5 h. They were then treated with the H2S (60 μM) for 10 min with or without glibenclamide pretreatment (10 μM, 30 min). Cell lysates were prepared and immunoblotted with antibodies specific for the phosphorylated forms of p38 and hsp27 or β-actin. Autoradiographs were scanned and band intensity quantified using image analysis software. n = 3–4; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. H2S. (C and D) Samples were processed as in A; pretreatment consisted of exposure to SB203580 (3 μM; 30 min). n = 3–4; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. H2S.
Role of Endogenously Produced H2S in Neovascularization.
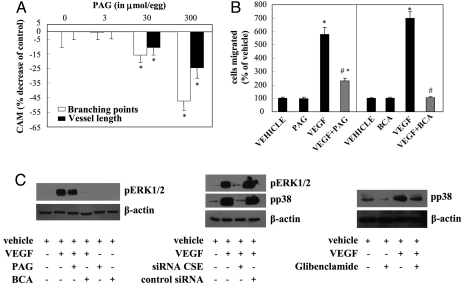
Treatment of CAM with H2S synthesis inhibitors PAG and BCA reduced H2S production (1.1 ± 0.1 nmol H2S/mg protein/min in vehicle; 0.22 ± 0.1 nmol H2S/mg protein/min in BCA; and 0.2 ± 0.1 nmol H2S/mg protein/min for PAG). Both agents dose-dependently decreased network length and vessel branching (Fig. 4A and Fig. S4A), suggesting that endogenous H2S is important for vascular network formation in vivo. Incubation of CAM with exogenously added H2S reversed the anti-angiogenic action of PAG and BCA (Fig. S4B). To test whether endogenously produced H2S contributes to the action of the prototypic angiogenic factor vascular endothelial growth factor (VEGF), cells were pretreated with PAG or BCA (Fig. 4B), or inhibitors of KATP channels (glibenclamide or 5-HD; Fig. S4C), before stimulation with VEGF. All of the above agents reduced or abolished VEGF-triggered EC motility, indicating that H2S produced by ECs contributes to the angiogenesis-related actions of VEGF. In contrast, pretreatment of cells with glibenclamide did not affect fibroblast growth factor-induced migration (Fig. S4C).
Fig. 4.
Endogenously produced H2S enhances angiogenesis. (A) CAMs were treated with the indicated dose of the H2S synthesis inhibitor PAG for 48 h and vascular network length and branching were determined. n = 36–45; *, P < 0.05 vs. vehicle. (B) ECs were serum starved overnight. Cells were then treated with PAG (3 mM) or BCA (0-6 μΜ) for 30 min. Cells were then trypsinized, placed in Transwells and allowed to migrate for 4 h in the presence of vehicle or VEGF (20 ng/mL). n = 5; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. VEGF. (C) ECs were serum starved for 5 h. Cells were then treated with PAG (3 mM), BCA (0-6 μΜ), or glibenclamide (10 μΜ) for 30 min and then treated with VEGF (20 ng/mL) for 10 min. Other cells were transfected with 5 nM CSE siRNA. After 24 h, siRNA-transfected or control cells were serum-starved for 5 h and then stimulated with VEGF (20 ng/mL) for 10 min. Cell lysates were prepared and immunoblotted with antibodies specific for the phosphorylated forms of ERK1/2 and p38. β-actin was used to ensure equal protein loading. Blots shown are representative from experiments performed at least twice.
In line with the ability of inhibitors of H2S synthesis or action to reduce VEGF-stimulated migration, we observed that PAG, BCA and glibenclamide reduced ERK1/2 and/or p38 phosphorylation (Fig. 4C). To obtain definitive confirmation of the role of endogenous H2S, we next used a siRNA approach to selectively reduce H2S production in ECs. The CSE siRNA attenuated CSE gene expression (averaged decrease of CSE protein levels was 59.8 ± 6.7%), leading to attenuated activation of MAPK (ERK1/2 and p38; Fig. 4C) and migration (Fig. 5B) in response to VEGF treatment.
Fig. 5.
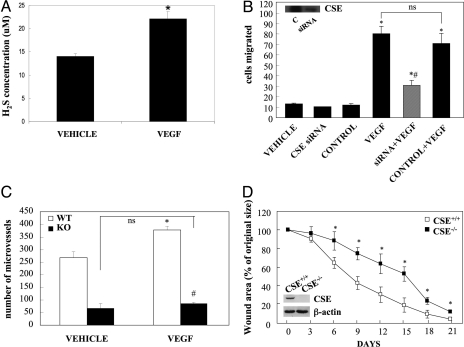
CSE is crucial for VEGF-stimulated angiogenesis and wound healing. (A) Production of H2S, as determined by methylene blue assay (15) in control ECs, and in response to VEGF (20 ng/mL) stimulation for 10 min. n = 5; *, P < 0.05 vs. vehicle. (B) Cells were transfected with 5 nM CSE siRNA or a control siRNA. Twenty-four hours post-transfection, cells were serum-starved overnight and then used in migration experiments in the presence or absence of VEGF (20 ng/mL). The inset shows a representative blot depicting the reduction in CSE protein levels. n = 5; *, P < 0.05 vs. vehicle and #, P < 0.05 vs. VEGF. (C) Aortic ring explants from CSE+/+ (WT) or CSE−/− (KO) mice were incubated in the presence or absence of VEGF (20 ng/mL). The number of new microvessels was determined by a blinded observer. n = 5; *, P < 0.05 vs. WT vehicle and #, P < 0.05 vs. WT VEGF. (D). Changes in total burn wound area over time. Four animals for CSE wild-type group and five for CSE knockout mice were used. Inset: blot showing lack of CSE protein expression in skin tissue of CSE−/− mice; *, P < 0.05 vs. CSE+/+.
Having proven that inhibition of CSE reduces VEGF responses, we sought to determine whether VEGF enhances H2S production. Indeed, exposure of HUVECs to VEGF resulted in accumulation of H2S in the culture medium (Fig. 5A). The role of CSE and H2S production in VEGF-triggered angiogenesis was further confirmed in mice with targeted disruption of the CSE gene (CSE KO mice). Using the in vitro aortic ring angiogenesis assay, we observed a reduction in the number of microvessels formed by cultured aortic rings from CSE knockouts, both basally and following VEGF stimulation, compared to littermate controls (Fig. 5C and Fig. S1B). The lack of an additive effect of maximal doses of VEGF and exogenously administered H2S with regards to cell motility (Fig. S4D) suggests that optimal amounts of this gaseous mediator are already produced following VEGF exposure.
H2S Promotes Wound Healing.
To study whether H2S administration exerts therapeutic benefits related to angiogenesis in vivo, we used a burn wound assay (30% of the total body surface area). Wound closure after 1 month was markedly improved in animals receiving daily topical administrations of H2S. Re-epithelialization, as a percent of original wound size, significantly increased from 16 ± 3% to 24 ± 1% (n = 6, P < 0.05). To determine the role of endogenously produced H2S we compared wound healing in the CSE−/− and CSE+/+ mice (Fig. 5D and Fig.S1C). Throughout the observation period, wound areas in CSE+/+ mice were consistently smaller than in CSE−/− mice, suggesting that healing is delayed when endogenous H2S production is suppressed.
Discussion
EC proliferation, migration, and differentiation are key properties associated with blood vessel formation (11). Previous reports have shown that H2S can affect the proliferation and survival of mammalian cells with experiments in vascular cells yielding opposing effects. H2S promotes apoptosis in smooth muscle cells (15), while it exerts a mitogenic effect in rat retinal ECs (16). Our results demonstrate that incubation of human ECs with H2S results in a concentration-dependent increase in cell number. Thus, similar to nitric oxide, H2S promotes the growth of EC, while inhibiting that of smooth muscle (17, 18). H2S also stimulates capillary morphogenesis to drive network-like formation of ECs in vitro. Moreover, H2S exerted a positive effect on endothelial migration, suggesting that H2S promotes the appearance of an angiogenic phenotype by the endothelium. Results from the CAM experiments further proved that exogenous H2S increases neovascularization. This is consistent with a report from Moore and colleagues, where H2S was shown to induce a dose-dependent effect on angiogenesis using the Matrigel model (16). The finding that H2S promotes angiogenesis is also in agreement with the ability of H2S to promote the healing of gastric ulcers and of colitic intestinal ulcerations (19, 20). Along these lines, in the present study, we observed that H2S promoted the healing of burn wounds, where angiogenesis occurs mainly during the proliferative phase of wound healing and is crucial in promoting re-epithelialization and closure. Thus, H2S donor compounds may exhibit therapeutic potential as stimulators of therapeutic angiogenesis and wound healing.
We have identified several downstream effectors of H2S in ECs. H2S has been reported to alter the activation status of ERK1/2 and p38 in a cell-type and stimulus-dependent manner. For example, H2S activates ERK1/2 in gastric epithelial cells (21), monocytes (22), and smooth muscle cells (15), while inhibits ERK1/2 phosphorylation in beta cells (23). In addition, H2S activates p38 in beta cells and smooth muscle (15), while it inhibits p38 phosphorylation in neutrophils (24) and microglia (25). In contrast to what was reported in transformed ECs (16) where exposure to H2S did not affect ERK1/2 phosphorylation, we found that incubation of HUVECs with H2S activates ERK1/2 as well as p38. Pharmacological inhibitors of MEK and p38 attenuated H2S-stimulated responses. Thus, in the conditions used in the current study, MAPK, but not PI-3K, pathways are involved in the H2S-stimulated migration in human ECs.
Some of the actions of H2S have been attributed to KATP channel opening (7, 10). EC express KATP channels both in the plasma membrane and in intracellular organelles (26, 27). The KATP channel inhibitors glibenclamide or 5-HD blocked the migratory response to H2S. Furthermore, incubation of cells with the KATP channel opener SG209 (28), induced a concentration-dependent migratory response, indicating that K+ efflux per se can drive EC motility. Another group has reported that blockade of Ca2+-activated K+ channels also attenuates EC growth and angiogenesis (29).
Hsp27 is a modifier of actin cytoskeleton which regulates cell migration (30–32). Phosphorylation of actin-bound hsp27 allows actin polymerization to proceed (33). Hsp27 is directly phosphorylated by MAPKAP (a p38 substrate) (34, 35). We report here that hsp27 is phosphorylated after exposure to H2S and this was blocked by p38 inhibition. Glibenclamide reduced p38 and hsp27 phosphorylation, suggesting the existence of a KATP channel/p38/hsp27 pathway that leads to migration. A role for KATP channels in regulating MAPK pathways has also been demonstrated in other experimental models previously (36). The finding that knockdown of hsp27 using siRNA inhibited the effects of H2S proves the existence of a KATP/p38/hsp27 axis regulating H2S migration.
To determine the contribution of endogenous H2S to angiogenesis, we used two different pharmacological inhibitors of H2S production, which both decreased the length of vascular networks, as well as their branching, suggesting that endogenously produced H2S plays a tonic stimulatory role in the angiogenetic process: it is conceivable, therefore, that H2S produced in the eggs may serve as a physiological hormone for the developing chicken embryo vasculature.
Since endothelial CSE activity is known to be stimulated by calcium/calmodulin (14), we hypothesized that exposure of ECs to the potent endogenous angiogenic agent VEGF, which promotes elevations in intracellular calcium levels (37), may lead to H2S release that in turn contributes to VEGF-stimulated angiogenesis-related properties of ECs. Indeed, we have shown here that exposure of HUVECs to VEGF stimulates the cellular release of H2S. Moreover, pretreatment of cells with PAG, glibenclamide, or CSE gene knockdown, attenuated VEGF signaling, as indicated by the reduction in ERK1/2 and p38 phosphorylation. In line with the ability to reduce VEGF signaling, inhibition of H2S production (PAG, BCA, or CSE siRNA) or action (glibenclamide) attenuated VEGF-induced migration. To determine whether endogenously produced H2S contributes to other angiogenesis-related actions of VEGF, we studied the ability of this growth factor to trigger an angiogenetic response in vascular tissues from CSE−/− mice. Exposure of aortic rings from wild-type animals to VEGF stimulated the growth of microvessels; this was not observed in vessels from CSE−/− mice, further confirming the role of endogenous H2S in neovascularization and in wound healing.
In summary, we have shown that H2S stimulates angiogenesis-related properties of ECs and blood vessel formation in vivo. H2S exerts its effects on ECs through KATP channels that in turn facilitate activation of MAPK pathways, leading to new blood vessel formation. Thus, we hypothesize that (a) H2S and other KATP channel activators may be useful in disease states associated with poor neovascularization and (b) that inhibition of endogenous H2S production may be useful in conditions of pathological/excessive angiogenesis.
Materials and Methods
EC Proliferation and Migration.
HUVECs were isolated from 3–5 umbilical cords and grown in M199 supplemented with 15% FBS, antibiotics, 5 U/mL heparin, and 150–200 μg/mL EC growth supplement (ECGS). Cells were treated with H2S (6–600 μM) and allowed to proliferate for 48 h. Cells were then trypsinized and counted. To measure cell migration, cells were serum-starved overnight, trypsinized, and added to Transwell inserts. After the treatment with various pharmacological agents, cells were allowed to migrate for 4 h at 37 °C. After fixation and staining, migrated cells were scored in eight random fields.
Matrigel Tube-Like Structure Formation Assay and In Vitro Angiogenesis Assay.
HUVECs (15,000 cells/well) were plated in 96-well plates precoated with 50 μL growth factor-reduced Matrigel. Following a 24 h-incubation, tube formation was quantified by image analysis software (Scion Image). Microvessel formation was also evaluated using the in vitro aortic ring angiogenesis assay. Each 3-mm aortic ring was housed in a 48-plate fibrin gel containing complete medium overnight. Medium was then removed and VEGF (20 ng/mL) or vehicle added for 72 h. Quantitative evaluation of newly formed structures was carried out via an inverted microscope.
Western Blots.
Cells were starved for 5 h and then treated with H2S or vehicle. Cell lysates were collected every 5 min for a duration time of 20 min. After SDS/PAGE, and transfer, membranes were immunoblotted with antibodies specific for the phosphorylated or total form of p38, ERK1/2 or hps27 or Akt. After incubation with secondary antibodies, band location was revealed with the use of a chemiluminescent substrate.
In Vivo CAM Angiogenesis Assay.
Fertilized White Leghorn chicken eggs were placed in an incubator as soon as embryogenesis started and kept under constant humidity at 37 °C. On day 4, a square window was opened in the shell and then sealed with adhesive tape. On day 9, an O-ring (1 cm2) was placed on the surface of the CAM and the substance tested was added inside this restricted area. After 48 h, CAM tissues were fixed in Carson's solution (saline-buffered formalin) and angiogenesis was evaluated using the NIH image analysis software.
Measurement of H2S Production.
H2S production rate was measured via the methylene blue assay (15).
Wound Healing Models.
Male Sprague–Dawley rats were anesthetized with ketamine (60 mg/kg) and xylazine (10 mg/kg) i.p. Rats were shaved, placed in a mold, and burned by submerging the back of the animal in scalding (95–99 °C) water for 10 s. Animals were treated with vehicle (n = 6), or daily s.c. injections of 300 μg/kg H2S in the volume of 50 μL per injection, at four equally spaced sites in the transition zone between burn site and healthy tissue. Re-epithelization was determined on day 31 by planimetry. For the murine studies, second generation of 16-week-old male CSE−/− and wild-type littermate mice were used. An approximate 100-mm2 scald wound (5% total body-surface area) was created on the dorsal surface of the animals using a heated metal stick. Wound size was determined every third day and quantified using AlphaEase FC (v5.0.1)
Data Analysis.
Data are expressed as means ± SEM. Statistical comparisons between groups were performed using ANOVA followed by a post-hoc or Student's t test.
Supplementary Material
Acknowledgments.
This work was supported by grants from the Greek Ministry of Education, Greek Secretariat of Research and Technology, and Ikaria Inc. (A.P.), National Institutes of Health Grant R01 GM060915 (to C.S.), and an operating grant from the Canadian Institutes of Health Research (R.W.) Z.Z. is supported by the Thorax Foundation.
Footnotes
Conflict of interest statement: C.S. is an officer and shareholder of Ikaria, Inc., a for-profit organization involved in the development of hydrogen sulfide-based therapeutic approaches. A. Papapetropoulos has received >$15,000 in grant support from Ikaria, Inc. The remaining authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908047106/DCSupplemental.
References
- 1.Fiorucci S, Distrutti E, Cirino G, Wallace J. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Moore P. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 5.d'Emmanuele di Villa Bianca R, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci USA. 2009;106:4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009 Jul 15; doi: 10.1093/jb/mvp111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackstone E, Roth M. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 9.Elrod JW, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanardo R, et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Angiogenesis in health and disease. Nature Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 16.Cai W, et al. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Garg U, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziche M, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Wallace J, Dicay M, McKnight W, Martin G. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa D, et al. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhi L, Ang A, Zhang H, Moore P, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi L, et al. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest. 2006;86:391–397. doi: 10.1038/labinvest.3700391. [DOI] [PubMed] [Google Scholar]
- 25.Hu L, Wong P, Moore P, Bian J. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 26.Malester B, et al. Transgenic expression of a dominant negative K(ATP) channel subunit in the mouse endothelium: Effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- 27.Katnik C, Adams DJ. Characterization of ATP-sensitive potassium channels in freshly dissociated rabbit aortic endothelial cells. Am J Physiol. 1997;272:H2507–H2511. doi: 10.1152/ajpheart.1997.272.5.H2507. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi T, Hamaguchi M, Imai S. 2-Nicotinamidoethyl acetate (SG-209) is a potassium channel opener: Structure activity relationship among nicorandil derivatives. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:235–239. doi: 10.1007/BF00167224. [DOI] [PubMed] [Google Scholar]
- 29.Grgic I, et al. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 30.Hedges J, et al. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 31.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 32.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Biol Chem. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benndorf R, et al. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 34.Guay J, et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin M, et al. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, et al. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 37.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.