Abstract
The incidence of skin cancer, including cutaneous melanoma, has risen substantially in recent years, and epidemiological and laboratory studies demonstrate that ultraviolet (UV) radiation is a major causative factor for this increase. UV damage also underlies photoaging of the skin, and those deleterious effects of UV can be, in part, prevented in skin with higher levels of constitutive pigmentation. We review the findings of a series of clinical studies we have made in recent years regarding the rapid and the long-term responses of the pigmentary system in human skin to UV exposure. Key words: ultraviolet, skin, photoprotection, pigmentation, photoaging
INTRODUCTION
The incidence of skin cancer, especially melanoma, has increased substantiallly in recent years, probably as a result, in part, from increased exposure of fair-skinned populations to ultraviolet radiation (UV) [NCI Seer Statistics, http://seer.cancer.gov/]. Epidemiological and laboratory studies provide convincing evidence that UV exposure is a major causative factor in melanomagenesis (Gilchrest et al., 1999; Landi et al., 2003) and certainly it is a critical factor in photoaging (Yaar and Gilchrest, 1998; Halder and Ara, 2003).
Constitutive pigmentation of human skin provides excellent protection against cellular and DNA damage due to UV exposure (Tadokoro et al., 2003; Miyamura et al., 2007; Brenner and Hearing, 2008) and that protection is due to a number of properties of the melanins produced. Melanin has a broad UV-visible absorption spectrum due to its strong scattering component (Kollias and Baqer, 1987; Chedekel, 1995). Melanin is produced by melanocytes residing at the basal layer of the epidermis but then is rapidly transferred to adjacent keratinocytes. Following the transfer, melanosomes are distributed towards the surface of skin, where they shield nuclear DNA in underlying cells from UV (reducing photodamage) and protect collagen and elastin in the dermis (reducing photoaging) (Pathak, 1995; Kobayashi et al., 1998). Constitutive pigmentation of skin plays a critical role in minimizing DNA damage from UV (Tadokoro et al., 2003; Tadokoro et al., 2005; Yamaguchi et al., 2006; Yamaguchi et al., 2008b) and in reducing the risk of photocarcinogenesis (Gilchrest, 1993; Gilchrest et al., 1999).
Our collaborative research groups have performed a series of clinical studies examining the effects of different wavelengths, doses and dose intervals of UV exposure on human skin of various racial/ethnic origins and phototypes. Those studies have been reported in the literature where interested readers can go for details (Tadokoro et al., 2003; Tadokoro et al., 2005; Yamaguchi et al., 2006; Miyamura et al., 2007; Yamaguchi et al., 2007; Brenner and Hearing, 2008; Miller et al., 2008; Wolber et al., 2008; Brenner et al., 2009; Yamaguchi et al., 2008a; Yamaguchi et al., 2008b; Coelho et al., 2009). Considering the sum of these studies, we are able to draw conclusions about the effects of UV on human skin and the kinetics of the biological responses elicited.
RESULTS and DISCUSSION
Clinical studies on the effects of UV on human skin
Subjects from a wide variety of skin phototypes, with different racial/ethnic backgrounds, were recruited for our studies, which examined the effects of individual or repetitive UV irradiation on human skin, and at various times (up to several years) after initial UV exposure. The sum of those analyses revealed that even within each racial/ethnic group there was a great range in the constitutive levels of skin pigmentation, that there was great variation in the ranges of MED and photosensitivity of the subjects in each group, and that there was also a wide range in ability to increase skin pigmentation in response to UV (termed facultative pigmentation), as well as the rate of increased pigmentation and its persistence.
The different time points examined after UV exposure also revealed 4 clear and distinct stages in the pigmentation responses.. (1) Immediate pigment darkening (IPD), which develops in minutes and can remain for several hours (Honigsmann et al., 1986; Routaboul et al., 1999), is gray/black in color and is thought to be due to the direct effects of UV on existing melanin or melanin precursors, perhaps oxidizing them to darker colors (Lim HW and Soter NA, 1993). (2) Persistent pigment darkening (PPD), which occurs within hours and remains for days (Moyal et al., 2000; Moyal et al., 2006) is thought to result from newly synthesized melanin in the epidermis (Tadokoro et al., 2003; Tadokoro et al., 2005). (3) Delayed pigmentation (DP), which develops in days and remains for weeks (Ortonne, 1990), results from prolonged increases in melanin content (Miller et al., 2008; Brenner et al., 2009). (4) Long-lasting pigmentation (LLP), which remains >9 months after initial UV exposure, results from prolonged activation of the pigmentary system (Brenner et al., 2009; Coelho et al., 2009).
Kinetics of pigmentary responses to UV in human skin
To evaluate the sequence of events that occurs in human skin that would increase pigmentation after 1 or more UV exposures, we examined biopsies of human skin of different phototypes at various times after a single or multiple UV exposure(s). Those biopsies were measured for melanin content (using Fontana Masson staining or chemical analysis) and for various melanocyte specific markers (e.g. MITF, TYR and MART1). Skin color was measured by reflectometry and other parameters, such as melanosome distribution and melanocyte density, were also evaluated. The composite results of the studies are summarized in Table 1.
TABLE 1.
Melanocyte markers studied (summary of results with skin phototypes 2–3)
| Marker | day 0 | day 1 | week1 | week 2 | week 3 | week5 | ≥ 9 months |
|---|---|---|---|---|---|---|---|
| MITF1 | + | ++ | +++ | ++ | + | + | + |
| TYR1 | + | + | ++ | +++ | ++ | ++ | + |
| Pmel171 | + | + | ++ | +++ | ++ | ++ | + |
| MART11 | + | + | ++ | +++ | ++ | ++ | + |
| Melanin 2 | + | + | ++ | ++ | ++ | ++ | + |
| Melanin 3 | + | + | + | + | ++ | ++ | + |
| Melanosome redistribution | + | + | ++ | + | + | + | + |
| Melanocyte density 4 | + | + | + | + | + | ++ | + |
| Visible skin color5 | + | ++ | +++ | +++ | +++ | +++ | + |
assessed as intensity of staining.
Melanin content as assessed by immunohistochemistry of Fontana Masson stain.
Melanin content as assessed by chemical analysis.
comparable results were obtained with stains for TYR, MART1, Pmel 17 and MITF.
measured as reflectance.
The sequence of events, when considered with the development of color in skin of phototypes II and III after UV exposure, provides a very clear picture of what occurs in the skin following UV exposure at the molecular level. The initial response noted (from the markers tested) is an increase in MITF levels, MITF being a transcription factor known as the master regulator of melanocyte function. Significant increases in MITF levels occur within 1 day of UV exposure and no other marker tested responds noticeably within that time frame, other than the dramatic and quick increase in visible skin color known as IPD (Figure 1). Since tyrosinase levels and melanin synthesis (determined immunohistochemically and by chemical analysis) do not increase within the first few days, it is clear that the earlier hypothesis that IPD represents an oxidation and/or polymerization of existing melanin (or melanin intermediates) was correct. MITF levels continue to increase up to 1 week after UV exposure but then begin to decline and, by week 3 (even with repeated UV exposure), MITF has returned to its baseline constitutive level.
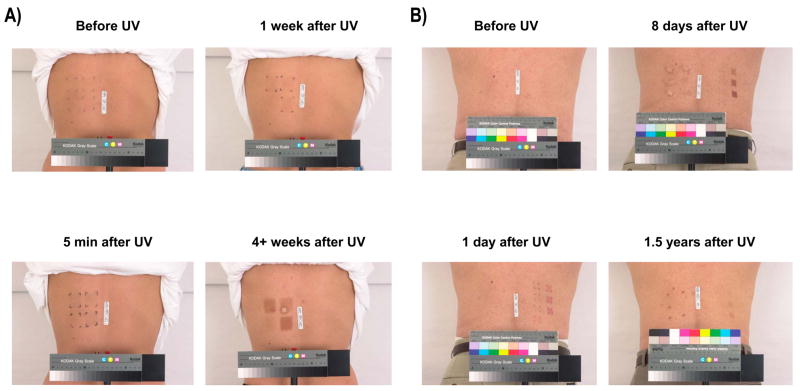
Figure 1.
Examples of phases of increased pigmentation in human skin following UV exposure. (A) subject T35; top left: control unirradiated skin prior to UV exposure; bottom left: IPD, 5 min after UV exposure; top right: PPD, 1 week after UV exposure; bottom right: DP, 4+ weeks after UV exposure; (B) subject S91; top left: control unirradiated skin; bottom left: 1 day after MED exposure; top right: PPD, 8 days after UV; bottom right: LLP, 1.5 years after UV exposure.
By week 1, the expression of tyrosinase, Pmel17 and MART1 (and other melanosomal proteins) has increased significantly, while the melanin content has increased only slightly (~10%). The major effect on pigmentation at this time point is a large redistribution of the melanosomes that existed prior to UV from the basal layer of the epidermis to layers higher up in the skin. In light of the very minor increase in melanin content at 1 week after UV exposure, it is clear that the visible increase in skin color (termed PPD, shown in Figure 1) is due to the upwards movement of melanosomes towards the surface of the skin, where the visible color increases and presumably, the melanin is more effective in preventing damage from further UV exposures to underlying cells.
By weeks 3 through 5, the melanocytic system becomes fully activated, levels of melanogenic proteins are maximal, levels of melanin synthesis are increased and even the density of melanocytes in the skin is increased (all at levels 2- to 3-fold higher than prior to UV exposure). The sum of these events leads to a pronounced increase in visible skin color known as DP (Figure 1). How long DP lasts varies from individual to individual and not much is known about the factors that determine it.
An interesting consideration is which wavelength region of UV causes the increased skin pigmentation, and in a separate study (Wolber et al., 2008), we compared the effects of UVA with UVB in eliciting increases in skin color. UVB is more effective at stimulating skin pigmentation than is UVA, but both wavelengths can elicit comparable increases in skin pigmentation with appropriate doses, although the mechanisms are distinct. UVA has no effect on melanin content, activation of melanocyte differentiation or other parameters measured, yet UVA exposure stimulates visible skin pigmentation significantly. Presumably, this is due to the oxidation of existing melanins and their precursors and/or to effects on the distribution of melanosomes in skin, perhaps from the known effects of UVA on cytoskeletal components (Jimbow and Fitzpatrick, 1975). Thus, UVA-induced increases in skin pigmentation are comparable to prolonged IPD. In contrast, UVB stimulates the melanogenic system, as discussed elsewhere in this paper.
Recently, our follow-up studies examined the same subjects from 9 months to 3 years after UV exposure (those individuals stated that they had no further significant UV exposure after the original protocol). Interestingly, in a significant portion of this population (~30%), visible increases in skin color remained in the areas that were previously UV-exposed. We have termed this phenomenon LLP, and studies are underway to determine why this occurs in some individuals and not in others, and to determine whether those factors might be involved in various hyperpigmentary diseases, particularly those that are UV-related.
In sum, our studies have determined that melanocyte density is comparable in dorsal skin of subjects from all racial/ethnic skin types (ranging from types 1 – 6), although the amount of melanins produced by those melanocytes varies significantly and results in the wide variety of human skin colors. Melanocyte density can be increased ~2-to 3-fold within 3–4 weeks of repetitive UV exposure. Visible skin pigmentation can be increased visibly 7- to 10-fold by repetitive UV exposure, although melanin content varies only ~2-fold, suggesting that the type(s) of melanin produced and/or their distribution in the skin is critical to the greater effect of UV on skin color. The long-term effects of UV on human skin can persist for years, even in the absence of further UV exposure. Since complete turnover of the epidermis usually occurs every 4–5 weeks, how the pigmentary system remains activated in some cases even years later should be studied in more detail. The mechanism(s) underlying LLP are a subject of great interest for future study, since they may provide important clues about the regulation of melanocyte differentiation and give further insights into abnormal hyperpigmentation of human skin in UV-related pigmentary diseases.
MATERIALS AND METHODS
Study subjects, UV irradiation and dosimetry
These studies involved volunteer subjects with skin phototypes 1–6, as reported in the initial studies for each protocol (Tadokoro et al., 2003; Miller et al., 2008; Wolber et al., 2008). The studies adhered to the Helsinki guidelines and were approved by the Research Involving Human Subjects Committees of the U.S. Food and Drug Administration and Beiersdorf AG and all subjects gave written, informed consent. Subjects (labeled ‘T’) from one of the studies (Miller et al., 2008) were UV-exposed 1 to 3 times per week for 4 to 5 weeks, while subjects (labeled ‘S’) from a different study (Tadokoro et al., 2003) received a single UV exposure, and subjects (labeled ‘B’) from yet another study (Wolber et al., 2008) were UV-exposed 5 times a week for 2 weeks.
Biopsies and Immunohistochemical analysis
Skin biopsies were taken from control and from UV-exposed sites at various times after UV exposure. Each biopsy was placed dermis side down on a Millipore filter and was then fixed in 4% formaldehyde, embedded in paraffin, sectioned at 3 μm-thickness, mounted on silane-coated glass slides, and then stained using immunohistochemistry, as previously described (Tadokoro et al., 2003; Yamaguchi et al., 2004; Tadokoro et al., 2005; Yamaguchi et al., 2006). Staining patterns were analyzed using a Leica DMRB/DMLD fluorescence microscope, and an internal control was used each time to control for reproducible antibody staining. Negative controls omitting the primary antibody were performed each time.
Melanocyte Density and Melanin Content
Melanocytes were counted following staining for tyrosinase, MITF, Pmel17, MART1 and other markers as detailed in the original articles, and their density along the epidermal:dermal border was determined as cells/mm. Fluorescence intensities for antibodies detecting melanocyte-specific markers were normalized against DAPI staining. For measurements of melanin content, specimens were stained by the Fontana-Masson method and were also measured by chemical analysis (Wolber et al., 2008). Transmitted light intensity was measured by the Leica DMRB\DMLD microscope and ScionImage software was used to analyze melanin quantity from integrated density in the skin sections, as previously described (Yamaguchi et al., 2006; Yamaguchi et al., 2008a).
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute at NIH, and by the Office of Science, Office of Women’s Health and the Center for Devices and Radiological Health, Food and Drug Administration (FDA).
Abbreviations
- DP
delayed pigmentation
- IPD
immediate pigment darkening
- LLP
long-lasting pigmentation
- PPD
persistent pigment darkening
- UV
ultraviolet radiation
References
- Brenner M, Coelho SG, Beer JZ, Miller SA, Wolber R, Smuda C, Hearing VJ. Long-term molecular changes in human skin after repetitive in situ UV irradiation. J Invest Dermatol. 2009;129:1002–1. doi: 10.1038/jid.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedekel MR. Photophysics and photochemistry of melanin. In: Zeise L, Chedekel MR, Fitzpatrick TB, editors. Melanin: Its Role in Human Photoprotection. Valdenmar Publ; Overland Park: 1995. pp. 11–22. [Google Scholar]
- Coelho SG, Zhou Y-C, Bushar HF, Miller SA, Zmudzka BZ, Hearing VJ, Beer JZ. Long-lasting pigmentation (LLP) of human skin, a new type of cutaneous response to UV. Pigment Cell Melanoma Res. 2009;22:239–41. doi: 10.1111/j.1755-148X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA. Sunscreens - a public health opportunity. New Eng J Med. 1993;329:1193–1194. doi: 10.1056/NEJM199310143291611. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. New Eng J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725–732. doi: 10.1016/s0733-8635(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Honigsmann H, Schuler G, Aberer W, Romani N, Wolff K. Immediate pigment darkening phenomenon. A reevaluation of its mechanisms. J Invest Dermatol. 1986;87:648–652. doi: 10.1111/1523-1747.ep12456326. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Fitzpatrick TB. Changes in distribution patterns of cytoplasmic filaments in human melanocytes during ultraviolet-mediated melanin pigmentation. J Cell Biol. 1975;65:481–488. doi: 10.1083/jcb.65.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Ohnishi T, Mori T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Kollias N, Baqer AH. Absorption mechanisms of human melanin in the visible, 400–720 nm. J Invest Dermatol. 1987;89:384–388. doi: 10.1111/1523-1747.ep12471764. [DOI] [PubMed] [Google Scholar]
- Landi MT, Baccarelli A, Tarone RE, Pesatori A, Tucker MA, Hedayati M, Grossman L. DNA repair, dysplastic nevi, and sunlight sensitivity in the development of cutaneous malignant melanoma. J Natl Cancer Inst. 2002;94:94–101. doi: 10.1093/jnci/94.2.94. [DOI] [PubMed] [Google Scholar]
- Lim HW, Soter NA. Clinical Photomedicine. Marcel Dekker, Inc; New York: 1993. [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Bushar HF, Yamaguchi Y, Hearing VJ, et al. Dynamics of pigmentation induction by repeated UV exposures: dose, dose interval and UV spectrum dependence. Br J Dermatol. 2008;159:921–30. doi: 10.1111/j.1365-2133.2008.08708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, Ito S, Smuda C, Passeron T, Choi W, Batzer J, Yamaguchi Y, Beer JZ, Hearing VJ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Moyal D, Chardon A, Kollias N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. (Part 1). Calibration of the method. Photodermatol Photoimmunol Photomed. 2000;16:245–249. doi: 10.1034/j.1600-0781.2000.160602.x. [DOI] [PubMed] [Google Scholar]
- Moyal D, Wichrowski K, Tricaud C. In vivo persistent pigment darkening method: a demonstration of the reproducibility of the UVA protection factors results at several testing laboratories. Photodermatol Photoimmunol Photomed. 2006;22:124–128. doi: 10.1111/j.1600-0781.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Ortonne JP. The effects of ultraviolet exposure on skin melanin pigmentation. J Intl Med Res. 1990;18:8C–17C. [PubMed] [Google Scholar]
- Pathak MA. Functions of melanin and protection by melanin. In: Zeise L, Chedekel MR, Fitzpatrick TB, editors. Melanin: Its Role in Human Photoprotection. Valdenmar Publ; Overland Park: 1995. pp. 125–134. [Google Scholar]
- Routaboul C, Denis A, Vinche A. Immediate pigment darkening: description, kinetic and biological function. Eur J Dermatol. 1999;9:95–99. [PubMed] [Google Scholar]
- Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function and regulation. J Invest Dermatol Suppl. 2001;6:99–104. doi: 10.1046/j.0022-202x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Gilchrest BA. Aging versus photoaging: postulated mechanisms and effectors. J Invest Dermatol Suppl. 1998;3:47–51. [PubMed] [Google Scholar]
- Yamaguchi Y, Beer JZ, Hearing VJ. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch Dermatol Res. 2008a;300 (Suppl 1):S43–S50. doi: 10.1007/s00403-007-0807-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Coelho SG, Zmudzka BZ, Takahashi K, Beer JZ, Hearing VJ, Miller SA. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp Dermatol. 2008;4:539–49. doi: 10.1111/j.1600-0625.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]