Abstract
Objective
Obesity and aging increase the risk of type 2 diabetes (T2D). We evaluated whether weight-loss therapy improves pancreatic endocrine function and insulin sensitivity in obese older adults.
Research Methods
Twenty-four obese (BMI:38±2 kg/m2) older (age:70±2 yr) adults completed a 6-month randomized, controlled trial. Participants were randomized to diet and exercise (treatment group) or no therapy (control group). Beta-cell function (assessed using the C-peptide minimal model), alpha-cell function (assessed by the glucagon response to an oral glucose load), insulin sensitivity (assessed using the glucose minimal model), and insulin clearance rate were evaluated by using a 5-hr modified oral glucose tolerance test.
Results
Body weight decreased in the treatment group, but did not change in the control group (−9±1% vs. 0±1%; P<0.001). Insulin sensitivity doubled in the treatment group and did not change in the control group (116±49% vs. −1±13%, P<0.05). Even though indices of β-cell responsivity to glucose did not change (P>0.05), the disposition index, which adjusts β-cell insulin response to changes in insulin sensitivity, improved in the treatment group compared with the control group (100±47% vs −22±9%; P<0.05). The glucagon response decreased in the treatment but not in the control group (−5±2% vs. 4±4%; P<0.05). Insulin secretion rate did not change (P>0.05), but insulin clearance rate increased (51±25%; P<0.05), resulting in lower plasma insulin concentrations.
Discussion
Weight-loss therapy concomitantly improves β-cell function, lowers plasma glucagon concentrations, and improves insulin action in obese older adults. These metabolic effects are likely to reduce the risk of developing T2D in this population.
INTRODUCTION
The prevalence of obesity and type 2 diabetes (T2D) in older Americans (age ≥65 yrs) has markedly increased in the last 25 years (1, 2). The increase in obesity and obesity-related metabolic complications present a major challenge to healthcare delivery systems by increasing the need for medical therapy, hospitalization, and chronic care in older adults (3). In fact, T2D in older adults is associated with increased rates of mortality, coronary heart disease, and functional disability (4).
Type 2 diabetes is caused by insulin resistance in conjunction with pancreatic β-cell failure. Aging is associated with both increased insulin resistance and decreased β-cell function (5–7). In addition, aging is associated with increased pancreatic α-cell glucagon production (7), which can contribute to hyperglycemia by increasing hepatic glucose production (8). Therefore, older adults who are also obese are particularly susceptible to developing T2D, because of the additive adverse effects of obesity and aging on insulin sensitivity and pancreatic endocrine function (5, 7, 9–11).
The primary purpose of this study was to test the hypothesis that weight-loss therapy (diet-induced weight loss and increased physical activity) improves pancreatic endocrine function and insulin action in obese older adults. Subjects were randomized to 26 weeks of weight-loss therapy or no therapy, and a 5-h modified oral glucose tolerance test (12) was used to simultaneously derive validated indices of β-cell function, insulin sensitivity, and glucagon secretion (13, 14).
METHODS
Study subjects
A total of 27 obese (body mass index [BMI] ≥ 30 kg/m2), older (age ≥ 65 years old) men and women enrolled in this study. The effects of diet and exercise therapy on physical function and metabolic coronary heart disease risk factors in these subjects were recently reported (15, 16). All subjects completed a comprehensive medical evaluation, including a medical history and physical examination, standard blood and urine tests, and a graded treadmill exercise stress test. Subjects were sedentary (i.e. did not participate in regular exercise more than twice a week) and reported having had a stable body weight (± 2 kg) for at least 1 year before the study. In addition, no subjects had any changes in medications for at least 6 months before starting the study. Subjects who had diabetes, severe or unstable cardiopulmonary disease, received treatment with corticosteroids, androgens, or estrogen-containing compounds in the last 6 months, or musculoskeletal impairment that precluded exercise training were excluded from participation. Most of the subjects enrolled in this study had impaired oral glucose tolerance.
This study was approved by the Human Studies Committee and the General Clinical Research Center (GCRC) Scientific Advisory Committee at Washington University School of Medicine. Written informed consent was obtained from each participant.
Study design
A Modified Oral Glucose Tolerance Test (MOGTT) and body composition analyses were performed in the Washington University GCRC at baseline and at 26 weeks in both the weight-loss therapy and control groups. The MOGTT was performed at ~0800 h, after subjects fasted overnight (12 hours). An intravenous catheter was placed into an antecubital vein, which was heated by using a thermostatically controlled box, to obtain arterialized venous samples (17). At time 0, participants ingested a 75-g glucose load. Blood samples were collected at −10, 0, 10, 20, 30, 60, 90, 120, 150, 180, 240, and 300 min after glucoseingestion to determine plasma glucose, insulin, C-peptide, and glucagon concentrations.
Total fat mass and trunk fat mass were measured by using dual energy X-ray absorptiometry (Delphi 4500-W; Hologic Inc, Waltham, MA), as previously reported (15).
Interventions
Subjects were randomized to 26 wks of treatment with a low-calorie diet and exercise training (treatment group; n = 17) or no treatment (control group; n = 10) in a ~1.5:1 ratio, by using a computer generated block random permutation procedure stratified for sex (18).
Control group intervention
Subjects assigned to the control group were instructed to maintain their usual diet and activities during the study period. These subjects were advised not to participate in any weight loss or exercise program and had minimal contact with our research team while participating in the study.
Treatment group intervention
Subjects assigned to the diet plus exercise group participated in weekly group behavioral therapy meetings and in a supervised exercise-training program (90-min sessions, 3 days/week) conducted within the Center for Human Nutrition’s Weight Management Program and Exercise Physiology Laboratory. Each participant was prescribed a balanced diet to provide an energy deficit of ~750 kcal/day. Daily energy requirement was determined by multiplying estimated resting energy expenditure by 1.3 (19). The diet contained approximately 30% of energy as fat, 50% as carbohydrate, and 20% as protein. Total calorie intake was adjusted to prevent more than a 1.5 % loss of body weight per week, with the goal of 10% weight loss at the completion of the study.
Subjects met weekly as a group with a dietitian, who was experienced in group behavioral therapy for obesity. Standard behavioral strategies, including goal setting, self-monitoring, stimulus control techniques, problem-solving skills, identification of high-risk situations, and relapse prevention training, were used to modify eating habits. In addition, subjects participated in supervised group exercise-training sessions on 3 nonconsecutive days each week at our indoor exercise facility. The exercise program focused on improving flexibility, endurance, strength and balance. Each session lasted 90 min and began with a 15 min warm-up of flexibility exercises, followed by 30 min of endurance exercise (primarily walking on a treadmill, step-ups, and stairmaster), 30 min of strength training, and 15 min of balance exercises.
Assessments
Biochemical measurements
Plasma glucose was measured immediately by using an automated glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Blood samples for measurements of insulin, C-peptide, glucagon were placed on ice, centrifuged at 4°C, and plasma stored at −70 C° for later batch assays in duplicate. Plasma insulin, C-peptide, and glucagon were measured by using double-antibody radioimmunoassays (Linco Research, St. Louis, MO).
Calculations
The total areas under the curve (AUC) for glucose, insulin, C-peptide, and glucagon were calculated by using the trapezoid method (20).
Insulin sensitivity (SI; dL · kg−1 · min−1 per pmol/L) was estimated from plasma glucose and insulin concentrations obtained during the MOGTT, by using the oral glucose minimal model (13, 21). This model measures the overall effect of insulin to stimulate glucose disposal and inhibit glucose production. Complete identification of model parameters requires using assumed values for glucose volume of distribution (V, 1.7 dL/kg) and fractional glucose effectiveness (p1, 0.014 min−1); the “piecewise linear” approach was used to estimate the time course profile of the rate of appearance of oral glucose assuming a constant fraction of glucose absorption (f, 0.86) (21).
Indices of β-cell function were estimated from plasma glucose and C-peptide concentrations obtained during the MOGTT, by using the oral minimal model of C-peptide secretion and kinetics (12, 22) and incorporating parameters for C-peptide kinetics and volume of distribution as measured by Van Cauter et al (23). This model calculates the insulin secretion rate (ISR, pmol /min) as a function of time and several indices of β-cell responsivity: 1) dynamic responsivity index (Φd [109]), which is an index of insulin secretion in response to the rate of change in glucose concentration; 2) static responsivity index (Φs [109 min−1]), which is an index of insulin secretion in response to a given glucose concentration; and 3) overall responsivity index (Φo [109 min−1]), which is a global sensitivity-to-glucose index of postprandial insulin secretion.
Disposition indices (DI) were calculated by multiplying each index of β-cell responsivity by insulin sensitivity, in accordance with Bergman et al.(24), to determine whether insulin secretion was appropriate for the degree of insulin resistance. Therefore, three different disposition indexes were calculated by multiplying Φd, Φs, and Φo by SI: 1) dynamic disposition index (DId); 2) static disposition index (DIs); and 3) overall disposition index (DIo) (12, 22).
Insulin clearance rate was calculated by dividing the mean ISR by the mean serum insulin (25).
Statistical Analyses
Baseline characteristics between groups were compared by using the t test for unpaired samples for continuous variables and the Fisher’s exact test for categorical variables. Analysis of variance was used to determine whether the change in outcomes was significantly different in response to intervention compared with control. Age and baseline values were entered as covariates in the analysis of variance. The t test for paired samples was also performed to determine whether there were significant within-group changes in outcomes. SPSS version 15.0 (SPSS Inc, Chicago, IL) was used for all statistical analyses. A P value < 0.05 was considered to be statistically significant. Results are reported as mean ± SE.
RESULTS
Of the 27 participants who started the study, 2 subjects in the treatment group dropped out because of difficulty maintaining dietary compliance and 1 subject in the control group dropped out because of relocation to another state. These participants dropped out before completing the post intervention MOGTT, and therefore were not included in the final analyses of the MOGTT parameters. Age, sex, body composition and metabolic characteristics at baseline were similar in the treatment and control groups (Table 1). As expected, even though subjects who had T2D were excluded from this study, the number of subjects who had impaired glucose tolerance was high in both the control group (7/9 [75%]) and the treatment group (13/15 [87%]), and was not different between groups (P=0.62).
Table 1.
Baseline Characteristics of the Study Participants
| Control Group (n = 9) | Treatment Group (n = 15) | P Value | |
|---|---|---|---|
| Age (years) | 71 ± 2 | 69 ± 1 | 0.37 |
| Sex (F/M) | 6/3 | 11/4 | 0.54 |
| Body weight (kg) | 101 ± 6 | 99 ± 4 | 0.81 |
| Body mass index (kg/m2) | 38 ± 2 | 38 ± 1 | 0.70 |
| Total fat mass (kg) | 46 ± 3 | 42 ± 2 | 0.20 |
| Truncal fat mass (kg) | 24 ± 2 | 22 ± 1 | 0.23 |
| Fasting glucose (mmol/L) | 5.5 ± 0.2 | 5.6 ± 0.2 | 0.96 |
| 2-h OGTT glucose (mmol/L) | 8.8 ± 0.8 | 9.6 ± 0.5 | 0.40 |
| 5-hour MOGTT* area under the curve | |||
| Glucose (103 min · mmol/L) | 2.1 ± 0.3 | 2.2 ± 0.3 | 0.41 |
| Insulin (103 min · pmol/L) | 10.9 ± 13.6 | 13.5 ± 15.8 | 0.28 |
| C-peptide (103 min · pmol/L) | 875 ± 79 | 858 ± 69 | 0.87 |
| Glucagon (103 min · pg/mL) | 38.4 ± 2.4 | 41.5 ± 2.3 | 0.37 |
| Insulin secretion rate (pmol/min) | 623 ± 56 | 654 ± 76 | 0.73 |
| Insulin clearance rate (L/min) | 12.3 ± 1.7 | 11.5 ± 0.6 | 0.21 |
| Insulin sensitivity, SI (10−5dL/kg/min per pmol/L) | 8.4 ± 1.6 | 7.3 ± 1.2 | 0.56 |
| Insulin secretion indices | |||
| Static β-cell responsivity, Φs (109 min−1) | 47.8 ± 4.7 | 44.1 ± 5.2 | 0.62 |
| Dynamic β-cell responsivity, Φd (1011) | 8.9 ± 3.5 | 7.8 ± 1.8 | 0.77 |
| Overall β-cell responsivity, Φo (109 min−1) | 24.1 ± 1.8 | 21.8 ± 1.5 | 0.20 |
| Insulin secretion adjusted for SI | |||
| Static DI†, DIs (106dl/kg/min2 per pmol/L) | 3.6 ± 0.6 | 3.0 ± 0.5 | 0.42 |
| Dynamic DI, DId (107dl/kg/min2 per pmol/L) | 5.4 ± 2.6 | 5.6 ± 1.7 | 0.94 |
| Overall DI, DIo (106dl/kg/min2 per pmol/L) | 1.8 ± 0.3 | 1.4 ± 0.3 | 0.20 |
Values are mean ± SE.
MOGTT = modified oral glucose tolerance test.
DI = disposition index.
At 26 weeks, the treatment group lost 9.0 ± 1.3 kg (9 ± 1 %) of their body weight, whereas body weight did not change in the control group (0.3 ± 0.9 kg [0.2 ± 0.9%]; P<0.001 between groups). Total fat mass (−7.1 ± 0.8 vs. 1.5 ± 1.4 kg [−18 ± 3 vs 2 ± 2 %]; P<0.001) and truncal fat mass (−4.2 ± 2.6 vs. 1.4 ± 1.2 kg [−20 ± 3 vs 4 ± 3 %]; P<0.001) decreased significantly in the treatment group and did not change in the control group.
At 26 weeks, both fasting plasma glucose concentration and plasma glucose concentration at 2 h of the OGTT decreased in the treatment group (4±4% and 4±12% decrease in values, respectively) compared with the control group (4 ± 2% and 8 ± 4% increase in values, respectively) (both comparisons p<0.05).
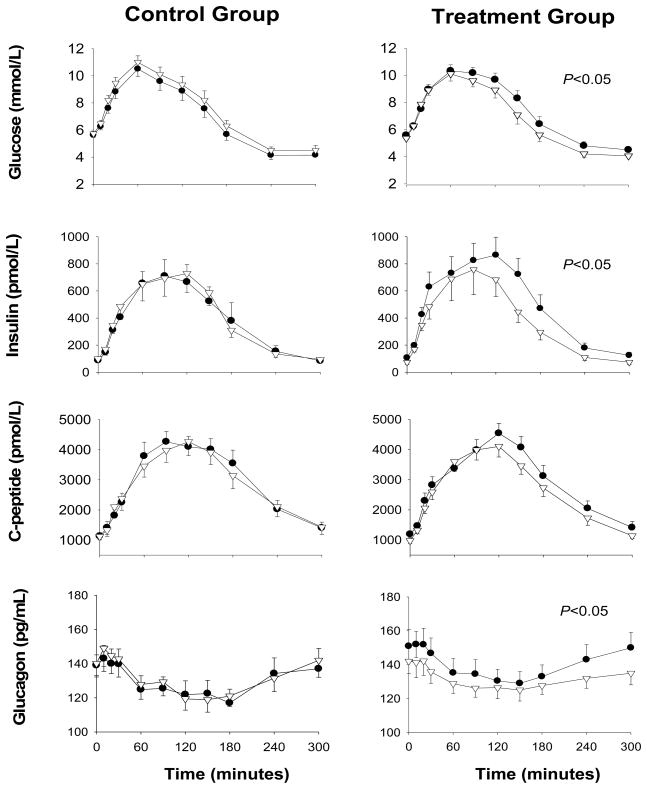
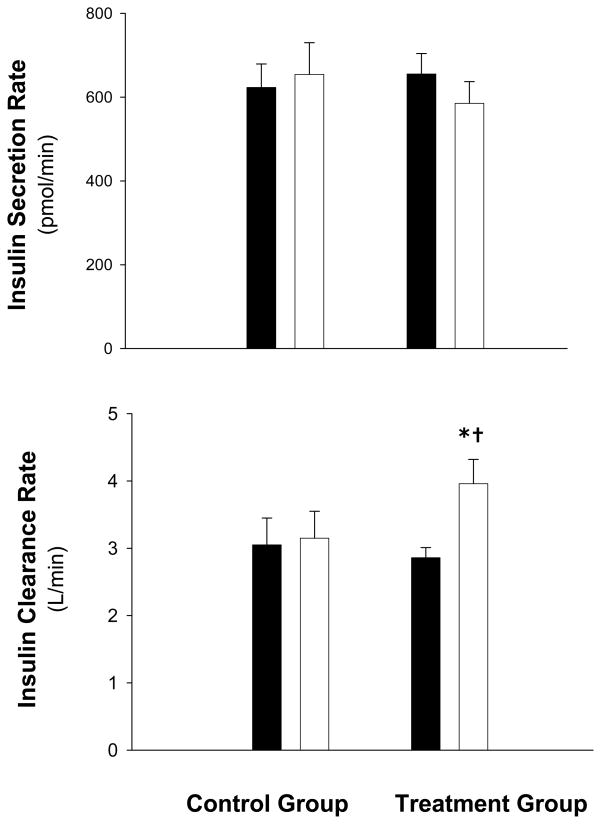
Both glucose AUC (−0.12 ± .24 vs. 0.08 ± .31 ×103 min · mmol/L [−5 ± 10 vs. 5 ± 15 %], P=0.03) and insulin AUC (−4.2 ± 0.9 vs. 4.2 ± 1.5 ×103 min · pmol/L [−26 ± 8 vs. 7 ± 14 %], P=0.04) decreased significantly in the treatment compared to the control group. (Figure 1). Despite the decrease in insulin AUC in the treatment group, C-peptide AUC did not change significantly (−75 ± 45 ×103 min · pmol/L; P=0.12). Accordingly, the insulin secretion rate which is derived from C-peptide did not change significantly, but plasma insulin clearance rate increased in the treatment group compared with the control group (4.4 ± 1.8 vs. 0.3 ± 0.6 L/min [51 ± 25 vs. 13 ± 13 %], P =0.045) (Figure 2). In addition, glucagon AUC decreased in the treatment compared with the control group (−2.6 ± 1.1 vs. 1.3 ± 1.2 ×103 min · mg/dL [−5 ± 2 vs. 4 ± 4 %], P=0.04) (Figure 1).
Figure 1.
Plasma glucose, insulin, C-peptide, and glucagon concentrations during a 5-hour modified oral glucose tolerance test in obese older subjects before (black circles) and after (white triangles) weight loss therapy (treatment group) or no therapy (control group). Values are mean ± SE. P values indicate the significance of the differences between area under the curve values before and after treatment and differences in the change in areas under the curve between groups.
Figure 2.
Insulin secretion and clearance rates in obese older subjects before (filled bars) and after (white bars) weight loss therapy (treatment group) or no therapy (control group). Values are mean ± SE. *Value significantly different from before treatment value, P <0.05. †Change from baseline was significantly different between the treatment and control group, P <0.05.
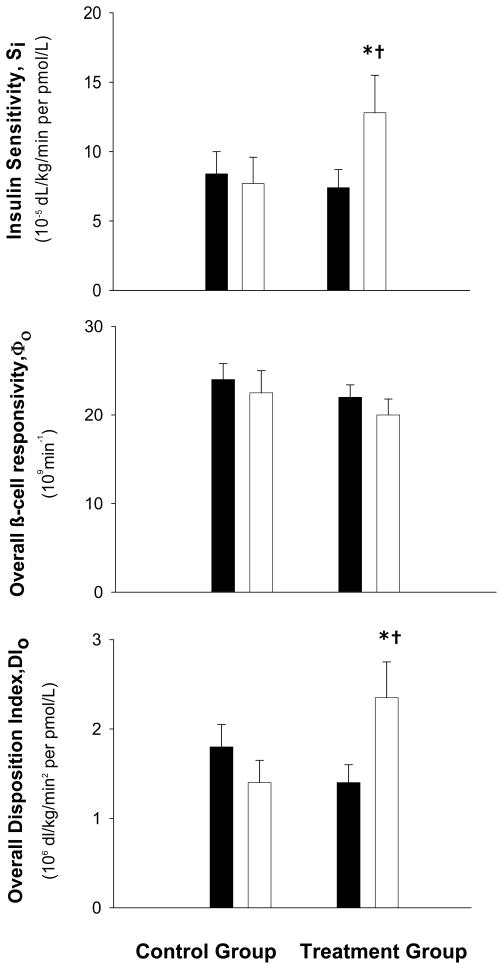
Mean values for SI increased in the treatment group but not in the control group (5.5 ± 2.6 vs. −8.0 ± 1.4 × 10−5 dL/kg/min perpmol/L [116 ± 49 vs. −11 ± 13 %]; P=0.04) (Figure 3). Despite the increase in SI in the treatment group, both the static and dynamic indices of β-cell responsivity did not change and were similar to the control group (Φs [3.8 ± 4.2 vs. −4.0 ± 5.1 × 109 min−1] and Φd, [−1.5 ± 2.2 vs. −4.1 ± 3.4 × 1011]; both P>0.05). Accordingly, the overall β-cell responsivity did not change in either group (Φo [−1.5 ± 1.7 vs. −1.6 ± 1.2 × 109 min−1]; P=0.97) (Figure 3). However, compared with the control group, the overall disposition index (DIo) increased significantly in the treatment group (0.9± 0.5 vs. −0.4 ± 0.3 × 106dL/kg/min2 per pmol/L [100 ± 47 vs. −22 ± 9 %]; P=.045) (Figure 3), which was primarily due to an increase in the static disposition index (DIs, 2.6 ± 0.8 vs. −0.4 ± 1.1 × 106dL/kg/min2 per pmol/L [153 ± 56 vs. −2 ± 25 %], P=.03).
Figure 3.
Insulin sensitivity, overall β-cell responsivity, and overall disposition index (insulin secretion adjusted for insulin sensitivity) in obese older subjects before (filled bars) and after (white bars) weight loss therapy (treatment group) or no therapy (control group). Values are mean ± SE. *Value significantly different from before treatment value, P <0.05. †Change from baseline was significantly different between the treatment and control group, P <0.05.
Improvements in SI and insulin clearance, and the reduction in glucagon AUC were no longer statistically significant (P >.05) after controlling for changes in either total body fat or truncal fat, by using analyses of covariance. Therefore, changes in both total fat and truncal fat were associated with improvements in these metabolic variables.
DISCUSSION
Pancreatic endocrine function deteriorates with age and contributes to impaired glucose homeostasis in obese older adults. The results from the present study demonstrate that lifestyle therapy (i.e. diet-induced weight loss and increased physical activity) improves both pancreatic β-cell (insulin secretion adjusted for insulin resistance in response to an oral glucose load) and α-cell function (glucagon secretion in response to an oral glucose load) and plasma insulin clearance in insulin-resistant obese older adults. Therefore, moderate diet induced weight loss in conjunction with regular physical activity, can ameliorate aging- and obesity- associated deterioration in pancreatic endocrine function in obese older adults who are at increased risk for T2D.
Although insulin resistance is commonly associated with obesity, the ability of the pancreas to compensate by increasing insulin secretion determines whether hyperglycemia occurs (26). Pancreatic β-cell function in relation to insulin sensitivity can be quantified by a product of the two variables, named the DI (24, 27). Most obese young adults do not have T2D because increased β-cell insulin secretion is able to compensate for the insulin resistance and DI is normal (24, 26). However, the aging process is accompanied by both insulin resistance and impaired insulin secretion.(5, 7, 10, 11) Therefore, inadequate β-cell responsivity to insulin resistance-induced hyperglycemia (decreased DI) leads to T2D. The data from the present study demonstrate that weight-loss therapy improves β-cell function in obese older adults, without altering the absolute rate of insulin secretion. The mechanisms responsible for the improvement in β-cell function are not clear, but could involve metabolic processes that reduce β-cell glucotoxicity and lipotoxicity (28, 29); lifestyle therapy caused a decrease in ambient blood glucose concentrations, and decreased circulating free fatty acids and triglycerides, as previously reported in these subjects (16).
Our findings support and extend data obtained from previous weight loss studies that evaluated pancreatic function. In one longitudinal study, 3 months of diet-induced weight loss improved β-cell function in overweight and obese older men (60–75 yr), (30). In other studies conducted in young and middle-aged adults, weight loss induced by treatment with bariatric surgery (31) or weight-loss medications (32) improved β-cell function. To our knowledge, the present study is the first randomized controlled trial to demonstrate that 6 months of lifestyle therapy improves both β-Cell and ά cell function, in obese (BMI ≥ 30 kg/m2) older (age ≥ 65 yr) adults, and therefore supports the beneficial effects of this intervention in older adults at increased risk for diabetes.
The MOGTT can be used to provide an assessment of insulin sensitivity (SI) by using a minimal model of glucose concentration as a function of time-variable insulin concentrations (13, 14). Weight loss therapy improved SI in our subjects who had both obesity- and aging-related insulin resistance. Therefore, these data demonstrate that insulin resistance is reversible in older adults, as has been shown in young and middle-aged adults (33, 34).
Weight loss reduced plasma glucagon concentrations in our study subjects. Hyperglucagonemia is commonly associated with both obesity and aging (7, 35). Excessive ά-cell secretion contributes to the metabolic disturbances in impaired glucose tolerance and T2D (35–37). Indeed, obese older adults in this study had elevated fasting plasma glucagon concentrations. Plasma glucagon concentration AUC during the 5-h MOGTT decreased after weight loss, which likely contributed to the observed improvement in glucose homeostasis (37).
The hyperinsulinemia associated with obesity is caused by an increase in insulin secretion and a decrease in insulin clearance (38). Aging is also associated with a reduction in insulin clearance (7, 39, 40). Our data show that weight loss can improve obesity- and aging- related abnormalities in insulin clearance. Assessment of ISR makes it possible to evaluate the rate of insulin clearance by calculating the ratio of the mean ISR to mean serum insulin (23, 25). Although ISR did not change, weight-loss therapy resulted in ~25% decrease in the plasma insulin concentration AUC during the 5-h MOGTT. Therefore, an increase in insulin clearance was responsible for the reduction in plasma insulin concentrations during the glucose challenge. Our data are consistent with data from studies conducted in younger obese adults that found weight loss can improve or normalize insulin clearance rate (41–43) through receptor-mediated insulin endocytosis in the liver or kidney (44). The decline in plasma FFA concentrations after weight loss could have contributed to the increase in insulin clearance from plasma, because fatty acids inhibit the action of insulin-degrading enzyme (44–46).
The increasing prevalence of obesity in older adults decreases their functional independence and increases health care costs (47). We have previously shown that weight loss and exercise can ameliorate frailty (15) and improve metabolic coronary heart disease risk factors (16) in obese older adults. The present results provide evidence that weight-loss therapy also improves pancreatic endocrine function, which can reduce the risk of developing T2D in obese insulin-resistant older adults (26).
Acknowledgments
This research was supported by the following grants AG025501, AG2116401, DK56341, DK20579, RR00036, and AG00078 from the National Institute of Health.
We thank the participants for their cooperation and the General Clinical Research Center staff for their skilled assistance in the performance of this study.
REFERENCE LIST
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–12. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 6.Roder ME, Schwartz RS, Prigeon RL, Kahn SE. Reduced pancreatic B cell compensation to the insulin resistance of aging: impact on proinsulin and insulin levels. J Clin Endocrinol Metab. 2000;85:2275–80. doi: 10.1210/jcem.85.6.6635. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–48. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 8.Fanelli CG, Porcellati F, Rossetti P, Bolli GB. Glucagon: the effects of its excess and deficiency on insulin action. Nutr Metab Cardiovasc Dis. 2006;16 (Suppl 1):S28–S34. doi: 10.1016/j.numecd.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 10.Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes. 2004;53:441–6. doi: 10.2337/diabetes.53.2.441. [DOI] [PubMed] [Google Scholar]
- 11.Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol. 1990;258(6 Pt 1):E937–E943. doi: 10.1152/ajpendo.1990.258.6.E937. [DOI] [PubMed] [Google Scholar]
- 12.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–8. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 13.Dalla MC, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–E643. doi: 10.1152/ajpendo.00319.2003. [DOI] [PubMed] [Google Scholar]
- 14.Dalla MC, Yarasheski KE, Caumo A, et al. Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab. 2005;289:E954–E959. doi: 10.1152/ajpendo.00076.2005. [DOI] [PubMed] [Google Scholar]
- 15.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of Weight Loss and Exercise on Frailty in Obese Older Adults. Arch Intern Med. 2006;166:860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 16.Villareal DT, Miller BV, III, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–23. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 17.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976;41:565–73. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- 18.Friedman LM, Furberg C, DeMets DC. Fundamentals of Clinical Trials. Littleton: John Wright PSG, Inc; 1980. [Google Scholar]
- 19.Harris JA, Benedict FF. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institution of Washington; 1919. [Google Scholar]
- 20.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Dalla MC, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002;49:419–29. doi: 10.1109/10.995680. [DOI] [PubMed] [Google Scholar]
- 22.Breda E, Toffolo G, Polonsky KS, Cobelli C. Insulin release in impaired glucose tolerance: oral minimal model predicts normal sensitivity to glucose but defective response times. Diabetes. 2002;51 (Suppl 1):S227–S233. doi: 10.2337/diabetes.51.2007.s227. [DOI] [PubMed] [Google Scholar]
- 23.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–77. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN, Ader M, Huecking K, Van CG. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51 (Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 25.Tillil H, Shapiro ET, Miller MA, et al. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol. 1988;254:E349–E357. doi: 10.1152/ajpendo.1988.254.3.E349. [DOI] [PubMed] [Google Scholar]
- 26.Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334:777–83. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 28.Yki-Jarvinen H. Glucose toxicity. Endocr Rev. 1992;13:415–31. doi: 10.1210/edrv-13-3-415. [DOI] [PubMed] [Google Scholar]
- 29.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–38. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 30.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab. 2004;89:2704–10. doi: 10.1210/jc.2003-031827. [DOI] [PubMed] [Google Scholar]
- 31.Guldstrand M, Ahren B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab. 2003;284:E557–E565. doi: 10.1152/ajpendo.00325.2002. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfalck AM, Hendel H, Rasmussen MH, et al. Minor long-term changes in weight have beneficial effects on insulin sensitivity and beta-cell function in obese subjects. Diabetes Obes Metab. 2002;4:19–28. doi: 10.1046/j.1463-1326.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- 33.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 35.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–10. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 36.Ahren B, Larsson H. Impaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrations. Diabetologia. 2001;44:1998–2003. doi: 10.1007/s001250100003. [DOI] [PubMed] [Google Scholar]
- 37.Larsson H, Ahren B. Glucose intolerance is predicted by low insulin secretion and high glucagon secretion: outcome of a prospective study in postmenopausal Caucasian women. Diabetologia. 2000;43:194–202. doi: 10.1007/s001250050029. [DOI] [PubMed] [Google Scholar]
- 38.Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol. 1983;245:E155–E159. doi: 10.1152/ajpendo.1983.245.2.E155. [DOI] [PubMed] [Google Scholar]
- 39.Pacini G, Beccaro F, Valerio A, Nosadini R, Crepaldi G. Reduced beta-cell secretion and insulin hepatic extraction in healthy elderly subjects. J Am Geriatr Soc. 1990;38:1283–9. doi: 10.1111/j.1532-5415.1990.tb03449.x. [DOI] [PubMed] [Google Scholar]
- 40.Fink RI, Revers RR, Kolterman OG, Olefsky JM. The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes. 1985;34:275–80. doi: 10.2337/diab.34.3.275. [DOI] [PubMed] [Google Scholar]
- 41.Letiexhe MR, Scheen AJ, Gerard PL, Desaive C, Lefebvre PJ. Insulin secretion, clearance and action before and after gastroplasty in severely obese subjects. Int J Obes Relat Metab Disord. 1994;18:295–300. [PubMed] [Google Scholar]
- 42.Letiexhe MR, Scheen AJ, Gerard PL, Desaive C, Lefebvre PJ. Postgastroplasty recovery of ideal body weight normalizes glucose and insulin metabolism in obese women. J Clin Endocrinol Metab. 1995;80:364–9. doi: 10.1210/jcem.80.2.7852491. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez J, Zuniga-Guajardo S, Zinman B, Angel A. Effects of weight loss in massive obesity on insulin and C-peptide dynamics: sequential changes in insulin production, clearance, and sensitivity. J Clin Endocrinol Metab. 1987;64:661–8. doi: 10.1210/jcem-64-4-661. [DOI] [PubMed] [Google Scholar]
- 44.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22:487–93. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 45.Jochen A, Hays J, Lee M. Kinetics of insulin internalization and processing in adipocytes: effects of insulin concentration. J Cell Physiol. 1989;141:527–34. doi: 10.1002/jcp.1041410311. [DOI] [PubMed] [Google Scholar]
- 46.Svedberg J, Bjorntorp P, Smith U, Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990;39:570–4. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- 47.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [also published in: Obes Res. 2005: 13:1849–1863] [DOI] [PubMed] [Google Scholar]