Abstract
AIM: To investigate whether silencing Fas-associated phosphatase 1 (FAP-1) expression enhances the efficiency of chemotherapy for colon carcinoma with oxaliplatin.
METHODS: Expression of FAP-1 in mRNA and protein was detected by reverse transcription polymerase chain reaction (RT-PCR) and flow cytometry. Small interfering RNA (siRNA) was designed according to the FAP-1 mRNA sequence. Cell proliferation was evaluated by methyl thiazolyl tetrazolium (MTT) assay. Anenxin V- and propidine iodine (PI) were assayed by flow cytometry for the detection of apoptosis.
RESULTS: The expression of FAP-1 was increased in SW480 cells after chemotherapy with oxaliplatin. Transfection of FAP-1 siRNA into SW480 cells silenced the expression of FAP-1 and consequently abolished the inhibitory function of Fas/FasL-mediated apoptosis pathway, thus increasing the efficacy of chemotherapy for colon carcinoma with oxaliplatin.
CONCLUSION: RNA interference combined with conventional chemotherapy is more effective against colon cancer.
Keywords: Colon carcinoma, Fas-associated phosphatase 1, RNA interference, Oxaliplatin, Chemotherapy
INTRODUCTION
The incidence of colon carcinoma is increasing worldwide[1]. Although great achievements have been made in surgery, chemotherapy and even some novel molecule-targeted drugs, such as bevacizumab (Avastin) used in treatment of colon carcinoma, their efficacy is still limited[2]. Since tumorigenesis is a multiple step event involving multiple genes, a single treatment modality just targets a part of the pathogenesis of colon carcinoma. The mechanism underlying the limited efficacy of the above treatment modalities still needs to be further explored in order to prolong the survival time of such patients.
Fas/FasL system is the essential pathway of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells to induce cell apoptosis[3]. Fas, an apoptotic message receptor, is belonged to the tumor necrosis factor family and expressed in many normal tissues and malignancies. Its expression pattern is mainly located in activated T lymphocytes and NK cells. By binding to Fas antibody or FasL, Fas-induced apoptotic pathway can be activated, initiating tumor cell apoptosis[4-6]. However, it has been reported that Fas receptors are highly expressed in colon carcinoma cells at both mRNA and protein levels, and FasL levels are high in blood and tissues of colon carcinoma patients[7,8], suggesting that colon carcinoma cells can escape from the immune clearance, resist to the cytotoxic activity of host immunocytes, and are, thus, insensitive to FasR- mediated apoptosis. Although the mechanism underlying the failure of immune system to protect humans against colon malignancies remains unclear, it has been recently shown that Fas-associated phosphatase 1 (FAP-1) may play an important role in the pathogenesis of colon malignancies[9]. FAP-1, a tyrosine phosphatase, inhibits FasR-mediated apoptosis. By interacting with the cytoplasmic death domain of Fas receptors, FAP-1 acts as a negative switch in the Fas pathway[10]. Transfection of FAP-1 into Fas-sensitive cells can block FasL-induced apoptosis[11]. Fas and FAP-1 are expressed in colon cancer tissue and the expression of FAP-1 is associated with resistance against Fas-mediated apoptosis and interrupting the correspondence between FAP-1 and Fas can reverse the anti-Fas inducing apoptosis function of FAP-1[12]. Down-regulating the expression of FAP-1 by interleukin 2 can promote the sensitivity of colon cancer cells to Fas-induced apoptosis[13] and interrupting FAP-1 also increases chemosensitivity to certain kinds of cancer[14], suggesting that FAP-1 can be used as a target for treatment of malignancies. These findings lead to a question of whether interrupting FAP-1 sensitizes chemotherapy for colon carcinoma.
It has been recently shown that RNA interference (RNAi) plays an important role in the treatment of malignancies, virus infection, and other diseases[15-19]. Small interfering RNA (siRNA) is small in size, and can easily infiltrate cell membranes and other structures. Its efficiency and specificity are higher than those of anti-sense oligonucleotide[20-23]. RNAi may be used as a novel gene therapeutic procedure combining with chemotherapy for colon carcinoma.
In this study, the expression of FAP-1 was up-regulated after treatment with oxaliplatin. Silencing FAP-1 by siRNA effectively reversed the apoptotic resistance and increased the efficacy of chemotherapy for colon carcinoma with oxaliplatin.
MATERIALS AND METHODS
Colon cancer cell line and culture
Human colon adenocarcinoma cell line SW480, obtained from Chinese Type Culture Collection Committee Cell Bank (Shanghai, China), was maintained in RPMI-1640 medium (GIBCO, Grand Island, NY, USA), supplemented with 10% heat-inactivated fetal calf serum (FCS) at 37°C in a humidified atmosphere containing 5% CO2.
FAP-1 siRNA design
Three different sequences of FAP-1 specific siRNA, including positive control glyceraldehyde phosphate dehydrogenase (GAPDH) siRNA and negative control siRNA, were designed and synthesized by Genepharma (Shanghai, China). The sequence of each siRNA is shown in Table 1.
Table 1.
siRNA sequence
| siRNA | From 5' to 3' |
| FAP-1 siRNA 1709 | Sense: CGAAGGAAAGUAAACAUAATT |
| Anti-sense: UUAUGUUUACUUUCCUUCGGT | |
| FAP-1 siRNA 6267 | Sense: CAGGUACAUUAAAGAUGAATT |
| Anti-sense: UUCAUCUUUAAUGUACCUGGA | |
| FAP-1 siRNA 3264 | Sense: GGGAGAUCACCUUAGUGAATT |
| Anti-sense: UUCACUAAGGUGAUCUCCCTT | |
| GAPDH positive control | Sense: GUAUGACAACAGCCUCAAGTT |
| Anti-sense: CUUGAGGCUGUUGUCAUACTT | |
| Negative control | Sense: UUCUCCGAACGUGUCACGUTT |
| Anti-sense: ACGUGACACGUUCGGAGAATT |
siRNA: Small interfering RNA; FAP-1: Fas-associated phosphatase 1; GAPDH: Glyceraldehyde phosphate dehydrogenase.
siRNA transfection
siRNA was transfected with a siPORT™ NeoFX™ transfection agent (AMBION) following its manufacturer’s instructions.
Detection of FAP-1 by reverse transcription polymerase chain reaction (RT-PCR)
RNA was harvested from colon cancer cells by extracting Trizol (Invitrogen) following its manufacture’s instructions. cDNA was synthesized from 2 μg of total RNA in a 20 μL reaction system containing 0.5 μL of PrimeScript™ RTase, 4 μL of 5 × PrimeScript™ buffer, 0.5 μL of RNase inhibitor, 1 μL of Oligo dT, 2 μL of dNTP, 11 μL of RNase free H2O. The mixture was incubated for 60 min at 42°C and then for an additional 30 min at 53°C. The unhybridized RNA was digested with 10 units of RNase H at 37°C for 10 min.
PCR was performed on cDNA using the sense and anti-sense primers to amplify FAP-1 and a house keeping gene, GAPDH. All primers were designed according to the published sequences: FAP-1: (sense) 5'-AGGTCTGCAGAGAAGCAAGAATAC-3' and (anti-sense) 5'-GAATACGAGTGTCAGACATGG-3'; GAPDH: (sense) 5'-AACGGATTTGGTCGTATTG-3' and (anti-sense) 5'-GGAAGATGGTGATGGGATT-3'.
The PCR conditions were as follows: denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for 30 s, at 50°C for 45 s, at 72°C for 1 min, and a final extension at 72°C for 7 min for FAP-1, and denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for 30 s, at 58°C for 45 s, at 72°C for 1 min, and a final extension at 72°C for 7 min for GAPDH. Primers were used at a final concentration of 0.1 μmol/L each, dNTPs at 50 μmol/L, MgCl2 at 1.5 mmol/L, Taq DNA polymerase at 1.0 μg per 50 μL reaction mixture. The 607 bp and 208 bp PCR products were the predicted FAP-1 and GAPDH, respectively, separated by electrophoresis on 2% agarose gel and stained with colloidal gold. The target bands were analyzed by densitometry. FAP-1 cDNA was semi-quantitated by densitometric comparison with GAPDH from the same sample.
Immunofluorescence analysis for FAP-1
Approximately 106 cells were incubated with 10 g/mL rabbit anti-human FAP-1 polyclone antibody (Santa Cruz) for 30 min at 4°C and washed with PBS containing 2% FCS. PE-conjugated secondary goat anti-rabbit antibody (Boster, Wuhan, China) was added to the cells for 30 min at 4°C. The cells were washed again with PBS containing 2% FCS and then the intensity of fluorescence was analyzed. Isotype-matched control antibody was used to determine the nonspecific binding. A total of 10 000 cells were examined for each determination. Data were expressed as relative fluorescence intensity (RFI = mean fluorescence intensity of cells stained with anti-FAP-1 pAb/mean fluorescence intensity of cells stained with control pAb).
Cell proliferation assay
Cell proliferation was evaluated by methyl thiazolyl tetrazolium (MTT) assay. SW480 cells were seeded into a 96-well plate at the concentration of 3000 cells per well. Oxaliplatin (Henrui Co, LTD, Jiangsu Province, China) was administrated at a concentration of 5 μg/mL 24 h after the cells were plated. The proliferation status of SW480 cells was observed at 24, 48, 72, and 96 h, respectively, after treatment with oxaliplatin. Each group was quadruplicates and its mean OD value was used to represent the proliferation status of the group. MTT (Merck) was dissolved in RPMI 1640 and prepared at 1 mg/L for use. The medium was removed, the cells were washed three times with PBS, and 100 μL MTT solution was added into each well and incubated in dark at 37°C. Then, the MTT solution was removed and 100 μL DMSO (Sigma) was added into each well to dissolve the remaining formazan by gently shaking the plate for 15 min. Finally, a 495 absorption value of each well was obtained with a spectrophotometer (Labsystems Dragon).
Detection of apoptosis by flow cytometry
Anenxin V-FITC kit (Bender Medsystems) and propidine iodine (PI) were used to calculate the cells undergoing apoptosis with a flow cytometer (Facscalibur, Becton Dickinson). Anenxin V (+) PI (-) represents apoptotic cells, whereas Anenxin V (+) PI (+) represents dead cells. The procedure was carried out according to the manufacturer’s instructions for the Anenxin V-FITC kit.
Statistical analysis
All experiments were performed in triplicate. The results were expressed as mean ± SE. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparisons among groups were performed by Bonferroni’s multiple-comparison t-test. P < 0.05 was considered statistically significant.
RESULTS
Oxaliplatin promoted FAP-1 expression in SW480 cells
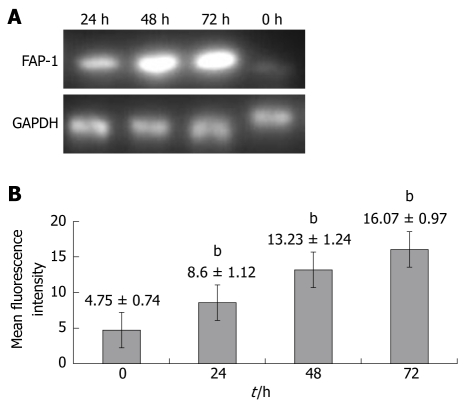
To investigate whether FAP-1 is resistant to chemotherapy for colon carcinoma with oxaliplatin, RT-PCR and flow cytometry were carried out to detect the FAP-1 expression in SW480 colon carcinoma cells at 0, 24, 48 and 72 h after chemotherapy for colon carcinoma with oxaliplatin. The FAP-1 expression was increased at both mRNA (Figure 1A) and protein levels (P < 0.01), and reached its peak at 48 h (Figure 1B).
Figure 1.
Expression of Fas-associated phosphatase 1 (FAP-1) mRNA (A) and protein (B) after oxaliplation administration. Oxaliplatin promotes FAP-1 expression of SW480 cells at 0, 24, 48 and 72 h after chemotherapy. bP < 0.01 vs 0 h group.
siRNA silenced FAP-1 expression
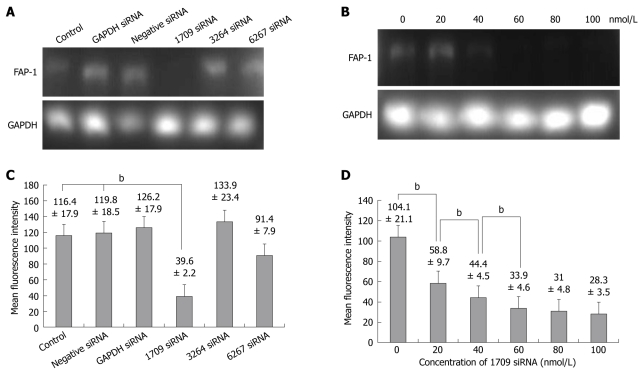
siRNA was transfected into SW480 cells with a transfection agent, siPORT(AMBION). Three sequences of FAP-1 siRNA (1709, 6267 and 3264) were designed. Forty-eight hours after transfection of siRNAs into SW480 cells, the FAP-1 expression at mRNA and protein levels was detected by RT-PCR and flow cytometry. The FAP-1 protein was expressed in siRNA 1709 group (Figure 2A and C) and at the concentration of 60 nmol/L (Figure 2B and D), respectively (P < 0.01).
Figure 2.
Small interfering RNA (siRNA) silencing FAP-1 expression in siRNA 1709 group (A and C) and at the concentration of 60 nmol/L (B and D). bP < 0.01 vs 0 h group.
FAP-1 siRNA combined with oxaliplatin inhibited proliferation of SW480 cells
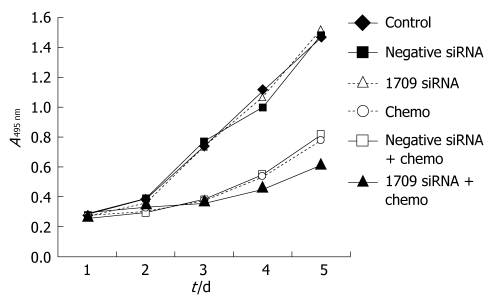
To investigate whether FAP-1 siRNA enhances the sensitivity of SW480 cells to oxaliplatin, cell proliferation was assayed in 6 groups including negative control group, negative siRNA group, siRNA 1709 group, oxaliplatin group, oxaliplatin+negative siRNA group, and oxaliplatin + siRNA 1709 group. Transfection was performed when the cells were seeded. After 24 h, the culture medium was removed and washed three times with PBS and oxaliplatin dissolved in the culture medium (5 μg/mL) was added. The culture medium was replaced daily to keep the consistent concentration of oxaliplatin. Cell growth was observed daily for five days. Transfection of negative siRNA and siRNA into SW480 cells did not inhibit cell proliferation. Transfection of oxaliplatin combined with transfection of negative siRNA reduced cell proliferation. The greatest proliferation inhibition was found after treatment with oxaliplatin combined with transfection of siRNA 1709 (Figure 3).
Figure 3.
FAP-1 siRNA increasing the inhibitory effect of oxaliplatin on proliferation of SW480 cells.
FAP-1 siRNA combined with oxaliplatin increased apoptosis of SW480 cells
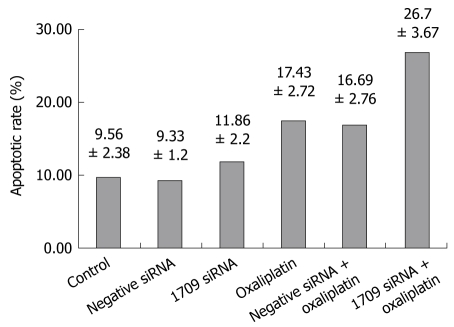
To investigate whether transfection of FAP-1 siRNA into SW480 cells combined with oxaliplatin increases apoptosis of colon carcinoma cells, the apoptotic rate of colon carcinoma cells was detected by flow cytometery and Anenxin V and PI immunofluorescence. Transfection was performed when the cells were seeded. After 24 h, the culture medium was removed and washed three times with PBS and oxaliplatin dissolved in culture medium at the concentration 5 μg/mL was added and the cells were harvested. The apoptotic rate of the negative control was 9.56% ± 2.38%, and similar in both siRNA transfection groups (P = 0.416). The apoptotic rate of oxaliplatin combined with siRNA 1709 transfection group was 26.7% ± 3.67%, which was higher than that of the oxaliplatin treatment group (P < 0.01, Figure 4), suggesting that oxaliplatin promotes FAP-1 expression in SW480 cells.
Figure 4.
FAP-1 siRNA enhancing the apoptosis inducing effect of oxaliplatin.
DISCUSSION
Colon cancer represents a major public health problem, resulting in more than 1 million new cases diagnosed each year and approximately a half million deaths worldwide. Colectomy is the only procedure that may cure colon carcinoma, but the 5-year survival rate mainly depends on the stage of tumor at the time of diagnosis. The majority of patients with colon carcinoma are at an advanced stage beyond surgical treatment when they visit a doctor[1]. For patients who cannot be cured by surgery, chemotherapy is another important and complementary treatment[24]. Among the chemotherapeutic drugs, oxaliplatin is commonly used in treatment of colon carcinoma. However, its efficacy, especially in patients at advanced stage, is still limited[2].
The main mechanism of action of oxaliplatin is mediated through the formation of DNA adducts[25-27]. When the platinum compound enters the cells, one chloride ligand is dissociated to form a reactive monoaquamonochloro complex, which reacts rapidly with the guanines on DNA to form monoadducts. The subsequent dissociation of the second chloro ligand allows conversion of the transiently formed monoadducts to a variety of stable diadducts[28,29]. The majority are intrastrand diadducts binding to a guanine residue[30,31]. Since intrastrand adducts are the most abundant adducts and capable of blocking both DNA replication and transcription, they are considered the major cytotoxic lesions. As a final result, oxaliplatin induces primary and secondary DNA lesions leading to apoptosis of human cancer cells[32].
It has been shown that DNA lesion repair mechanism, over-expression of copper transporters, and enhanced drug detoxification result in an increased chemo-resistance to oxaliplatin[33,34]. However, the mechanism may be more complicated. Some researchers hold that the major process leading to chemotherapy resistance is the ability of cancer cells to evade cell death signals[35]. In our study, the expression of FAP-1, a negative switch in Fas- mediated apoptosis, was elevated in SW480 colon cancer cells after treatment with oxaliplatin. We quantified the transcription level only by densitometry rather than by RT-qPCR. The role of MMP7 (matrix metallopeptidase 7) and its cross-talk with the FAS/FASL system during the acquisition of chemo-resistance to oxaliplatin have been reported[36]. Raymond D[37] also showed that oxaliplatin can activate the Notch-1 signaling pathway in colon cancer cells and enhance its chemo-resistance to SW480 colon cancer cells, indicating that the functional disorder of the Fas apoptosis pathway mediated by FAP-1 elevation may protect SW480 cells against apoptosis and is involved in chemo-resistance effect.
In our study, since FAP-1 was elevated after treatment with oxaliplatin and might account for chemo-resistance, the FAP-1 expression was inhibited by RNA interference to make clear whether it sensitizes chemotherapy. The apoptotic rate of oxaliplatin combined with siRNA transfection was higher than that of oxaliplatin only. The greatest proliferation inhibition was found in the group of oxaliplatin combined with siRNA transfection, suggesting that the elevated FAP-1 expression is involved in the mechanism enabling SW480 cells to be insensitive to oxaliplatin treatment. Based on the fact that siRNA used to silence the expression of FAP-1 and treatment with oxaliplatin increased the apoptosis of SW480 cells and reduced their proliferation, we can develop a novel therapeutic measure to enhance the efficacy of chemotherapy. The similar phenomenon was also observed in other malignances. Etodolac, a selective cyclo-oxygenase-2, can enhance carboplatin-induced apoptosis of human tongue carcinoma cells by down-regulating FAP-1 expression[38] and sphingosine kinase isoforms can regulate oxaliplatin sensitivity to human colon cancer cells through ceramide accumulation and Akt activation[39]. Secretase inhibitors have been recently used to abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells, which can enhance chemo-sensitivity[37]. In the present study, FAP-1 siRNA combined with oxaliplatin reduced the proliferation of colon carcinoma SW480 cells compared with oxaliplatin alone. No study is available so far on the Fas/FasL system and FAP-1 interacting to influence cell proliferation. We hold that the higher reduction of proliferation is due to the enhanced apoptotic rate of FAP-1 siRNA combined with oxaliplatin treatment, decreasing the number of cells.
Since the pathogenesis of colon carcinoma remains largely unclear, a variety of chemotherapies have been designed to inhibit tumor growth. So far, no single strategy can solve all the complicated problems in the treatment of colon carcinoma. Our study is an attempt to integrate gene therapy targeting FAP-1 and conventional chemotherapy for colon cancer.
In conclusion, oxaliplatin increases the expression of FAP-1. RNAi can knockdown FAP-1 and sensitize chemosensitivity, and RNA interference combined with conventional chemotherapy is more effective against colon cancer.
COMMENTS
Background
Colon cancer represents a major public health problem, resulting in more than one million new cases diagnosed each year and approximately a half million deaths worldwide. Chemotherapy is an important and complementary treatment modality for colon carcinoma. Among the chemotherapeutic drugs, oxaliplatin is a commonly used in treatment of colon carcinoma, but its efficacy, especially in patients at advanced stage, is still limited.
Research frontiers
The mechanism underlying oxaliplatin chemo-resistance is complicated. DNA lesion repair mechanism, over-expression of copper transporters, and enhanced drug detoxification cannot fully explain its mechanism. In this study, Fas-associated phosphatase-1 (FAP-1) was elevated in colon carcinoma cells after oxaliplatin treatment, implicating that the functional disorder of the Fas apoptosis pathway mediated by FAP-1 elevation may protect colon carcinoma cells against apoptosis and is involved in the chemo-resistance effect. Chemotherapy can be sensitized by inhibiting FAP-1 expression with RNA interference.
Innovations and breakthroughs
Since the pathogenesis of colon carcinoma remains largely unclear, a variety of chemotherapeutic treatment modalities available have been designed. So far, no single treatment modality can solve all the complicated problems. This study is an attempt to integrate gene therapy targeting FAP-1 and conventional chemotherapy for colon cancer.
Applications
Oxaliplatin can increase the expression of FAP-1. RNAi can knockdown FAP-1 and sensitize chemosensitivity. This in vitro study showed RNA interference combined with conventional chemotherapy is more effective against colon cancer.
Terminology
FAP-1 is a tyrosine phosphatase, which inhibits FasR-mediated apoptosis. By interacting with the cytoplasmic death domain of Fas receptors, FAP-1 acts as a negative switch in the Fas pathway. Transfection of FAP-1 into Fas-sensitive cells can block FasL-induced apoptosis.
Peer review
This study investigated the important factors that inhibit the apoptotic effect of oxaliplatin on colorectal cancer cells, which is of significance in the treatment of colon carcinoma.
Footnotes
Supported by Research grants from the Science and Technology Foundation of Guangdong Province, No. 2006B36002010, 2008B030301092, 2009B030801005; the Foundation of Health Department of Guangdong Province, No. A2005226; the foundation of Guangzhou Science and Technology Bureau, No. 2009Y-C011-1; the Natural Science Foundation of Guangdong Province, No. 7001592; and the National Natural Science Foundation of China, No. 30973505
Peer reviewer: Jamie Murphy, MRCS (Eng.), MA, Lecturer in Colorectal Surgery, Centre for Academic Surgery, Royal London Hospital, 3rd Floor Alexandra Wing, London, E1 1BB, United Kingdom
S- Editor Wang YR L- Editor Wang XL E- Editor Lin YP
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Carrato A. Adjuvant treatment of colorectal cancer. Gastrointest Cancer Res. 2008;2:S42–S46. [PMC free article] [PubMed] [Google Scholar]
- 3.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 6.Halapi E. Oligoclonal T cells in human cancer. Med Oncol. 1998;15:203–211. doi: 10.1007/BF02787202. [DOI] [PubMed] [Google Scholar]
- 7.von Reyher U, Sträter J, Kittstein W, Gschwendt M, Krammer PH, Möller P. Colon carcinoma cells use different mechanisms to escape CD95-mediated apoptosis. Cancer Res. 1998;58:526–534. [PubMed] [Google Scholar]
- 8.O'Connell J, Bennett MW, O'Sullivan GC, Roche D, Kelly J, Collins JK, Shanahan F. Fas ligand expression in primary colon adenocarcinomas: evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J Pathol. 1998;186:240–246. doi: 10.1002/(SICI)1096-9896(199811)186:3<240::AID-PATH173>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Shin MS, Park WS, Kim SY, Kim HS, Lee JH, Han SY, Lee HK, Park JY, Oh RR, et al. Immunohistochemical localization of FAP-1, an inhibitor of Fas-mediated apoptosis, in normal and neoplastic human tissues. APMIS. 1999;107:1101–1108. doi: 10.1111/j.1699-0463.1999.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 10.Ungefroren H, Kruse ML, Trauzold A, Roeschmann S, Roeder C, Arlt A, Henne-Bruns D, Kalthoff H. FAP-1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J Cell Sci. 2001;114:2735–2746. doi: 10.1242/jcs.114.15.2735. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 12.Yao H, Song E, Chen J, Hamar P. Expression of FAP-1 by human colon adenocarcinoma: implication for resistance against Fas-mediated apoptosis in cancer. Br J Cancer. 2004;91:1718–1725. doi: 10.1038/sj.bjc.6602136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song E, Chen J, Antus B, Wang M, Xie Y, Yao H, Exton MS. Interleukin-2 enhances susceptibility of colon cancer cells to FasR mediated apoptosis by up-regulating Fas receptor level and down-regulating FAP-1 expression. Int J Immunopathol Pharmacol. 2000;13:113–122. [PubMed] [Google Scholar]
- 14.Wang B, Zheng WG, Xin XY, Qi RY, Yu YC, Cao YX. [Combinative effects of FAP-1 antisense oligonucleotide and carboplatin on apoptosis of ovarian cancer cell SKOV3] Ai Zheng. 2004;23:885–889. [PubMed] [Google Scholar]
- 15.Lieberman J, Song E, Lee SK, Shankar P. Interfering with disease: opportunities and roadblocks to harnessing RNA interference. Trends Mol Med. 2003;9:397–403. doi: 10.1016/S1471-4914(03)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlomai A, Shaul Y. Inhibition of hepatitis B virus expression and replication by RNA interference. Hepatology. 2003;37:764–770. doi: 10.1053/jhep.2003.50146. [DOI] [PubMed] [Google Scholar]
- 17.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- 18.Li K, Lin SY, Brunicardi FC, Seu P. Use of RNA interference to target cyclin E-overexpressing hepatocellular carcinoma. Cancer Res. 2003;63:3593–3597. [PubMed] [Google Scholar]
- 19.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 20.Grünweller A, Wyszko E, Bieber B, Jahnel R, Erdmann VA, Kurreck J. Comparison of different antisense strategies in mammalian cells using locked nucleic acids, 2'-O-methyl RNA, phosphorothioates and small interfering RNA. Nucleic Acids Res. 2003;31:3185–3193. doi: 10.1093/nar/gkg409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–1004. doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- 22.Aoki Y, Cioca DP, Oidaira H, Kamiya J, Kiyosawa K. RNA interference may be more potent than antisense RNA in human cancer cell lines. Clin Exp Pharmacol Physiol. 2003;30:96–102. doi: 10.1046/j.1440-1681.2003.03801.x. [DOI] [PubMed] [Google Scholar]
- 23.Garber K. Better blocker: RNA interference dazzles research community. J Natl Cancer Inst. 2003;95:500–502. doi: 10.1093/jnci/95.7.500. [DOI] [PubMed] [Google Scholar]
- 24.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson NP, Hoeschele JD, Rahn RO. Kinetic analysis of the in vitro binding of radioactive cis- and trans-dichlorodiammineplatinum(II) to DNA. Chem Biol Interact. 1980;30:151–169. doi: 10.1016/0009-2797(80)90122-2. [DOI] [PubMed] [Google Scholar]
- 26.Butour JL, Mazard AM, Macquet JP. Kinetics of the reaction of cis-platinum compounds with DNA in vitro. Biochem Biophys Res Commun. 1985;133:347–353. doi: 10.1016/0006-291x(85)91882-0. [DOI] [PubMed] [Google Scholar]
- 27.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–1979. [PubMed] [Google Scholar]
- 28.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 29.Schaller W, Reisner H, Holler E. Kinetic investigation of the DNA platination reaction: evidence for a transient adduct between deoxyribonucleic acid and cis-platinum(II) Biochemistry. 1987;26:943–950. doi: 10.1021/bi00377a039. [DOI] [PubMed] [Google Scholar]
- 30.Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986;25:3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- 31.Fichtinger-Schepman AM, van der Veer JL, den Hartog JH, Lohman PH, Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- 32.Faivre S, Chan D, Salinas R, Woynarowska B, Woynarowski JM. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem Pharmacol. 2003;66:225–237. doi: 10.1016/s0006-2952(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 33.Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol. 2002;42:317–325. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 34.Chen CC, Chen LT, Tsou TC, Pan WY, Kuo CC, Liu JF, Yeh SC, Tsai FY, Hsieh HP, Chang JY. Combined modalities of resistance in an oxaliplatin-resistant human gastric cancer cell line with enhanced sensitivity to 5-fluorouracil. Br J Cancer. 2007;97:334–344. doi: 10.1038/sj.bjc.6603866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- 36.Almendro V, Ametller E, García-Recio S, Collazo O, Casas I, Augé JM, Maurel J, Gascón P. The role of MMP7 and its cross-talk with the FAS/FASL system during the acquisition of chemoresistance to oxaliplatin. PLoS One. 2009;4:e4728. doi: 10.1371/journal.pone.0004728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng RD, Shelton CC, Li YM, Qin LX, Notterman D, Paty PB, Schwartz GK. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69:573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima K, Nariai Y, Yoshimura Y. Etodolac, a selective cyclo-oxygenase-2 inhibitor, enhances carboplatin-induced apoptosis of human tongue carcinoma cells by down-regulation of FAP-1 expression. Oral Oncol. 2005;41:77–81. doi: 10.1016/j.oraloncology.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto S, Nakamura M, Osawa Y, Kono S, Itoh Y, Okano Y, Murate T, Hara A, Ueda H, Nozawa Y, et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]