Abstract
The transcription factor Gata4 is essential for normal heart morphogenesis and regulates the survival, growth, and proliferation of cardiomyocytes. We tested if Gata4 can specify cardiomyocyte fate from an uncommitted stem or progenitor cell population, by developing a system for conditional expression of Gata4 in embryonic stem cells. We find that in embryoid body cultures containing even a low ratio of these cells, expression of Gata4 is sufficient to enhance significantly the generation of cardiomyocytes, via a non-cell-autonomous mechanism. The Gata4-expressing cells do not generate cardiac or other mesoderm derivatives. Rather, Gata4 expression directs the development of two types of Sox17+ endoderm. This includes an epCam+Dpp4+ subtype of visceral endoderm. In addition, Gata4 generates similar amounts of epCam+Dpp4− definitive endoderm enriched for Cxcr4, FoxA2, FoxA3, Dlx5 and other characteristic transcripts. Both types of endoderm express cardiac-inducing factors, including WNT antagonists Dkk1 and Sfrp5, although the visceral endoderm subtype has much higher cardiac inducing activity correlating with relatively enhanced levels of transcripts encoding BMPs. The Gata4-expressing cells eventually express differentiation markers showing commitment to liver development, even under conditions that normally support mesoderm development. The results suggest that Gata4 is capable of specifying endoderm fates that facilitate, with temporal and spatial specificity, the generation of cardiomyocyte progenitors from associated mesoderm.
Keywords: cardiogenesis, specification, progenitors, WNT, DKK1, BMP, liver
Introduction
Understanding the transcriptional and signaling programs that specify cardiomyocyte fate from uncommitted progenitors may provide important clues to impact cellular strategies for treating failing or infarcted heart tissue. GATA factors comprise a small family of highly conserved zinc finger transcription factors that play critical roles in the development of the cardiovascular, hematopoietic, digestive, and reproductive systems (Patient and McGhee, 2002). Based on its expression in early precardiac mesoderm, and the phenotypes caused by various gain and loss-of-function experiments, Gata4 is considered a key regulator of cardiogenesis (Pikkarainen et al., 2004). Indeed, mutations in the Gata4 gene cause human congenital cardiomyopathies, including valve and septal defects (Garg et al., 2003; Rajagopal et al., 2007). However, the genetic programs under Gata4 control are not well understood. This is a challenging problem to address genetically, since Gata4 expression is not restricted to a specific lineage, there is potential compensation from co-expressed sister genes (Gata5 and Gata6), and it could potentially function either cell-autonomous or non-cell-autonomous with respect to cardiac lineages.
The murine Gata4 knockout is embryonic lethal, and while there is a heart phenotype, this is secondary due to defects in extra-embryonic endoderm that affect embryonic folding (Kuo et al., 1997; Molkentin et al., 1997). Gata4 deficient ES cells retain the ability to differentiate into cardiomyocytes in vitro, and the analysis of chimeric mice hosting knockout cells proved that Gata4 is not required for cardiomyocyte differentiation (Narita et al., 1997a). However, Gata4 does function in cardiac cells. Conditional knockout mice, lacking Gata4 in specified cardiomyocytes, and transgenic mice expressing only 30% of the normal levels of GATA4 in the heart, both display atrioventricular canal defects and a hypoplastic ventricular myocardium. The ventricular hypoplasia is thought to be associated with defects in cardiac morphogenesis and cardiomyocyte proliferation, rather than differentiation (Pu et al., 2004; Zeisberg et al., 2005). Tetraploid complementation was used to rescue the extra-embryonic defects in Gata4 null embryos, in order to analyze the function of Gata4 during mouse organogenesis (Watt et al., 2004). These embryos lack proepicardium and show defects in cardiac morphogenesis, including a hypoplastic ventricular myocardium. Likewise, we showed a morphogenetic defect in zebrafish depleted for Gata4 (Holtzinger and Evans, 2005). Gata4 continues to be required beyond embryogenesis, since heterozygous mutant adult mice are hypersensitive to pressure-induced stress overload (Bisping et al., 2006). In addition to its requirement for epicardial and myocardial function, Gata4 is also essential in endocardial cells for cushion EMT and valve development (Rivera-Feliciano et al., 2006).
In sum, Gata4 is not required for cardiomyocyte specification. However, gain-of-function experiments suggest that Gata4 encodes sufficient activity to affect cardiomyocyte fate. Forced expression of Gata4 enhances cardiogenesis during Xenopus embryogenesis (Jiang and Evans, 1996) or in P19 embryonal carcinoma cells (Grepin et al., 1997). Furthermore, Gata4 expression in Xenopus ectodermal explants is sufficient to induce differentiation of cardiomyocytes, even after initial commitment of the explants to epidermal fate (Latinkic et al., 2003). Gata4-null ES cells generate cardiomyocytes, yet less efficiently compared to wild-type cells (Narita et al., 1997a). In the gain-of-function experiments Gata4 might direct programs normally regulated by other GATA factors, or the sum total of GATA factors. Regardless of the mechanism, these experiments suggest that Gata4 is a viable candidate for enhancing cardiogenesis from a progenitor cell population.
Cardiomyocytes are derived from mesoderm, yet their specification during early embryogenesis is dependent on inductive signals from endoderm that develops in close association with presumptive cardiac mesoderm (Foley et al., 2006). Thus, ablation of the anterior endoderm in Xenopus embryos results in a loss of myocardium due to a failure of cardiomyocyte specification (Nascone and Mercola, 1995). Gata4 also regulates growth of endoderm-derived organs, including gut, liver and pancreas in zebrafish (Holtzinger and Evans, 2005), consistent with the analysis of knockout mice rescued by tetraploid complementation (Watt et al., 2007). Wild-type endoderm is sufficient to rescue cardiogenesis in both mouse (Narita et al., 1997b) and chick (Ghatpande et al., 2000) embryos lacking normal Gata4 expression in the mesoderm. Therefore, Gata4 functions in both mesoderm and endoderm, consistent with a role in regulating the emerging lineages derived from a common precursor germ-layer, referred to as the mesendoderm (Loose and Patient, 2004).
The embryonic stem (ES) cell system provides an accessible model to study the early stages of murine cardiomyocyte specification, in the context of embryoid body (EB) development. Indeed, a recent study showed that co-culturing murine ES cell-derived EBs with BMP2 and a combination of visceral endoderm-like cells increases significantly the generation of cardiomyocytes (Bin et al., 2006). However, it is not known if Gata4 is relevant to this or other inductive mechanisms during cardiogenesis. An experimental challenge is the fact that Gata4 functions in both extra-embryonic and embryonic endoderm. Direct over-expression of Gata4 in murine ES cells causes them to differentiate into extra-embryonic endoderm (Fujikura et al., 2002; Zhang et al., 2007), precluding investigation of function during EB development. We therefore generated a system to conditionally express Gata4 in cultured EBs, and evaluate its ability to direct cardiomyocyte fate.
Material and methods
ES cell line derivation and culture
The embryonic stem cell lines AinV15 and AinV18 were described (Kyba et al., 2002) and we used these parental ES cells (referred to generically as AinV cells) to generate the Gata4-inducible lines (and they functioned equivalently as controls). The iresEGFP fragment from pIRES2EGFP (Clontech) was isolated following restriction with XhoI and NotI and inserted into the plox vector to generate plox-iresEGFP. A cDNA fragment encoding a flag-tagged Gata4 open reading frame (Gata4ORF) was cloned into the HindIII and XbaI sites of the KS plasmid (Stratagene) to create KS-FLAG-Gata4ORF. The FLAG-Gata4ORF was then inserted into the XhoI site of plox-iresEGFP (confirming the appropriate orientation). 20 µg of the resulting targeting construct (plox-FLAG-Gata4ORF-iresEGFP) was co-electroporated with 20 µg of the pSalk-CRE expression vector into 8 × 106 AinV cells. Stably transfected cells were selected using increasing concentrations of G418 (Cellgro; up to 400 µg/ml). In addition to harvesting pools of clones, individual clonal lines were also selected by limiting dilution in 96 well plates. In each case, proper integration of the transgene was confirmed by PCR analysis of genomic DNA using the following primers: fwd: 5’-CTAGATCTCGAAGGATCTGGAG-3’; rev: 5’-ATACTTTCTCGGCAGGAGCA-3’.
ES cells were maintained on irradiated mouse embryonic fibroblasts (MEFs) in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 20% ES-qualified fetal calf serum (Gemini), penicillin, streptomycin, LIF (2% conditioned medium) and 1.5×10−4 M monothioglycerol (MTG; Sigma). For the generation of embryoid bodies, ES cells were depleted from MEFs, trypsinized and plated at day 0 at a concentration of 12000 cells/ml in ethylene oxide treated dishes (VWR Scientific), in Iscove’s Modified Dulbecco Medium (IMDM) supplemented with 15% FCS (Atlas), 2mM L-glutamine (Gibco/BRL), 10% protein-free hybridoma medium (PFHMII; Gibco-BRL), 0.18mg/ml transferrin (Roche), 50ng/ml ascorbic acid (Sigma), 4.5×10−4 M MTG, penicillin, streptomycin. Transgene expression was induced by addition of doxycycline (Sigma) into the culture media at a final concentration of 1 µg/ml.
Western blotting
20 µg of total protein extract derived from embryoid bodies was electrophoresed through a 10% NuPAGE Bis-Tris gel using the XCell SureLock Mini-Cell system (Invitrogen) and transferred to a PVDF nylon membrane (Biorad). For detection of Flag-tagged protein, membranes were incubated with an anti-FLAG M2-HRP conjugated monoclonal antibody (Sigma) at a 1:1000 dilution in blocking buffer. For GATA4 detection, membranes were incubated with a rabbit anti-mouse GATA4 primary antibody (Sigma) at a 1:500 dilution, and an HRP-goat anti rabbit secondary antibody (Biorad), at a 1:10,000 dilution in blocking buffer. Specific bands were detected using the Western Blotting Luminol Reagent (Santa Cruz). To confirm equal loading, gels were stained in Coomassie Blue.
Gel mobility shift assays
Oligonucleotides used as probe or cold competitors were PAGE purified. The upper strand oligomer was end-labeled with [γ–32P]-ATP, purified through a G-25 Sephadex column (Roche), and annealed to its complementary lower strand. The competitors were prepared identically, without labeling. The specific competitor was the unlabeled probe. The non-specific competitor contains mutations in the GATA binding sites. The sequences of the top strands are as follows: probe and specific competitor: 5’-AGCTTGCGGATAAGATAAGGCCGGAATTCA-3’; non-specific competitor: 5’-AGCTTGCGCTGAACTGAAGGCCGGAATTCA-3’. Binding reactions and mobility shift assays were carried out as described (Evans et al., 1988).
Differentiation assays
For evaluating cardiomyocyte differentiation, EBs were harvested at day 6 and plated onto gelatin-coated dishes in IMDM supplemented with 2mM L-glutamine, 10% PFHMII, 0.18mg/ml transferrin, 50 ng/ml ascorbic acid, 4.5x10– 4 M MTG, penicillin, streptomycin. At day10, the EBs were evaluated visually in blinded samples to quantify the number of beating clusters per total number of EBs. For titration assays, EBs were derived from AinV and iGATA4 ESCs in the following ratios respectively: 1:0, 1:1, 1:3, 1:10, and 1:50. EBs were subsequently processed as described above.
For evaluating hematopoiesis, cells were isolated from EBs at day 6 of development and were plated at a concentration of 100,000 cells/ml into 1% methylcellulose supplemented with 15% plasma-derived serum (PDS; Antech), 5% PFHMII, 2mM L-glutamine, 0.18mg/ml transferrin, 25ng/ml ascorbic acid, 4.5x10–4 M MTG, 4U/ml erythropoietin, penicillin, streptomycin. Primitive erythroid colonies were scored from blinded samples at day 11.
Flow cytometry
EBs were trypsinized and single cells were analyzed on a BD FACSCalibur cytometer (BD Biosciences). For cell death assays, the dissociated cells were processed with the Annexin V kit (BD Pharmingen) according to the manufacturer’s instructions. In some experiments, fixed cells were stained with anti-cardiac TroponinT primary antibody (cTnT; NeoMarkers, Fremont, CA) and Cy3-conjugated donkey anti-mouse IgG as secondary antibody (Jackson ImmunoResearch) in PBS+5% saponin (Sigma). The number of cTnT positive cells and EGFP expressing cells were analyzed on a BD FACSCalibur cytometer (BD biosciences). In some experiments EBs were trypsinized and single cells were stained on ice with anti-epCAM (eBioscience) and anti-DPPIV (R & D) antibodies, diluted 1:10 and 1:200, respectively at a concentration of 1 × 106 cells per 100 µl sorting buffer, consisting of 1X D-PBS, 2.5mM EDTA, 25mM HEPES pH7, and 1% FBS. 7AAD (BD Biosciences) was added to the sorting buffer after washing and the cells were sorted using a FACS vantage cell sorter (BD Biosciences). Analysis of flow cytometric data was performed using Flo Jo (Tree Star). For sorting assays, trypsinized cells from induced day 4 iGATA4 EBs were sorted into six well ultra low cluster plates (Corning). Trypsin-dissociated cells from day 2 AinV EBs were added at a 1:1 ratio to a final concentration of 5×105 cells per 4 ml EB media. Reaggregated EBs were subsequently processed as described for evaluation of cardiomyocyte differentiation.
Quantitative real-time PCR
Total RNA was isolated from EBs (Qiagen RNeasy mini kit). cDNA synthesis was performed using the Transcriptor kit (Roche) and qPCR completed on a Lightcycler 480 II (Roche) using the Lightcycler 480 Sybr Green Kit (Roche). Ct values were calculated using the second derivative max algorithm and relative fold change determined using the ΔΔCt method (Livak and Schmittgen, 2001), based on the median value from a triplicate set. Each value was normalized to levels of Gapdh transcripts. The primers used are listed in Supplemental Table ST1.
Results
An embryonic stem cell line allows conditional expression of Gata4
We adapted the system established by Daley and colleagues (Kyba et al., 2002) to generate an ES cell line in which Gata4 is placed under doxycycline-inducible control. The parental AinV cell line expresses constitutively the reverse (inactive) tetracycline transactivator protein from the Rosa26 locus, and has an engineered targeting site placed upstream of the HPRT locus that contains a tetracycline-regulated promoter. We cloned a cDNA encoding flag-tagged Gata4 into the targeting vector in frame with a downstream IRES–EGFP sequence, which facilitates evaluation of transgene expression by monitoring green fluorescence. Flanking the construct is a lox site and an initiator ATG that, upon correct site-specific transgene integration, establishes neomycin resistance to the AinV cells (Fig. 1A).
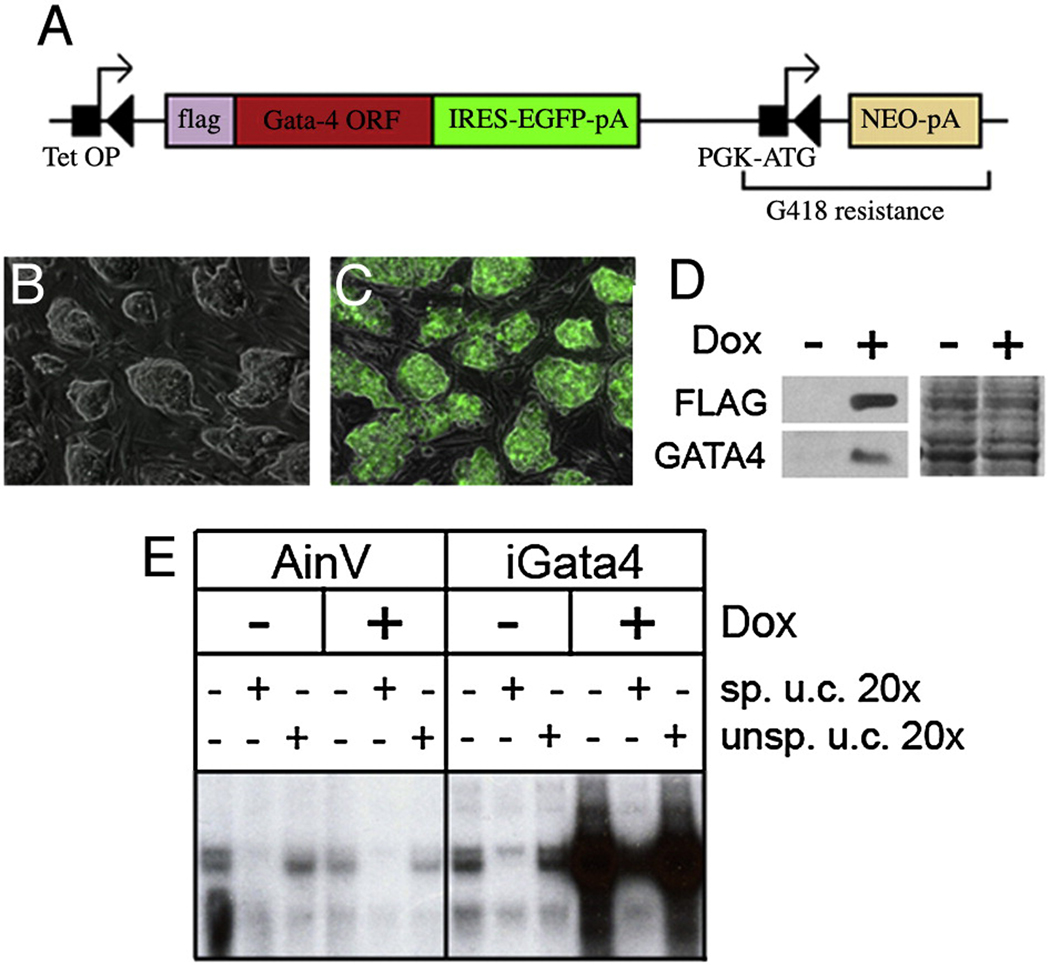
Fig. 1. Characterization of the iGATA4 ES cell lines.
(A) Cre-mediated recombination between lox sites facilitates integration of the Gata4 cDNA into the targeting site of parental AinV cells. The FLAG-Gata4ORF-iresEGFP transgene is flanked by a lox site (left black triangle) and a phosphoglycerokinase promoter (PGK-ATG). The target site in AinV ES cell contains a tetracycline response element (tetOP) followed by a lox site and a neomycin resistance gene lacking its start codon (NEO-pA). Upon site-specific integration, the transgene is placed under control of the tetracycline response element. Note that PGK-ATG lies in frame with NEO-pA to establish neomycin resistance. (B–E) Representative results using a clonal iGATA4ES cell line. Essentially identical results were obtained using pooled clones or several independent lines derived by serial dilution cloning. (B, C) Merged 10x phase and fluorescence images of uninduced (B) and induced (C) iGATA4ES cells. GFP expression only in induced cells indicates successful induction and non-leakiness of transgene expression. (D) Left panel: Western blot analysis using total cell extracts from uninduced (−) or induced (+) iGATA4ES-derived embryoid bodies. Protein detection was performed using a monoclonal anti-FLAG antibody or an anti-GATA4 antibody, as indicated. Right panel: Coomassie Blue staining of the western blotted gel. (E) Gel mobility shift analysis using nuclear extracts from parental cells (AINV), or iGATA4ES cells, under uninduced (−) or induced (+) conditions. The specific competitor (sp. u.c.) is a 20x excess of unlabeled probe. The non-specific competitor (unsp. u.c.) is a 20x excess of unlabeled probe containing mutations in the GATA-binding site.
AinV cells were co-electroporated with the Gata4-IRES-Gfp targeting vector and a Cre expression vector, and neomyocin-resistant clones were selected; PCR assays of genomic DNA confirmed site-specific integration of the transgene (data not shown). The cell lines carrying the Flag-Gata4-IRES-Gfp transgene are referred to as iGATA4ES cells. Transgene expression was induced by addition of doxycycline to the culture medium and throughout this study similar results were obtained using pooled clones, or using any of several independent derived clonal lines. The absence of GFP expression in uninduced iGATA4ES cells shows that there is no detectable basal (leaky) expression of the transgene (Fig. 1B) while strong GFP expression in the cells cultured with doxycycline indicates appropriate conditional expression of the transgene (Fig.1C). Essentially all of the iGATA4ES cells express GFP (and by inference GATA4) according to flow cytometry (>95%, data not shown). Western blotting experiments using anti-FLAG or anti-GATA4 antibodies confirmed ectopic expression of GATA4 upon induction (Fig.1D). We also performed gel mobility-shift assays using nuclear extracts prepared from the parental AinV cells or iGATA4ES cells cultured under either uninduced (control) or induced conditions (Fig. 1E). A much higher level of binding activity is detected using nuclear extracts from induced iGATA4ES cells. A site-specific unlabeled probe competes effectively for the induced Gata4 activity, while a non-specific DNA fails to compete with the labeled probe, confirming that the induced GATA4 protein binds specifically to its target DNA sequence (Fig. 1E).
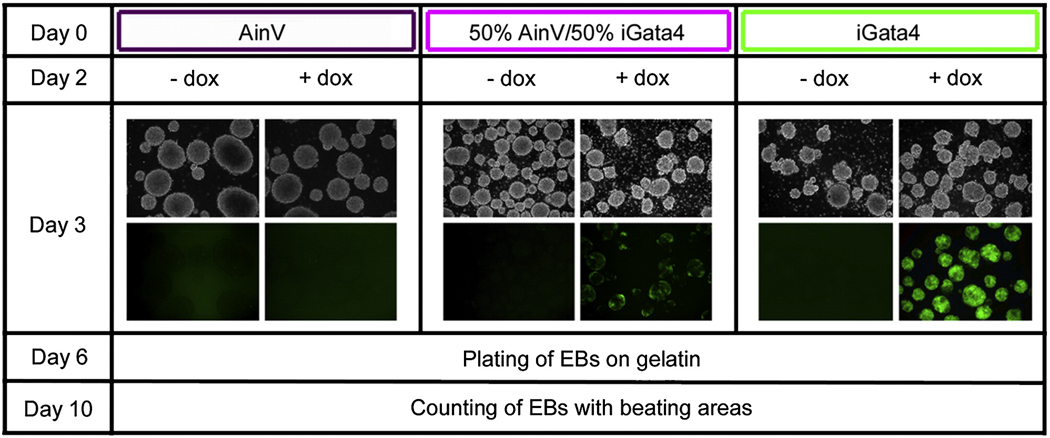
We next established an assay to determine if Gata4 expression influences ES-derived cardiogenesis during EB development. In this assay, EBs are grown in suspension for six days and then plated onto gelatin, under conditions (with serum) that are permissive for mesoderm development, but do not enhance differentiation of cardiac progenitors into beating cardiomyocytes. Gata4 was induced starting at day 2 during the initial 6 days of EB development, and at day 10 cardiogenesis was assessed by counting the number of EBs that develop with beating foci. Cardiomyocyte assays were set up in parallel, using three different cell populations (Fig. 2). The first group represents a negative control consisting of EBs derived from the parental AinV cells that do not contain an inducible transgene. The second population of EBs is derived from an equal mixture comprised of 50% AinV cells and 50% iGATA4ES cells. The third population of EBs is derived entirely from iGATA4ES cells. As shown in Fig. 2, when doxycycline is added to the cultures at day 2 of EB development, at day 3 the AinV EBs (group 1) do not express GFP. In the mixed population (group 2), induced EBs display patches of GFP expression, while EB cultures established with exclusively the iGATA4ES cells (group 3) are homogeneously bright green. In the absence of induction there is no detectable expression of GFP, indicating that the transgene is not leaky. We predicted that if Gata4 can function in a cell-autonomous manner to specify cardiomyocytes, doxycycline induction should generate increased numbers of beating foci in group 3 EBs, and this should be less efficient or ineffective in the group 2 or group 1 EBs, respectively. This is not what we observed.
Fig. 2. Experimental strategy for evaluating cell autonomy of Gata4 function within EBs.
Three populations of EBs were initiated in parallel at day 0: the first population is derived only from AinV parental ES cells, a second is comprised of an equal mixture of 50% AinV and 50% iGATA4ES cells, and a third is comprised entirely of iGATA4ES cells. At day 2, EBs were induced (+ dox) while control samples were left uninduced (− dox). At day 3, Gata4 transgene expression is monitored by observation of GFP expression (top panels: 10x phase; bottom panels: corresponding views under fluorescence). At day 6, EBs were plated onto gelatin under permissive conditions that allow differentiation (beating) of the cardiac progenitors. At day 10, the number of EBs containing beating foci was counted for each experimental condition.
Gata4 is sufficient to induce cardiogenesis but does so only in a non-cell-autonomous manner
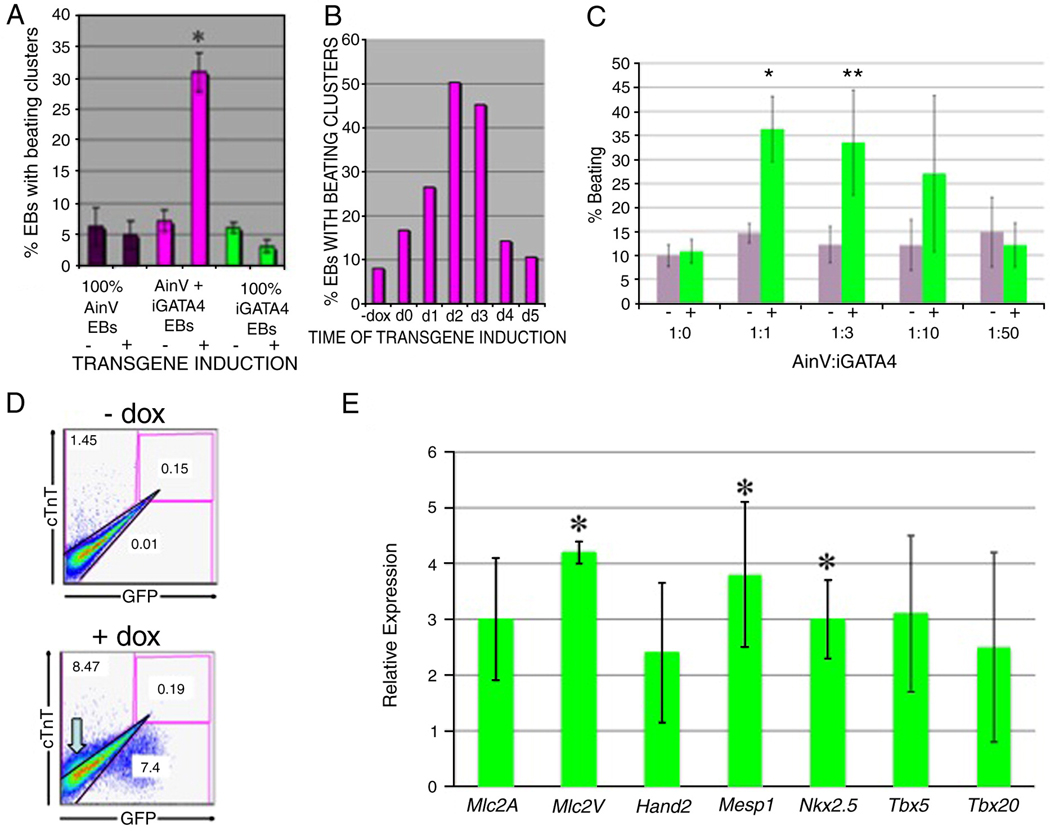
As expected, in the absence of doxycycline induction, the number of beating EBs generated was low, and similar for each of the three groups, regardless of the starting cell population (Fig. 3A). In addition, induction with doxycycline had no effect on the generation of beating foci for either group 1 (AinV cells) or group 3 (iGATA4ES cells). In fact, group 3 (derived from 100% iGATA4ES cells) showed a consistent decrease in beating foci to very low numbers approaching zero. However, in the case of group 2, containing EBs derived from an equal mixture of AinV and iGATA4ES cells, there is a significant 5 to 6-fold increase in the number of beating EBs (p<0.01). This result supports a hypothesis that Gata4-expressing cells do not develop into cardiomyocytes, but rather that they induce other ES-derived cells to a cardiomyocyte fate. For our assay, we chose day 2 for induction since this is the time during EB development that mesoderm is expected to be specified toward defined fates (Gadue et al., 2005). Since Gata4 appears to function non-cell-autonomously we tested a variety of different induction times during culture of the mixed EBs. Results from a representative experiment are shown in Fig. 3B, demonstrating that induction at around day 2–3 is an optimal time to generate a significant increase in cardiomyocyte specification. Based on the timing (day 2–3) the results indicate that Gata4 affects cardiogenesis at a relatively early progenitor state, for example at the mesendoderm or mesoderm stage, rather than later differentiation stages when cardiomyocyte progenitors are already committed (Kattman et al., 2007). We tested if cardiac induction was dependent on the ratio of Gata4-expressing cells, since a non-autonomous effect would not be expected to be strictly dependent on the number of inducing cells. When the ratio of iGATA4ES cells present in the mixed EBs is lowered 3-fold, the level of induction is similar and significant (Fig. 3C), and even EB cultures with only 1/10 of the cells expressing Gata4, although more variable, show a trend toward induction. Flow cytometry was used to measure quantitatively the number of cardiac troponinT-expressing cardiomyocytes, and this confirmed a cellular increase of approximately 6-fold when Gata4 expression was induced, and only in the mixed (group 2) EBs (Fig. 3D). The cTnT-positive cells are GFP negative, consistent with the commitment of the parental AinV cells in the mixed group to cardiac fate. The number of double-positive GFP+cTNT+ cells is negligible in both uninduced and induced EB samples.
Fig. 3. Induction of Gata4 enhances cardiomyogenesis, but only in mixed EBs.
Experiments were carried out as described in Fig. 2. (A) The relative percentage of EBs with beating foci were determined from 3 distinct EB populations (group 1: 100% Ainv; group 2: 50% AinV / 50% iGATA4ES (mixed); group 3: 100% iGATA4ES) cultured with or without induction. Gata4 induction at day 2 in mixed EBs results in a significant increase in the number of beating EBs at day 10 (asterisk, p<0.01). These data represent the combined results of 4 independent assays. Error bars represent the standard error of the mean. (B) Shown are results from a single but reproducible representative experiment in which mixed EBs were either left uninduced (−Dox) or induced at different days as indicated (d1 = day 1, etc.). EBs were cultured until day 6, plated for cardiomyocyte assays and beating foci counted at day 10. In a reproducible manner, maximal foci are seen with induction at day 2–3. (C) Efficient induction of cardiogenesis occurs over a range of iGATA4 ESC ratios. EBs were derived from starting ratios of parental AinV and iGATA4 ESCs (Ainv: GATA4, respectively), as indicated. At day 6, EBs were replated on gelatinized petri dishes in media supporting cardiomyocyte differentiation. The resulting EB clusters were counted at day 10. Shown is the combined result of three independent experiments. * p<0.006 and ** p<0.033 when comparing day 2 uninduced (−, grey bars) to the induced (+, green bars) group using Student's T-test. Even at the 1:10 ratio there is a clear trend toward induction, although it is more variable and did not show statistical significance. Error bars represent the standard error of the mean. (D) Shown is a representative example of flow cytometry using a 1:1 ratio of iGATA4ES:Ainv EBs that were either left uninduced (−dox) or induced at day 2. The EBs were plated at day 6, and at day 10 the EBs were harvested, dissociated, and evaluated concurrently for GFP expression (fluorescence) and cTnT expression (using intracellular antibody staining). The cTnT-positive cell population is indicated by the block grey arrow in the +dox panel. The induced EBs show typically 10% cardiac cells (compared to 1% in control samples), although this is likely an under-estimate, since the beating foci containing cardiomyocytes are difficult to dissociate, and only single cells were counted. The GFP+ cells fall outside the cTnT gate, and the percentage of double-positive cells in the induced samples is never above background. (E) Quantitative real time PCR analysis for cardiac markers, as indicated, in day 6 induced mixed EBs normalized to levels in day 6 uninduced controls. Shown are results from three independent experiments, with relative expression data log2 transformed. Error bars indicate the standard error of the mean with asterisks denoting statistical significance (p<0.05). In other cases, a single sample gave a relatively high difference. While this limited statistical significance, the trend for all cardiac markers is nevertheless clear.
We evaluated expression levels for markers by quantitative real-time PCR using cDNA isolated from the mixed (group 2) control or induced EBs, and confirmed that cardiac differentiation is enhanced by expression of Gata4 (Fig. 3E). Expression levels for cardiomyocyte markers Mlc2A and Mlc2V are increased significantly, but only when Gata4 expression is induced. This also correlates with increased levels of early cardiac commitment genes, since expression levels for markers of the early cardiac lineage including Hand2, Mesp1, Nkx2.5, Tbx5, and Tbx20 are all increased in the mixed (group 2) EBs upon induction of Gata4. In total, the data indicates that Gata4 expands the number of cells that commit to the cardiac fate.
Gata4 expression increases specifically cardiac progenitors rather than expanding mesoderm derivatives generally
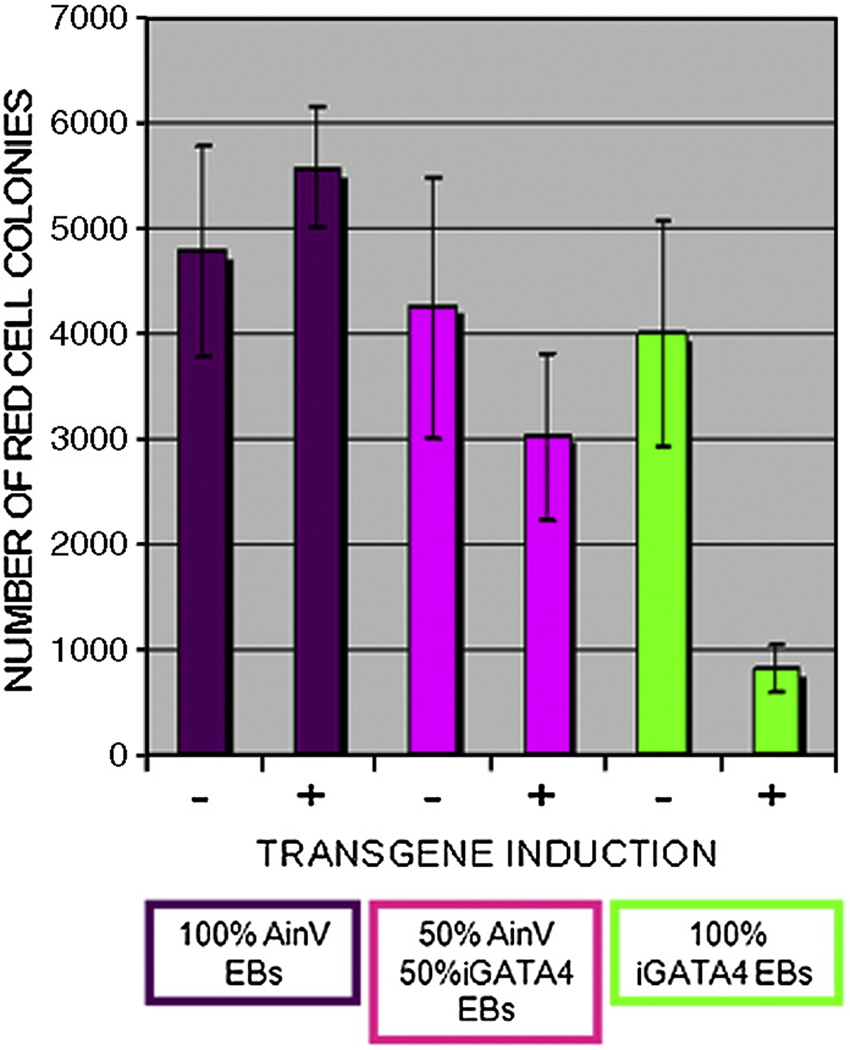
One possibility is that Gata4 expands the number of early EB progenitors that commit to a mesoderm fate, for example by influencing the fate of mesendoderm in the EB cultures during the equivalent of embryonic gastrulation. In this case, other mesoderm derivatives besides cardiac would be expected to increase. It is well established that in developing EBs the hemangioblast, a common progenitor for primitive hematopoietic and vascular endothelial cells, develops from a cell population within the mesoderm that is distinct from the cardiovascular progenitors (Kattman et al., 2007). We therefore assessed the effect of Gata4 expression on commitment to the hematopoietic fate, by analyzing the ability of induced EBs to generate differentiated primitive erythroid colonies. We compared the three experimental groups described previously (Fig. 2) in the presence or absence of Gata4 induction, except that at day 6 the EBs were dissociated and progenitors were cultured for 5 days in methylcellulose, under conditions that promote erythroid colony development (Fig. 4). As expected, all three uninduced groups generate a similar number of erythroid colonies, and this is equivalent in the samples obtained from induced AinV control cells. However, the cells plated from group 2 (mixed) EBs that had been induced to express Gata4 generate significantly less erythroid colonies compared to the same samples that had been left uninduced (p<0.05). Even more striking, the EBs derived from iGATA4ES cells (group 3) show a major reduction in erythroid colony formation compared to any of the uninduced EBs (p<0.05). These results indicate that mesoderm in the mixed EBs are relatively defective at hematopoietic development, due to the presence of the Gata4-expressing cells. Since Gata4 expression induces cardiac fate through a non-cell-autonomous mechanism, this does not occur through an increase of mesoderm formation, but rather by directing specification of early progenitors toward a cardiac fate.
Fig. 4. Gata4-induced signals suppress hematopoietic progenitors.
Erythroid colony assays were carried out using the three different EB populations (100% Ainv; 50% AinV / 50% iGATA4ES (mixed); 100% iGATA4ES) cultured with (+) or without (−) induction by doxycycline. Gata4 induction at day 2 of iGATA4ES or mixed EBs results in a significant decrease in the number of primitive erythroid colonies (p<0.01 and p<0.05 respectively). These data represent the combined results of 4 independent colony assays. Error bars represent the standard error of the mean.
Gata4 directs development of ES-derived EBs into endoderm
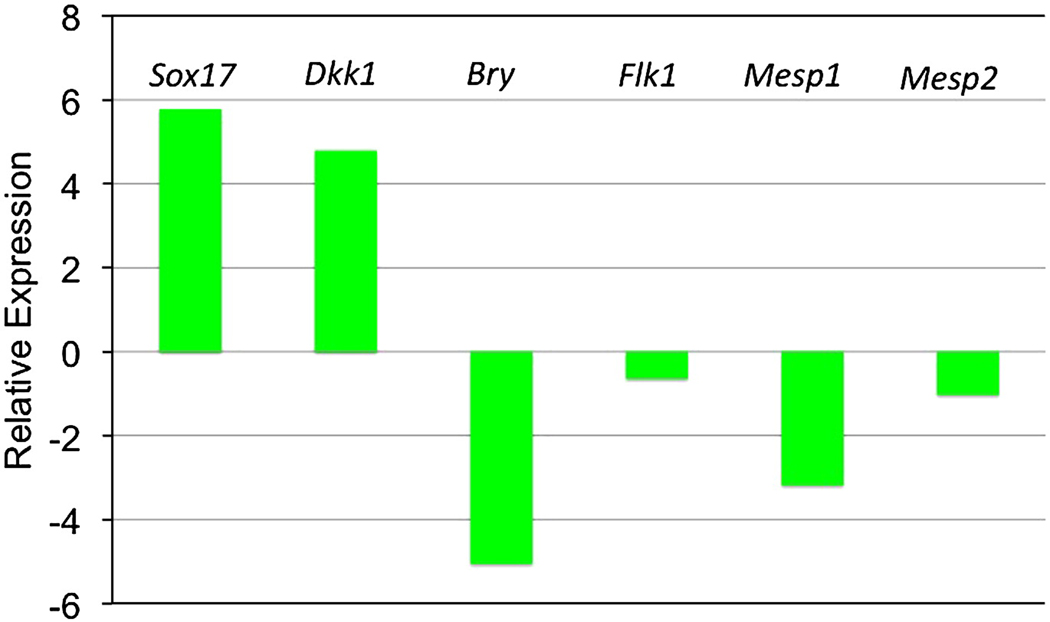
The ES-derived cells expressing Gata4 do not differentiate into cardiomyocytes, yet they influence indirectly cardiomyocyte fate. We therefore carried out marker analysis to investigate the fate of Gata4-expressing cells in the mixed EBs. For this purpose, we separated by FACS the GFP+ and GFP− cells from mixed EB cultures (group 2) at day 3, following 24 hr of incubation with doxycycline. These cells were harvested and used to generate cDNA for qPCR analysis (Fig. 5). The GFP+ cells (iGATA4ES-derived) expressed more than 50-fold and 25-fold enhanced levels of the early endoderm markers Sox17 and Dkk1, respectively, compared to the GFP− cells (AinV-derived). In contrast, the iGATA4ES cells expressed dramatically reduced levels of mesoderm markers Bry and Mesp1, and relatively reduced levels of mesoderm markers Flk1 and Mesp2, compared to the AinV-derived cells. These results strongly support the idea that Gata4 functions between day 2 and day 3 in the mixed EBs to direct in a cell-autonomous manner endoderm fate.
Fig. 5. In EBs that are enhanced for cardiogenesis, the Gata4-expressing cells commit to endoderm, not mesoderm.
Shown are representative qPCR results using cDNA derived from cells sorted out of mixed EBs (group 2, 50% iGATA4ES / 50% AinV). EBs were induced with doxycycline at day 2, and at day 3 the GATA4-expressing cells were sorted as GFP+ and compared to levels of transcripts (as indicated) in the GFP−. The normalized expression data is shown log2 transformed. The GATA4− expressing GFP+ cells express relatively high levels of endoderm markers Sox17 and Dkk1, and relatively low levels for the mesoderm markers. Multiple independent experiments gave similar results.
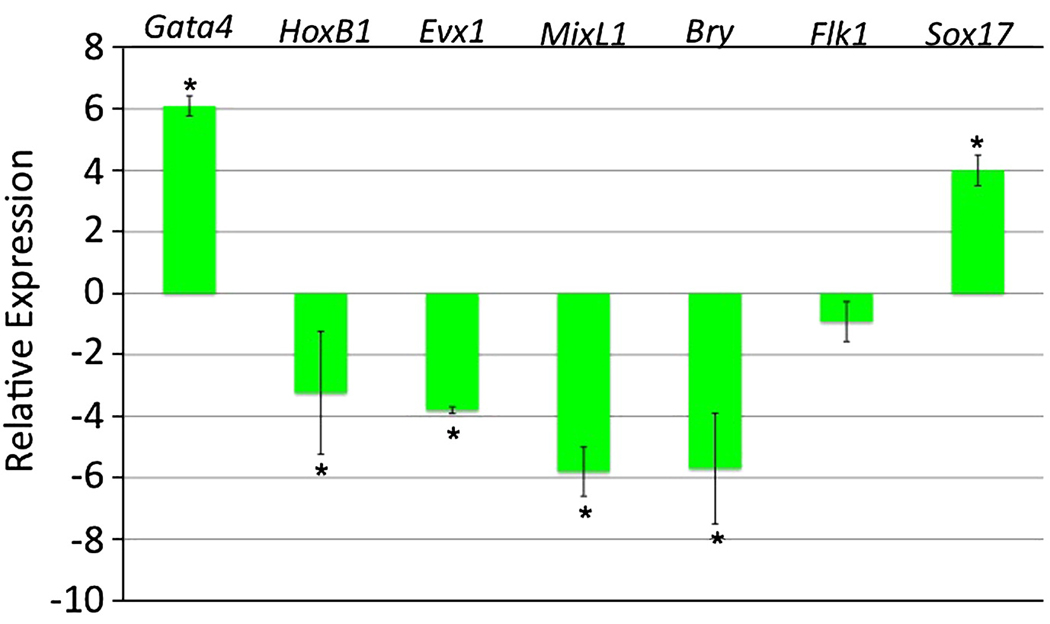
Gata4 was shown previously to be necessary and sufficient for visceral endoderm differentiation from murine embryonic stem cells (Fujikura et al., 2002; Soudais et al., 1995). However, the Sox17+ endoderm generated in iGATA4 ES cells is caused by induction starting at day 2 of EB development, when cells may be responsive to signals that commit toward a definitive endoderm or mesoderm fate. We therefore carried out transcript profiling experiments with EBs derived entirely from iGATA4ES cells either left uninduced or induced with doxycycline at day 2. RNA was purified at day 3 from these cultures and used to generate cDNA. The relative expression levels for specific early markers were evaluated by quantitative real-time PCR (Fig.6). The EBs that had been induced to express Gata4 show a 70-fold increase in Gata4 transcript levels. In contrast, the same induced samples express much lower levels of transcripts for the posterior primitive streak markers HoxB1 and Evx1, as well as the early mesoderm markers MixL1 and Bry. Sox17 transcript levels are substantially increased. These results confirm, as shown for the mixed cultures, that Gata4 expression directs the development of ES-derived progenitors toward generation of endoderm and not mesoderm.
Fig. 6. In EBs derived entirely of iGATA4ES cells, induction of Gata4 generates Sox17+ cells and a relative loss of mesoderm.
Shown are accumulated qPCR results from three independent experiments measuring transcripts at day 3 for cells from EBs induced to express GATA4 protein at day 2. Levels from induced EBs were compared with uninduced controls and the relative differences are plotted with the data log2 transformed. Early mesoderm markers are repressed, while Sox17 transcript levels are increased. Error bars indicate the standard error of the mean with asterisks denoting statistical significance (p<0.05).
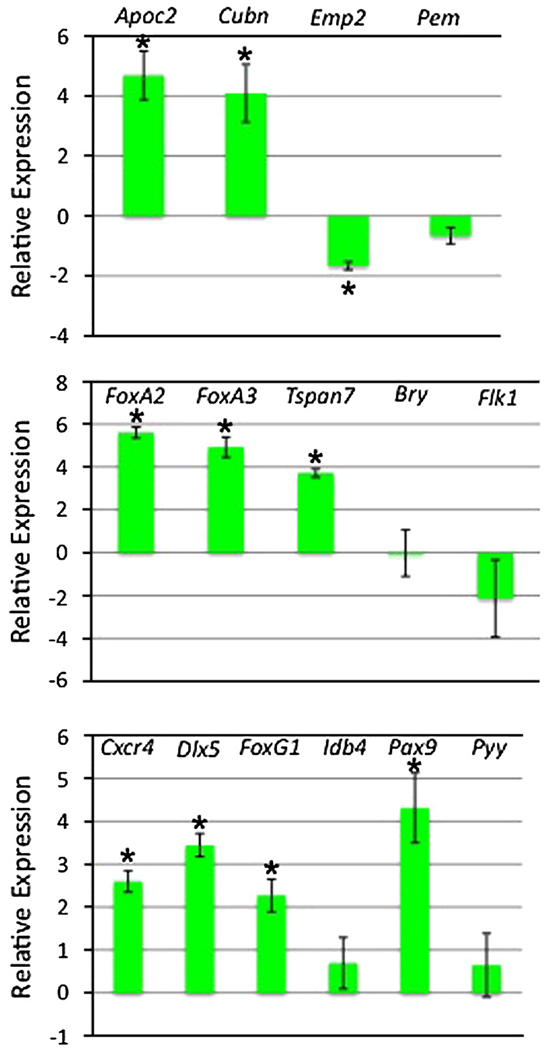
However, a closer evaluation of endoderm markers revealed additional complexity. Visceral and definitive endoderm lineages are closely related and share the expression of many pan-endoderm markers, including Sox17 and Dkk1. Several groups have sought recently to better characterize genes that distinguish the endoderm sub-types, through expression profiling of isolated embryonic endoderm tissues (Hou et al., 2007; Sherwood et al., 2007). We used this information to focus on markers that should be relatively enriched in visceral or definitive endoderm (Fig. 7). RNA was prepared control and induced samples at day 6 and evaluated by qPCR. The expression levels for the visceral endoderm markers Pem and Emp2 are reduced in EBs that are induced at day 2 to express Gata4. In contrast, the transcript levels of Apoc2 and Cubn are substantially increased by Gata4 expression, suggesting that the iGATA4 EBs generate a sub-type of visceral endoderm. Meanwhile, definitive endoderm markers Cxcr4, Dlx5, FoxG1, and Pax9 are all significantly increased, while modest increases are noted also for Idb4 and Pyy, indicating that Gata4-expressing EBs are also directed toward a definitive endoderm fate. Cxcr4 is a marker that clearly distinguishes definitive endoderm from extra-embyronic endoderm (Morrison et al., 2008; Takenaga et al., 2007).
Fig. 7. Markers for both visceral and definitive endoderm are enhanced when GATA4 expression is induced at day 2 of EB development.
Shown are results from three independent experiments using three different iGATA4ES subclones. Cells were harvested at day 6 and qPCR was performed to measure relative fold change comparing the values from each subclone to its uninduced control, shown in the graphs with the data log2 transformed. Top panel: A subset of defined visceral endoderm markers are increased in levels (Apoc2, Cubn), while others (Emp2, Pem1) are not, Middle panel: Expression of pan-endoderm markers (FoxA2, FoxA3, Tspan7) are increased whereas by day 6 Bry and Flk1 transcript levels are unchanged or downregulated. Bottom panel: definitive endoderm marker levels are generally increased (Cxcr4, Dlx5, FoxG1, Idb4, Pax9, Pyy). Error bars indicate the standard error of the mean with asterisks denoting statistical significance (p<0.05). In some other cases, a single sample gave a relatively high difference. While this limited statistical significance, the trend is nevertheless clear.
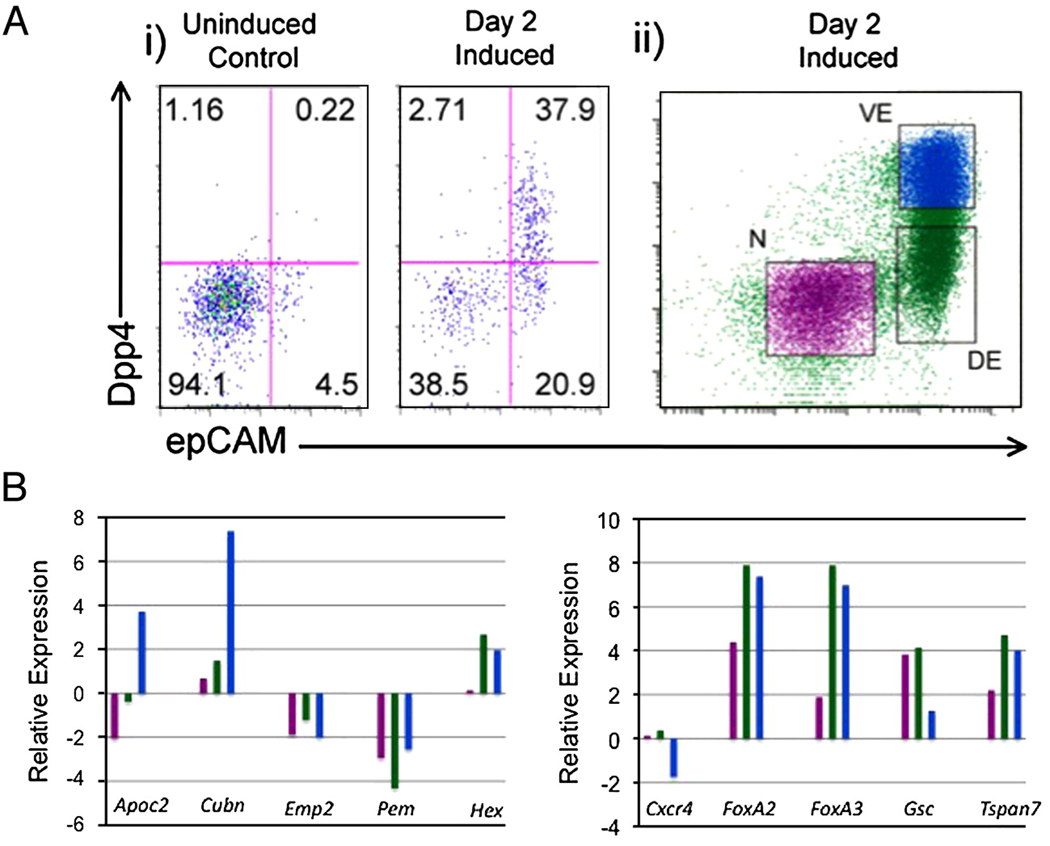
Since Gata4-expression appears to direct development in EBs of both primitive and definitive endoderm, we determined if both sub-types could be quantified by flow cytometry. We used a double-staining protocol suggested by Sherwood et al. (2007) to distinguish the cell types. Staining for epCAM identifies all embryonic endoderm sub-types, while high levels of DPP4 specifically characterize visceral endoderm. As shown in a representative flow experiment (Fig. 8Ai), both endoderm sub-types are readily distinguished and increased in numbers when iGATA4ES cells are induced to express Gata4. In EBs induced at day 2, we reproducibly find approximately 60% of the cells are by day 4 epCAM+ endoderm. Approximately 50% of these endoderm cells are DPP4+ (visceral endoderm), with the other half being DPP- (definitive endoderm). When these two sub-types were sorted (Fig. 8Aii) we could validate their character by qPCR analysis (Fig. 8B). For example, only the double positive cells are enriched for Apoc2 and Cubn, the single positive epCAM+ cells are enriched for Cxcr4 and Gsc transcripts (compared to the double-positive cells, but similar to the double-negative mesoderm-containing population, as expected), while both endoderm populations are highly enriched for pan-endoderm markers Hex, Sox17, FoxA2, FoxA3, and Tspan7. Consistent with the whole EB samples, the double-positive cells are not enriched for the visceral endoderm markers Emp2 or Pem.
Fig. 8. GATA4 induces a mixture of visceral and definitive endoderm sub-types.
(A) Shown is the flow cytometry strategy, with live cells gated for epCAM (endoderm) and Dpp4 (visceral endoderm). (i) A representative experiment is shown of an uninduced iGATA4ES control and the same cells induced to express GATA4 at day 2 of EB development. Cells were harvested and analyzed at day 4 on a FACSCalibur cytometer. This experiment was repeated (n = 3) with similar results using independent subclones. (ii) Gating strategy used in the preparative sorting experiments with iGATA4 EBs induced at day 2 and dissociated at day 4 of development. Cells were gated as negative (N, epCAM-/Dpp4−), definitive endoderm (DE, epCAM+/Dpp4−), or visceral endoderm (VE, epCAM+/Dpp4+) and populations were sorted with a BD FACSVantage cell sorter. Secondary analyses showed the populations are greater than 90% pure (not shown). (B) Shown are representative qPCR analyses to validate the sorting strategy. The relative transcript levels are plotted with the data log2 transformed, compared to those in the control uninduced sample. Double negative and VE and DE endoderm samples are labeled as in panel Aii. Left panel: Known VE markers, Right panel: Known pan and DE markers. Both definitive and visceral endoderm sorted cells show expression of pan-endoderm markers (FoxA2, FoxA3, Tspan7) whereas the expression of definitive endoderm (Cxcr4, Gsc) or visceral endoderm (Apoc2, Cubn) marker levels are relatively enhanced in their respective sorted populations. As expected, the double-negative sample, containing mesoderm, is also positive for Cxcr4 and Gsc. Both endoderm subtypes demonstrate expression of the anterior endoderm marker Hex and decreased levels of visceral endoderm markers Emp2 and Pem1. Equivalent results were obtained in at least 3 independent experiments.
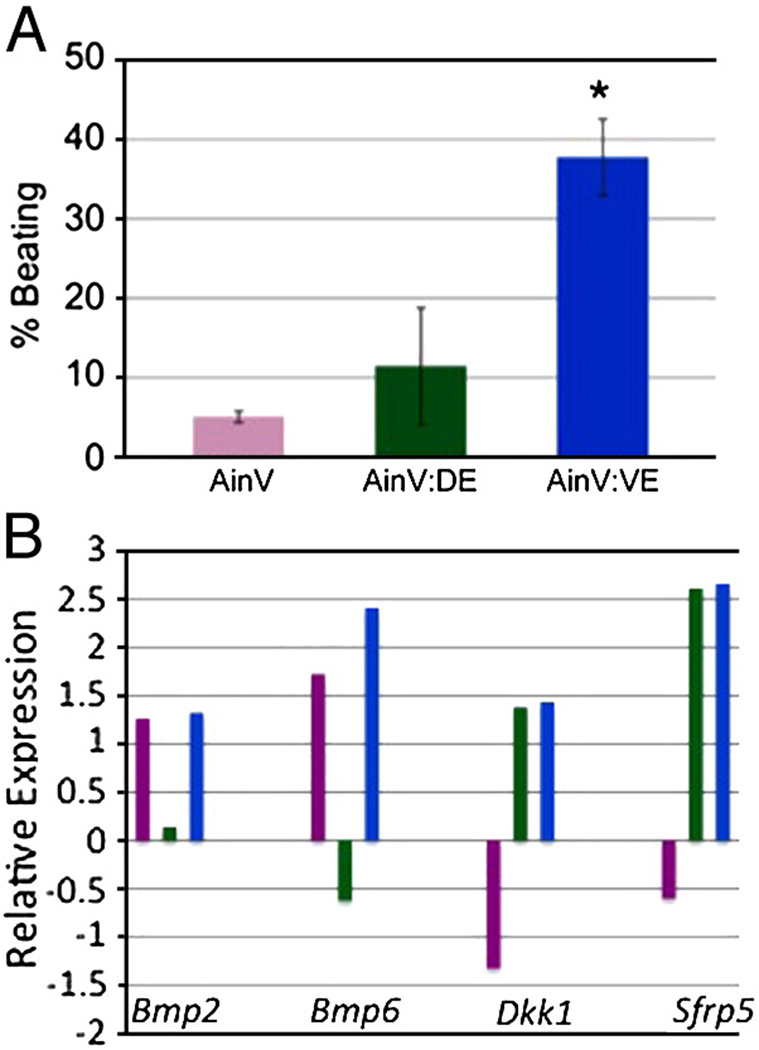
The ability to distinguish by flow cytometry distinct types of endoderm allowed us to test their respective cardiac inducing activities. For this purpose, iGATA4ES cells were used to generate EBs that were induced with doxycycline at day 2, and the day 4 epCAM+Dpp− (definitive endoderm) and epCAM+Dpp+ (visceral endoderm) cells were purified by flow cytometry. These cells were used separately to make EBs by re-aggregation with equal numbers of cells derived from EBs made using the parental AinV line. The re-aggregated EBs were cultured in the standard assay to evaluate the development of beating foci and cardiomyocytes. As shown in Fig. 9A, both types of endoderm had cardiac-inducing activity, but this was low and variable in the case of the definitive endoderm. In contrast, the bulk of the cardiac-inducing activity appears to derive from the visceral endoderm sub-type, which gave results similar to those using unsorted iGATA4ES cells. Since the two endoderm samples are closely related, we considered whether downstream targets regulated by Gata4 might include secreted cardiac-inducing signaling molecules. Preliminary microarray experiments, comparing transcript patterns generated using cDNA from uninduced and induced iGATA4ES-derived EBs identified significant increases in expression for Dkk1, Sfrp5, Bmp2, and Bmp6(data not shown). This was confirmed quantitatively by qPCR using independent samples that were sorted to compare the visceral and definitive endoderm derivatives (Fig. 9B). Both Dkk1 (Marvin et al., 2001; Schneider and Mercola, 2001) and Sfrp5 (Finley et al., 2003) are known WNT inhibitors, and WNT inhibition is a key step of cardiac induction. BMPs have also been implicated upstream of a cardiac-inducing pathway (Bin et al., 2006; Shi et al., 2000). Interestingly, we find that both visceral and definitive sub-types express high and similar levels of transcripts for the WNT antagonists, while the more active (cardiac-inducing) visceral endoderm is relatively enriched for BMP transcripts.
Fig. 9. The bulk of the cardiogenic inducing activity from GATA4-expressing cells is in the visceral endoderm population.
(A) Cell populations were sorted at day 4 as described in Fig. 8 and were then mixed with dissociated cells from day 2 AinV EBs in a 1:1 ratio at a concentration of 1.25 × 105 cells/ml. Reaggregated EBs were plated on gelatinized petri dishes four days later in media supporting cardiomyocyte differentiation. The resulting EB clusters were counted at day 10 (relative to the responding AinV cells). Shown are the combined results of two independent experiments. The asterisk indicates p<0.011 when comparing AinV:VE to AinV using Student's T-test. (B) Differential expression of cardiac inducing molecules in the two endoderm populations. Shown are representative qPCR results using cDNA generated from sorted cell populations. Cells were derived and sorted as described in Fig. 8, and the levels relative to uninduced controls are plotted with the data log2 transformed. Both endoderm sub-types express enhanced levels of the Wnt antagonists Dkk1 and Sfrp5, whereas the visceral endoderm, compared to definitive endoderm, shows relatively increased levels of Bmp2 and Bmp6 transcripts. Equivalent results were obtained in at least three independent experiments.
Gata4 expression directs the development of endoderm with liver potential
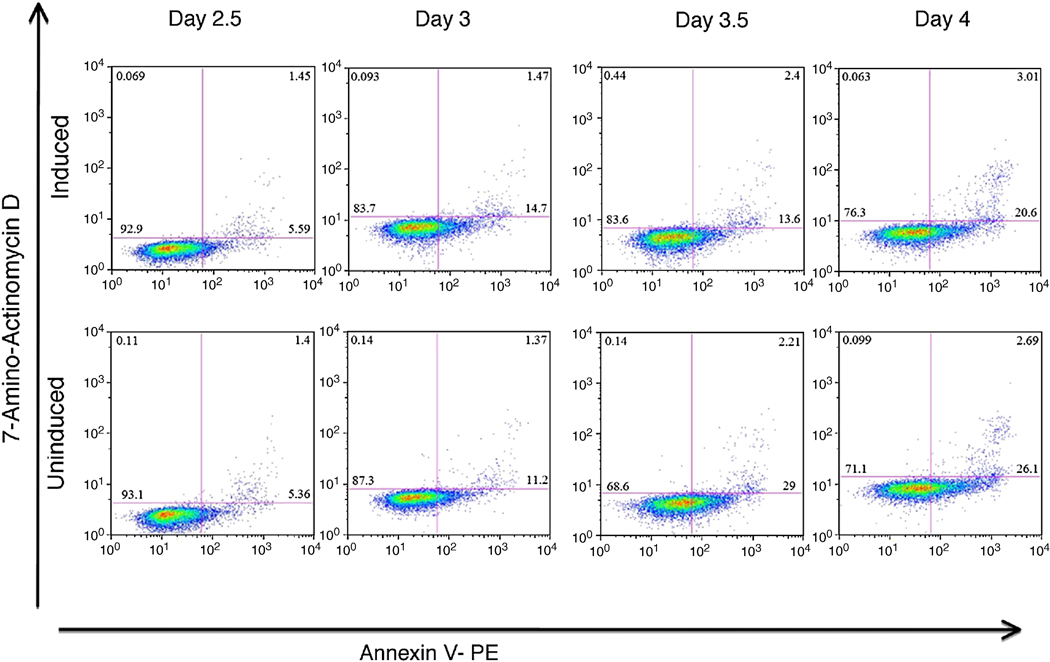
We considered if Gata4 leads to selection of cells that are already endoderm fated, for example by loss of mesoderm. In this case we would expect to see differences in cell survival in EBs induced to express Gata4. We generated EBs using the iGATA4ES line, induced Gata4 expression at day 2 with doxycycline, and harvested cells at 12, 24, 36, or 48 hr for quantitative evaluation of cell death and apoptosis by flow cytometry. As shown from a representative experiment in Fig. 10, there is not a significant change in the amount of cells that are 7-AAD or Annexin V positive at any time point, comparing the uninduced and induced samples. Total cell numbers in the EB cultures do not vary significantly in the control or doxycycline-induced conditions, and there is no evidence of differential cell death Since the EBs generate considerably more endoderm than mesoderm, Gata4 appears to actively direct uncommitted cells toward endoderm fates.
Fig. 10. Gata4 does not selectively induce apoptosis in EB cultures.
EBs were generated from the iGATA4ES line and either left uninduced or induced at day 2 to express Gata4. EBs were harvested at day 2.5, 3, 3.5, or 4 as indicated, dissociated, and cells evaluated by flow cytometry for cell death (7-AAD) and apoptosis (Annexin V). These data are from a representative experiment that was reproducible with equivalent results.
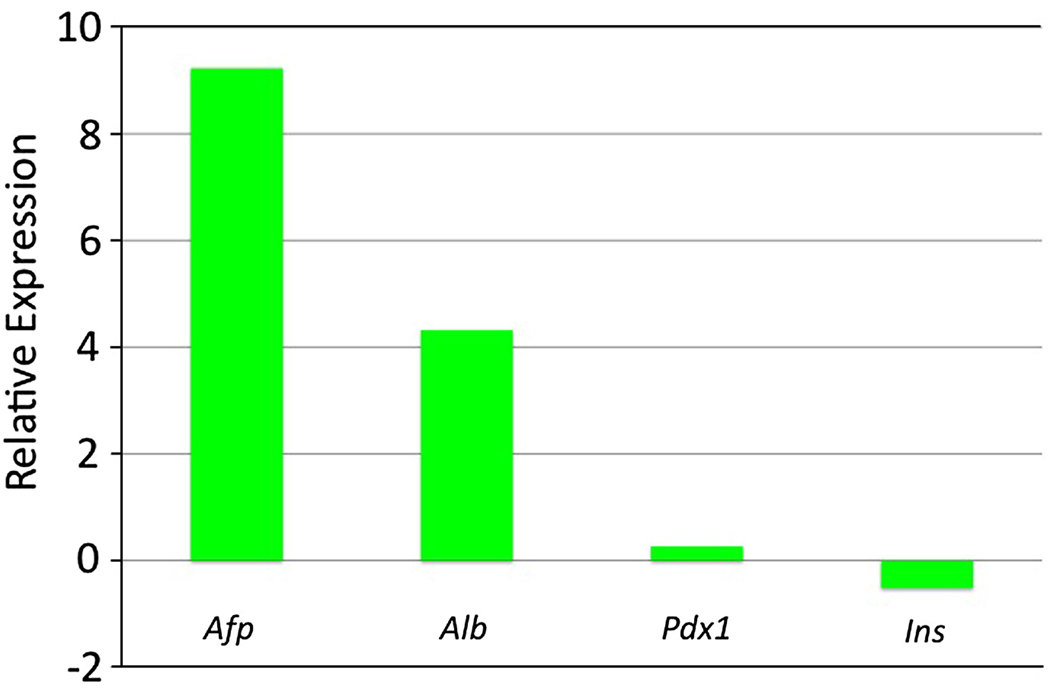
Finally, we allowed the GATA4-expressing cells to remain in culture to evaluate the eventual fate of the endoderm-committed cells. The iGATA4ES-derived EBs were induced at day 2 and allowed to develop in culture to day 17. Analysis of transcript levels by qPCR showed that these EBs differentiate with a significant bias toward liver fate (Fig. 11). In contrast to day 6 EBs, these cultures no longer show enhanced levels of visceral endoderm markers. Instead, the transcript levels are enhanced 620-fold for Afp and 20-fold for Alb in the induced samples compared to the uninduced control EBs. In contrast, there was little if any relative difference in the transcript levels for the pancreatic markers Pdx1 or Ins.
Fig. 11. The GATA4-expressing EBs eventually are highly enhanced for liver markers.
The EBs derived entirely from iGATA4ES cells were induced to express Gata4 at day 2, plated at day 10 under conditions that normally support mesoderm development, and harvested at day 17. Shown are representative qPCR results analyzing cDNA isolated from induced EBs compared with levels of transcripts (as indicated) in the uninduced control cells. The relative levels are plotted with the data log2 transformed. The GATA4-expressing cells have relatively high levels of transcripts for liver markers Afp and Alb, and relatively unchanged levels for the pancreas markers Pdx1 and Ins. Multiple independent experiments gave equivalent results.
Discussion
Heart failure associated with the loss of contractile cardiomyocytes is a major cause of mortality and morbidity, and strategies that facilitate efficient generation of cardiomyocytes from ES cells provide an attractive approach for developing cell-based therapies (Chien et al., 2008; Murry and Keller, 2008). An important goal is to identify the appropriate molecular pathways that can enhance the specific and efficient specification and differentiation of cardiomyocytes. We hypothesized that Gata4 might stimulate this process, since it is an early cardiomyocyte marker and is recognized as a key regulator of cardiomyocyte growth and survival (Heineke et al., 2007; Liang and Molkentin, 2002). Our results using conditional expression in an ES cell model confirm that Gata4 expression enhances significantly the commitment of progenitor cells to a cardiomyocyte fate. However, we found that the mechanism is indirect, and Gata4 induces cardiomyocyte differentiation in a non-cell-autonomous manner. The study suggests that Gata4 directs expression of target genes, including Dkk1 and Bmp6 encoding secreted factors with the potential to stimulate cardiogenesis.
A role for GATA factors in regulating endoderm development reflects an evolutionarily conserved pathway. GATA factors are essential for endoderm specification in worms and flies (Maduro and Rothman, 2002; Murakami et al., 2005), and ectopic expression of the C. elegans or Drosophila endodermal Gata genes induces endoderm development in Xenopus ectoderm explants (Murakami et al., 2005; Shoichet et al., 2000). Similarly, Xenopus Gata4 regulates gut-specific genes (Gao et al., 1998) and induces endoderm gene expression in ectoderm explants (Afouda et al., 2005). Embryonic explant assays implicated anterior endoderm as a tissue from which cardiac inductive cues are derived, including members of the NODAL, BMP, FGF and WNT signaling pathways (for review, see (Lough and Sugi, 2000). Explant assays in Xenopus identified DKK1 as a secreted factor required for expression of Hex1 in endoderm associated with presumptive cardiac mesoderm (Foley et al., 2006). Consistent with these data, the ES-derived cardiac-inducing cells specified by Gata4 express and secrete high levels of antagonists for canonical WNT/beta-catenin signaling, specifically Dkk1 and Sfrp5. Using ELISA assays, the culture medium from day 6 induced EBs derived from iGATA4ES cells was shown to contain approximately 14-fold the levels of DKK1 compared to medium from sister EBs that were not induced (not shown). We attempted to evaluate the ability of conditioned medium from the iGATA4ES cells to induce cardiogenesis, and the necessity of Dkk1 for cardiac induction in the EB system using DKK1-specific blocking antibodies, but in both cases the results were equivocal, possibly for technical reasons related to reagent penetrance, or because of variable compensation from other antagonists (e.g. Sfrp5) or factors (e.g. Bmp6). While Gata4 clearly functions non-cell-autonomously, we do not know if the secreted factors function to regulate the Gata4-induced endoderm in a paracrine fashion, as suggested by Xenopus endoderm studies (Foley et al., 2006). We established cultures with iGATA4ES EBs separated by transwell filters from EBs derived from parental Ainv ES cells. When induced with doxycycline the transwell cultures failed to demonstrate significant induction of cardiogenesis. Together, these negative results suggest that Gata4-induced signals may be complex and/or function by a juxtaparacrine mechanism during EB development. Forced expression of a cardiac mesoderm specification gene, MesP1, is sufficient to induce cardiac fate, by a DKK1-mediated pathway (David et al., 2008). Therefore, it is possible that both mesoderm and endoderm associated with the presumptive cardiac region integrate cardiac induction using common signals. Expression of Gata4 in the mixed EBs is sufficient to drive transcript levels for Dkk1, Hex, and MesP1.
The timing of WNT antagonism is of critical importance to regulating cardiogenesis (Eisenberg and Eisenberg, 2006). The ES cell/embryoid body system is proving useful for dissecting this problem in the mouse, given the spatio-temporal complexity of the early embryo, and since the expression of key regulatory genes can be directed with temporal specificity. With respect to WNT signaling, early canonical WNT signals are essential for generation of Flk1+ mesoderm, including presumptive cardiac mesoderm, so that application of DKK1 from the earliest stages blocks expression of Gata4 (Lindsley et al., 2006). Using conditional expression of WNT pathway modulators in zebrafish, WNT signaling prior to gastrulation was shown to promote cardiac fate, while later signaling during gastrulation represses heart formation (Ueno et al., 2007). In our EB assay, induction of Gata4 expression around day 2–3 is optimal for inducing cardiogenesis. While this is a relatively early time point, the subsequent accumulation of functional WNT antagonists (requiring target gene transcription, translation, secretion, etc.) around day 4–6 is more consistent with the later (post-gastrulation) requirement to inhibit WNT signaling. Importantly, the comparison of visceral and definitive endoderm subtypes at day 4 of EB development suggests that WNT antagonism expression is not sufficient for efficient induction, and implies that another, perhaps earlier signal (For example, BMP-mediated) integrates with subsequent WNT inhibition. Other factors, yet to be identified, may also be required.
Continued WNT signaling (along with VEGF) directs mesoderm toward a hematopoietic fate (Nostro et al., 2008). Our data suggests that Gata4 has a dominant activity to direct mesendoderm toward an endoderm fate, even under culture conditions that normally support mesoderm at the expense of endoderm development (Kubo et al., 2004). During mesoderm development in our mixed EB cultures, factors induced by Gata4 commit cells toward a cardiac fate, since timed expression of Gata4 is sufficient to enhance cardiomyocyte fate while at the same time repressing the hematopoietic program. BMP signaling enhances hematopoiesis, so that the repression of hematopoietic fate could be mediated by suppression of WNT signaling. Experiments in Xenopus and ES cell models implicate Wnt11 as a non-canonical signal that is required subsequently for promoting cardiogenesis (Afouda et al., 2008; Ueno et al., 2007).
Inhibition of the endoderm specification gene Sox17 in ES cells by RNA interference results in suppression of cardiomyocyte differentiation, without altering mesoderm formation. Sox17 induces cardiomyogenesis in a non-cell-autonomous manner through the endodermal cardiogenic factor Hex (Liu et al., 2007), consistent with the Xenopus studies of Mercola and colleagues. In the Sox17 study, the levels of Gata4 were not affected by the lack of Sox17. On the other hand, induced Gata4 expression in our EB system increases significantly the levels of Sox17 and Hex. Therefore, our results indicate that Gata4 acts upstream of Sox17 for the production of endoderm-derived heart-inducing factors. It should be noted that Gata4 does not function only in the endoderm. Embryo-proper and cardiac conditional knockout experiments have clearly demonstrated Gata4 functions for proliferation and survival of cardiomyocytes (see Introduction). While the function in mixed EB cultures for Gata4 is for development of cardiac-inducing endoderm, it remains to be known if Gata4 in the mesoderm cells regulates the response to endoderm-derived cardiac-inducing signals. Forced expression of Gata4 directly in embryonic mesoderm is not sufficient to induce cardiac fate, but does promote cardiac specification and differentiation when co-expressed in non-cardiac mesoderm with Tbx5 and Baf60c (Takeuchi and Bruneau, 2009).
In summary, our data show that Gata4 expression is sufficient to specify cardiomyocyte fate in ES-cell derived progenitors, although it does so by an indirect mechanism. Rather than directing mesoderm toward a cardiac fate, Gata4 (even when expressed during EB cultures that promote mesoderm) directs the development of cardiac-inducing visceral endoderm and definitive endoderm with hepatocyte potential. Recent lineage tracing experiments in the mouse showed, contrary to previous dogma, that visceral endoderm incorporates extensively into the epiblast-derived definitive endoderm and incorporates into the developing gut tube (Kwon et al., 2008). It will be interesting to know if the visceral endoderm subtype generated by Gata4 in EBs relates to this process. We note that forced expression of Gata4 in EB cultures could alter the normal kinetics of cell specification, proliferation and/or differentiation, and it is therefore possible that Gata4 does not normally function in this manner. The current study is purposefully meant to explore “functional capability” rather than normal development. Yet since Gata4 is expressed in visceral and definitive presumptive foregut endoderm, and in the associated cardiogenic mesoderm, it may play a central role through co-regulating both the character of cardiac-associated inductive endoderm, and the cardiac mesoderm itself. This would permit the appropriate spatial and temporal presentation of BMPs, WNT antagonists and other signals to promote cardiomyocyte development at the right time and place. In addition to the developmental relevance of this study, we established an assay to identify additional Gata4-dependent factors that can impact the generation of cardiomyocytes and possibly hepatocytes from ES cells.
Supplementary Material
Acknowledgements
We thank George Daley and his colleagues for generously providing the parental cell lines and targeting vector. The authors are grateful for experimental advice and reagents from Brian Zafonte, Gordon Keller, and Valerie Gouon-Evans, comments on the manuscript from Gordon Keller, Steve Kattman, Stephan Irion, Cristina Nostro, and Valerie Gouon-Evans, and to Ingrid Torregroza for technical assistance. Anonymous reviewers provided key suggestions to improve the study. We thank Sergei Rudchenko and Stanka Semova, of the Hospital for Special Surgery Flow Cytometry Core Facility, for technical assistance. This work was supported by a grant to T.E. from the National Institutes of Health (HL64282). G.E.R. was supported by an NIH Training Grant (GM07491). Each of the authors contributed significantly to the study. A.H. devised the strategy, carried out experiments, and wrote the manuscript. G.E.R. carried out experiments and wrote the manuscript. T.E. devised the strategy, carried out experiments, and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afouda BA, Ciau-Uitz A, Patient R. GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development. 2005;132:763–774. doi: 10.1242/dev.01647. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Bin Z, Sheng LG, Gang ZC, Hong J, Jun C, Bo Y, Hui S. Efficient cardiomyocyte differentiation of embryonic stem cells by bone morphogenetic protein-2 combined with visceral endoderm-like cells. Cell Biol Int. 2006;30:769–776. doi: 10.1016/j.cellbi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103:14471–14476. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H, et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988;85:5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley KR, Tennessen J, Shawlot W. The mouse secreted frizzled-elated protein 5 gene is expressed in the anterior visceral endoderm and foregut endoderm during early post-implantation development. Gene Expr Patterns. 2003;3:681–684. doi: 10.1016/s1567-133x(03)00091-7. [DOI] [PubMed] [Google Scholar]
- Foley AC, Gupta RW, Guzzo RM, Korol O, Mercola M. Embryonic heart induction. Ann N Y Acad Sci. 2006;1080:85–96. doi: 10.1196/annals.1380.008. [DOI] [PubMed] [Google Scholar]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. Exp Hematol. 2005;33:955–964. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18:2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Ghatpande S, Ghatpande A, Zile M, Evans T. Anterior endoderm is sufficient to rescue foregut apoptosis and heart tube morphogenesis in an embryo lacking retinoic acid. Dev Biol. 2000;219:59–70. doi: 10.1006/dbio.1999.9601. [DOI] [PubMed] [Google Scholar]
- Grepin C, Nemer G, Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development. 1997;124:2387–2395. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzinger A, Evans T. Gata4 regulates the formation of multiple organs. Development. 2005;132:4005–4014. doi: 10.1242/dev.01978. [DOI] [PubMed] [Google Scholar]
- Hou J, Charters AM, Lee SC, Zhao Y, Wu MK, Jones SJ, Marra MA, Hoodless PA. A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE) BMC Dev Biol. 2007;7:92. doi: 10.1186/1471-213X-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:258–270. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Adler ED, Keller GM. Specification of multipotential cardiovascular progenitor cells during embryonic stem cell differentiation and embryonic development. Trends Cardiovasc Med. 2007;17:240–246. doi: 10.1016/j.tcm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130:3865–3876. doi: 10.1242/dev.00599. [DOI] [PubMed] [Google Scholar]
- Liang Q, Molkentin JD. Divergent signaling pathways converge on GATA4 to regulate cardiac hypertrophic gene expression. J Mol Cell Cardiol. 2002;34:611–616. doi: 10.1006/jmcc.2002.2011. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, Niu Z, Chen M, Schwartz RJ, Schneider MD. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:3859–3864. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loose M, Patient R. A genetic regulatory network for Xenopus mesendoderm formation. Dev Biol. 2004;271:467–478. doi: 10.1016/j.ydbio.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Rothman JH. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol. 2002;246:68–85. doi: 10.1006/dbio.2002.0655. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Morrison GM, Oikonomopoulou I, Migueles RP, Soneji S, Livigni A, Enver T, Brickman JM. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Murakami R, Okumura T, Uchiyama H. GATA factors as key regulatory molecules in the development of Drosophila endoderm. Dev Growth Differ. 2005;47:581–589. doi: 10.1111/j.1440-169X.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development. 1997a;124:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997b;189:270–274. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. GATA transcription factors in the developing and adult heart. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Rajagopal SK, Ma Q, Obler D, Shen J, Manichaikul A, Tomita-Mitchell A, Boardman K, Briggs C, Garg V, Srivastava D, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43:677–685. doi: 10.1016/j.yjmcc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Jitianu C, Cleaver O, Shaywitz DA, Lamenzo JO, Chen AE, Golub TR, Melton DA. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Shi Y, Katsev S, Cai C, Evans S. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224:226–237. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- Shoichet SA, Malik TH, Rothman JH, Shivdasani RA. Action of the Caenorhabditis elegans GATA factor END-1 in Xenopus suggests that similar mechanisms initiate endoderm development in ecdysozoa and vertebrates. Proc Natl Acad Sci U S A. 2000;97:4076–4081. doi: 10.1073/pnas.97.8.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Takenaga M, Fukumoto M, Hori Y. Regulated Nodal signaling promotes differentiation of the definitive endoderm and mesoderm from ES cells. J Cell Sci. 2007;120:2078–2090. doi: 10.1242/jcs.004127. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed trans-differentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Zhao R, Li J, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7:37. doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ye X, Zhang H, Ding M, Deng H. GATA factors induce mouse embryonic stem cell differentiation toward extraembryonic endoderm. Stem Cells Dev. 2007;16:605–613. doi: 10.1089/scd.2006.0077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.