Abstract
Purpose
Poor control of intermittent exotropia (IXT) has been considered an indication for surgical intervention, and poor distance stereoacuity may be an indicator of poor control. We evaluated two new measures of distance stereoacuity, the Frisby Davis Distance (FD2) test and Distance Randot® (DR) test, both of which have been previously validated in normal and strabismic subjects, and we compared stereoacuity to scores on a recently described control scale.
Design
Prospective case series.
Participants
25 consecutive patients with IXT.
Methods
Office-based control was graded at distance and near on a 0 to 5 scale and distance control ranged from 1 (recovery in 1–5 seconds after monocular occlusion) to 4 (>50% spontaneously tropic). Stereoacuity was measured using the FD2 and DR tests at distance and the Preschool Randot® and Frisby tests at near.
Main outcome measures
Distance stereoacuity measured using the FD2 and DR tests.
Results
Measurable distance stereoacuity thresholds in IXT were poor with the DR test and excellent with the FD2 test (medians: nil and 40”, p<0.0001). Near stereoacuity was excellent with both the Preschool Randot® and Frisby (medians: 60” and 60”, p=0.99). There was poor correlation between distance control score and either FD2 (rs=0.1, p=0.6) or DR (rs=0.3 p=0.2). Control scores correlated with magnitude of deviation at distance (rs=0.5, p=0.02) and near (rs=0.5, p=0.01).
Conclusions
The real world contour-based targets of the new distance FD2 test appear to stimulate fusion in IXT, even when distance control is poor. In contrast, the new Polaroid vectograph-based Distance Randot® test is very sensitive to disturbances of binocularity. Two new distance stereoacuity tests appear sensitive to opposite ends of the IXT spectrum; FD2 performance deteriorates when the patient is constantly tropic whereas DR performance deteriorates at the earliest stages of intermittency.
Introduction
Although intermittent exotropia (IXT) is a common form of childhood strabismus,1, 2 the indications for surgery are unclear.3 Authors of retrospective case series have reached opposing conclusions, some suggesting prolonged observation, since visual acuity and near stereoacuity are preserved in the vast majority of cases without intervention,4 and others suggesting that surgery should be performed early before the deviation becomes constant.5, 6
The proportion of time that the deviation is manifest and the ease of re-fusion after dissociation has been termed “control.” Such control has been graded in a variety of ways by previous investigators; some use a 3-point office-based scale (excellent or good, moderate or fair and poor),7, 8 and others have suggested a scale that involves both parental report and in-office observation.9
Assessing distance stereoacuity has been advocated as a measure of control in IXT, in contrast to near stereoacuity which is often preserved until late in the course of the condition.7 Stathacopoulos et al7 described the association of poor distance stereoacuity with IXT, compared with normal controls. Nevertheless, they found7 no significant relationship between their 3-point office-control scale and level of distance stereoacuity. Moreover, the Baylor Visual Acuity Tester (BVAT) and Binocular Vision System (BVS), used in their study, are no longer widely available and newer methods of measuring distance stereoacuity have been introduced.
We evaluated measurable distance stereoacuity thresholds in patients with IXT using two new tests, the Frisby Davis Distance (FD2) test10 and Distance Randot® (DR) test,11, 12 which have been previously validated in normal and strabismic subjects.11–13 We compared measurable distance stereoacuity thresholds with a newly described office control scale14 that has finer gradations of control than previous scales.
Methods
For the present study, Institutional Review Board (IRB)/Ethics Committee approval was obtained. All experiments and data collection were conducted in a manner compliant with the Health Insurance Portability and Accountability Act.
Evaluation of control
For each patient, the “control” of exotropia was evaluated using a recently described “control scale” assigning a value from 0 (phoric) to 5 (constantly tropic) for both distance (3 m) and near (1/3 m) fixation (Table 1).14
Table 1.
Control score description and instructions.
| Control Score | Control Score Description |
|---|---|
| 5 | Constant exotropia during a 30-second observation period (before dissociation) |
| 4 | Exotropia >50% of the time during a 30-second observation period |
| 3 | Exotropia <50% of the time during a 30-second observation period |
| 2 | No exotropia unless dissociated (10 seconds): recovery in > 5 seconds |
| 1 | No exotropia unless dissociated (10 seconds): recovery in 1–5 seconds |
| 0 | Pure phoria: < 1 second recovery after 10-second dissociation |
| Instructions: | |
| The control scale is for both distance (3m) and near (1/3m) fixation, which combined, yields an overall score ranging from 0 to 10. 14 The fixation objects are accommodative, and age appropriate, such as small stickers and videos for younger children and letters for older children and adults. | |
| Levels 5 to 3 are assessed during a 30-second observation period. If exotropia is observed, testing stops and the control score is recorded as 5, 4, or 3 at that distance. If no exotropia is observed during the 30-second observation period, testing continues. | |
| Levels 2 to 0 are then assessed and graded as the worst of three rapidly successive 10-second periods of occlusion. An occluder is first placed over the right eye for 10 seconds and the time required to re-fusion is noted. The left eye is then occluded for 10 seconds and the time to refusion is similarly measured. A third occlusion trial is performed on the eye that required the longest time to re-fuse. The worst of the three 10-second trials is recorded, resulting in a control score of 2, 1, or 0 at that distance. | |
| If the patient has a micro-esotropia by simultaneous prism and cover test, but exodeviation by alternate cover test,25 the scale applies to the exodeviation. | |
Methods of measuring stereoacuity
Stereoacuity was measured using two tests at distance fixation and two tests at near fixation. The Frisby Davis Distance (FD2),10, 13 a “real depth test,” and the Distance Randot® test (DR),11 a Polaroid vectograph test, were used to test distance stereoacuity. The near Frisby15 and the Preschool Randot® 16 were used to measure near stereoacuity. All 4 tests were administered using previously described presentation protocols.11, 13, 16, 17 Two targets at each level had to be identified correctly to be scored as having passed that level.11, 13, 16, 17
The FD2 test and DR test were administered at 3 meters and yielded thresholds of 20 to 200 secarc and 60 to 800 secarc, respectively. The Preschool Randot® test was administered at 40 cm and yielded thresholds of 40 to 800 secarc. The 3 plates of the near Frisby were administered at a range of distances yielding thresholds of 40 to 400 secarc. If the subject failed the largest disparity on a test, the stereoacuity was recorded as “nil” for that test.
Patients
For the present study of stereoacuity and control, consecutive patients with intermittent exotropia (IXT) were recruited at two sites. Only patients with truly intermittent deviations were included, excluding patients with a constant tropia (control score of 5) or a pure phoria (control score of zero) at both distance and near. The study was designed in this way to avoid the bias of evaluating correlations of control and stereoacuity when including patients with pure phorias associated with excellent stereopsis or constant large-angle tropias associated with an inevitable absence of stereoacuity.
We only included patients with visual acuity of 20/40 or better in each eye and no amblyopia (defined as at least 0.2 logMAR difference between eyes). Visual acuity was measured using the Amblyopia Treatment Study visual acuity protocol18 with surrounded HOTV optotypes for children under seven years of age. For children older than seven years and for adults, visual acuity was measured using the electronic Early Treatment for Diabetic Retinopathy Study method19 or a Snellen chart. All patients were tested in their habitual spectacle correction. These visual acuity criteria were used so as not to bias against the Randot® tests, where the individual component dots might not be resolvable at poorer acuities. Twenty-five patients were included, ranging in age from 3 to 68 years. Sixteen of these 25 were children 3 to 17 years, mean 7.9 years ± 3.7 years (SD), and 9 were adults mean 48.4 years ± 17.7 years. Distance exodeviations ranged from 8 to 70 prism diopters (pd) measured using the alternate prism and cover test. Near deviations ranged from 4 to 70 pd with no patient having a near deviation more than 10 pd greater than their distance deviation, thereby excluding patients with convergence insufficiency. Four of 25 (16%) would have been defined as distance type exotropia with a distance deviation of at least 10 pd greater than the near deviation, by alternate prism and cover test. Patients who had more than 2 prism diopters of vertical deviation were excluded.
Statistical methods
Stereoacuity thresholds were compared between methods by grouping thresholds (Table 2) to comparable levels in order to eliminate any bias of different possible minimums and maximums and using non-parametric Wilcoxon signed rank tests. Actual thresholds were used when reporting median values and evaluating correlations using non-parametric Spearman rank methods.
Table 2.
Classification of stereoacuity thresholds into 3 groups to eliminate potential bias of different minimums and maximums.
| Group classification |
FD2 levels (secarc) |
Distance Randot® Levels (secarc) |
Near Frisby levels (secarc) |
Preschool Randot® levels (secarc) |
|---|---|---|---|---|
| Nil | Nil | Nil | Nil | |
| Coarse / nil | - | 800 | - | 800 |
| - | 400 | 400 | 400 | |
| 200 | 200 | 200 | 200 | |
| Moderate | 160 | - | - | - |
| - | 100 | 100 | 100 | |
| 80 | - | - | - | |
| 60 | 60 | 60 | ||
| Fine | 40 | - | 40 | 40 |
| 20 | - | - | - | |
Results
Distance stereoacuity
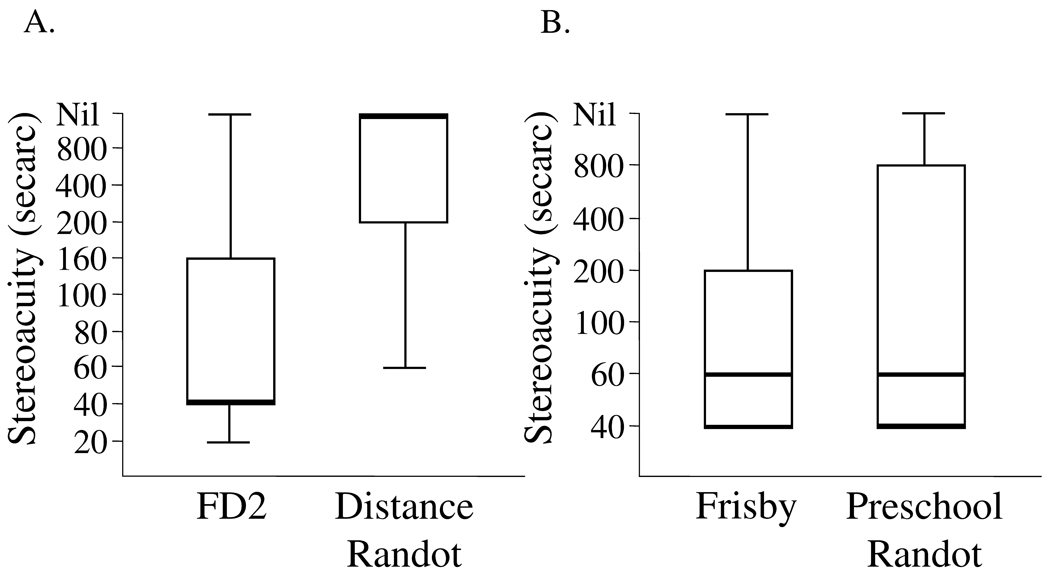
In these patients with IXT, measurable distance stereoacuity thresholds were poor with the DR test and excellent with the FD2 test (medians: nil and 40”, p<0.0001). Figure 1A
Figure 1.
Box and whiskers plots of measurable distance stereoacuity thresholds (A) measured using the Frisby Davis Distance (FD2) test and Distance Randot® (DR) test in 25 patients with intermittent exotropia and near stereoacuity (B) measured using the near Frisby test and Preschool Randot® test. Center bold lines represent the median, top and bottom of the boxes represent quartiles and the whiskers represent the extreme values.
Near Stereoacuity
As expected in patients with IXT, measurable near stereoacuity thresholds were excellent with the Preschool Randot® and Frisby (medians: 60” and 60”, p=0.99). Figure 1B
Control scores and angle of deviation
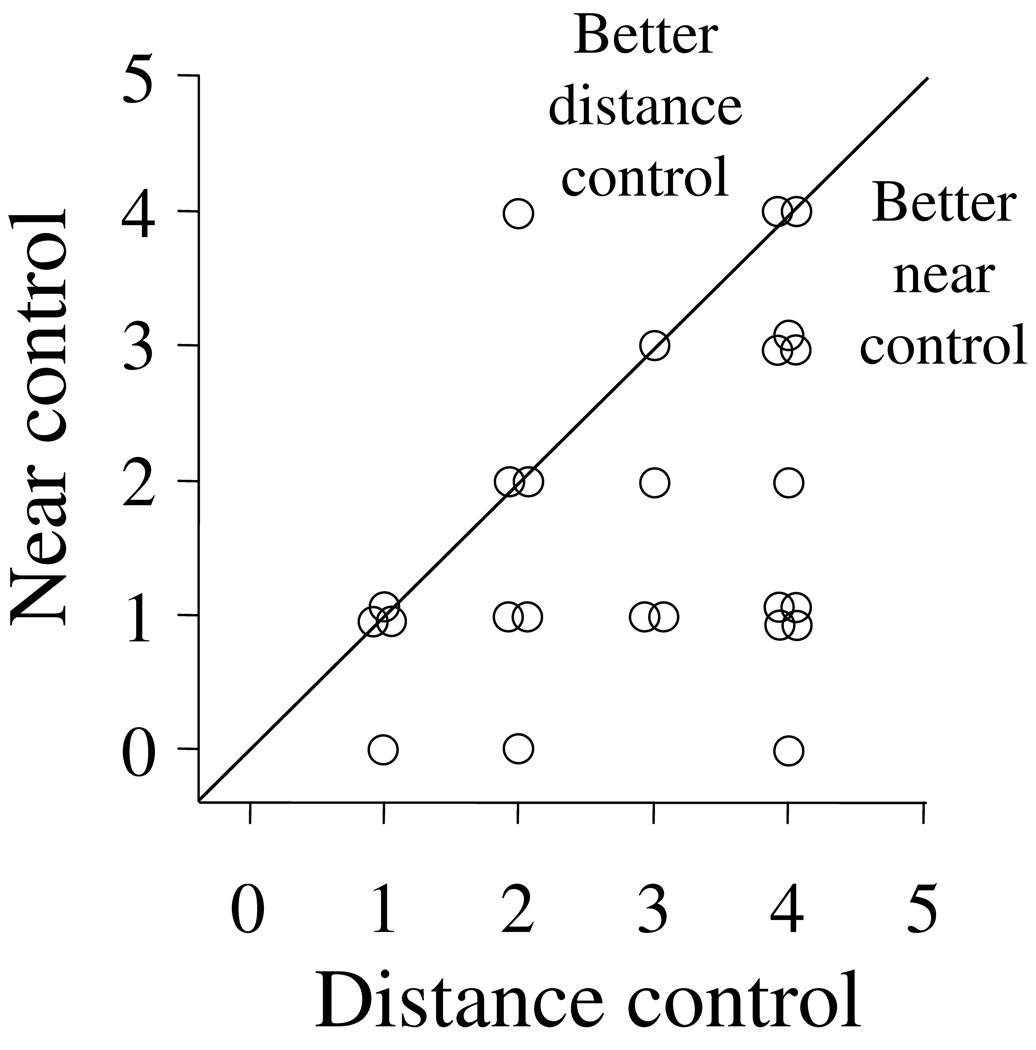
Control scores ranged from 1 to 4 at distance fixation (since patients with constant exotropia, control score of 5, were excluded from the primary cohort), and all but one patient had distance control that was the same or worse than the control at near control (Figure 2).
Figure 2.
Relationship between distance and near control. Typical of patients with IXT, all but one patient had distance control that was worse than, or the same as, near control.
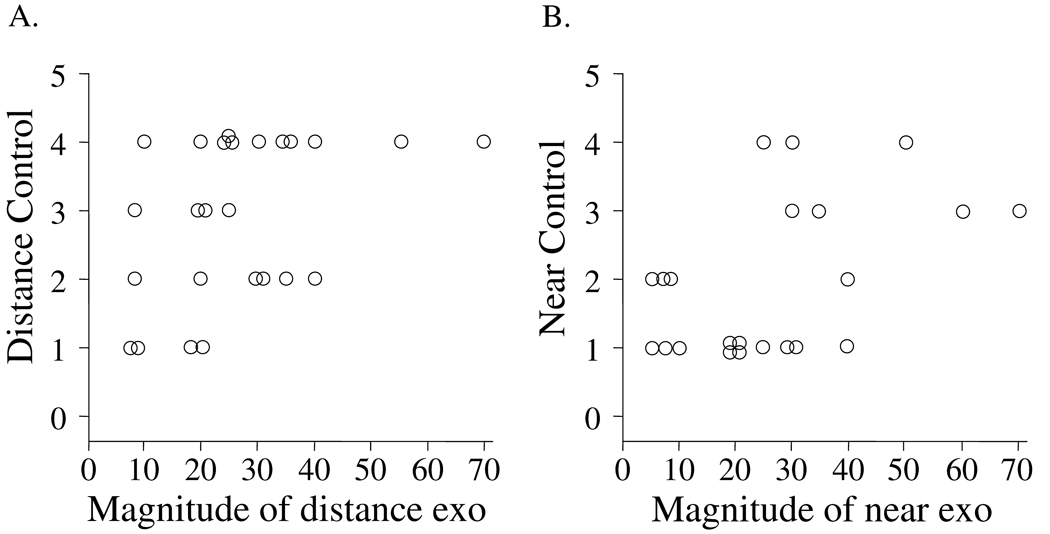
There was a positive correlation between the magnitude of deviation by alternate prism and cover test with the control score at both distance (Figure 3A, rs=0.5, p=0.02), and near (Figure 3B, rs=0.5, p=0.01). Nevertheless, there were notable exceptions; patients with the same angle of deviation might exhibit different control scores (Figures 3A and 3B). This individual variability in control for the same deviation corresponds to common clinical experience, where a patient with a given angle of exodeviation may have either excellent or poor control.
Figure 3.
Relationship between control and angle of exodeviation by alternate prism and cover test at distance fixation (A) and near fixation (B). Although there was a positive correlation (distance rs = 0.5, p=0.02, near rs = 0.5, p=0.01), there were notable examples of patients who had the same angle of deviation by alternate cover test and either excellent or poor control.
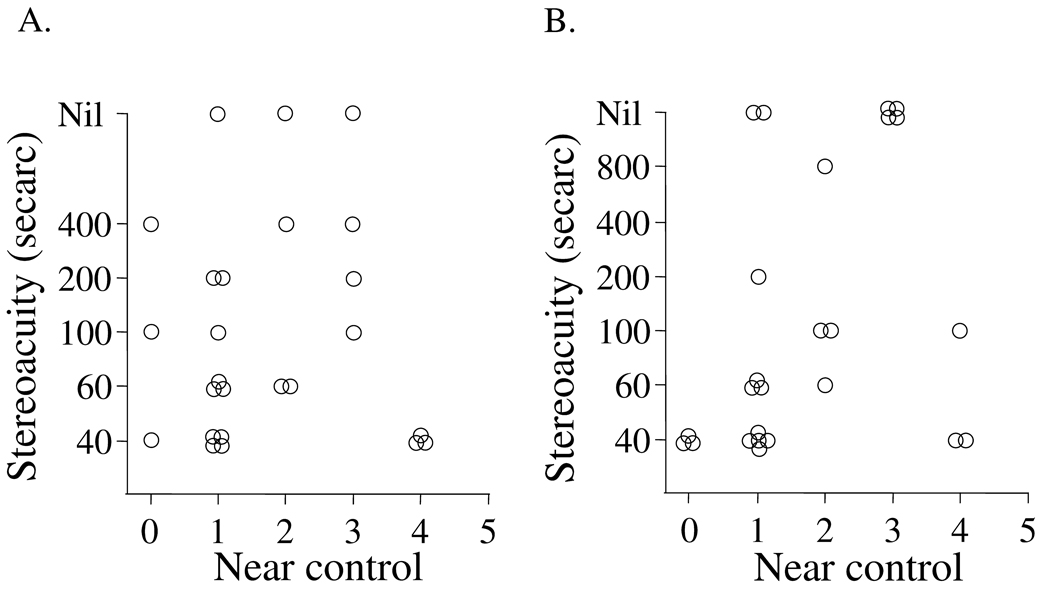
Control scores and distance stereoacuity
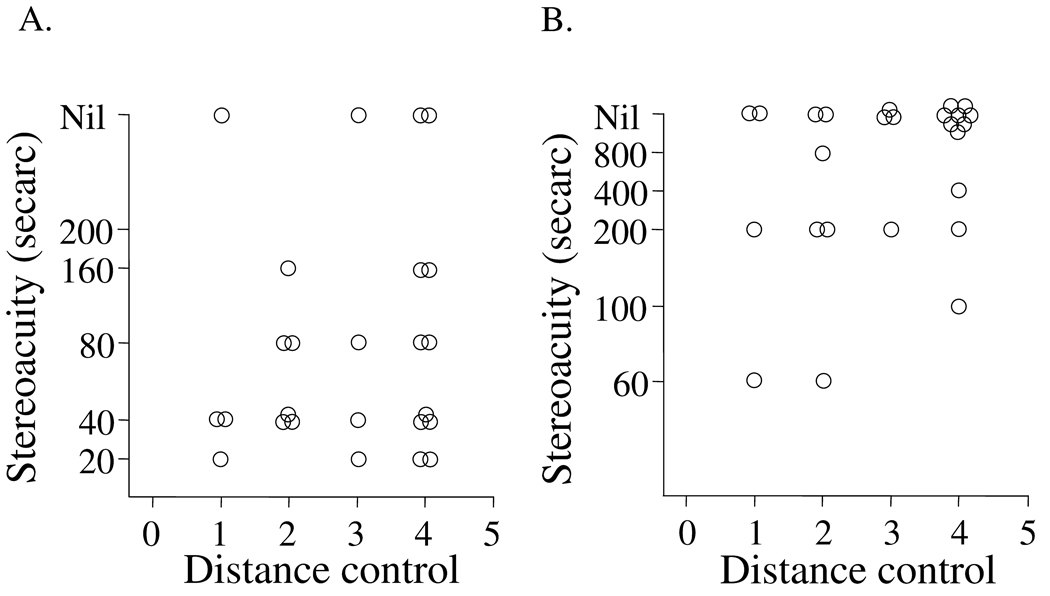
Performance on the FD2 test was generally good but the correlation with distance control score was poor (rs=0.1, p=0.6, Figure 4A). One possible reason for the poor correlation was that most patients had excellent stereoacuity with the FD2, regardless of control score. In contrast to the FD2, performance on the DR test was generally poor and correlation with the distance control score was also poor (rs=0.3 p=0.2, Figure 4B). With the DR test, the distributions of stereoacuity scores were clustered around "nil," and this contributed to the overall poor correlation with control scores.
Figure 4.
Relationship between distance control score and measurable distance stereoacuity thresholds either measured using the Frisby Davis 2 (FD2) test (A) or Distance Randot® (DR) test (B). The performance on the FD2 test was generally good, but the correlation with control score was poor (rs=0.1, p=0.6). Performance on the DR test was generally poor and the correlation with the control score was similarly poor (rs=0.3 p=0.2).
In order to evaluate the correlations of stereoacuity with control across a broader spectrum of control, a secondary analysis was performed, including 8 additional patients with constant XT of 10 to 50 pd at distance and 4 to 55 pd at near (control score 5 at distance and near), who were seen during the study period and also met the visual acuity and other inclusion criteria. All of these patients had “nil” stereoacuity on each of the 4 stereotests, as expected in patients with constant moderate or large angle deviations. When these patients were included, as a distinct separate secondary statistical analysis of correlation between distance control score and distance stereoacuity, the correlations became significant (rs=0.5, p=0.001 for FD2, rs=0.5, p=0.006 for Distance Randot®). But care should be taken in interpreting this finding, because, while performance on both tests is severely disrupted by constant XT (control score 5), the results of this secondary analysis are not applicable to examining the sensitivity of the tests to disruption by intermittent exotropia. Subsequent analyses return to the primary cohort of truly intermittent exotropia.
Control scores and near stereoacuity
Although performance on the near Preschool Randot® test and near Frisby were excellent overall, there were still differences between patients that might be partially explained by control. However, the correlation of Near Frisby and near control was poor (rs=0.02, p=0.9, Figure 5A) and the correlation of near Preschool Randot® and near control was only modest (rs=0.4, p=0.04, Figure 5B).
Figure 5.
Relationship between near control score and measurable near stereoacuity thresholds either measured using the near Frisby test (A) or the Preschool Randot® test (B). The performance on both tests was generally excellent, but the correlation with control score was poor with the near Frisby (rs= 0.02, p=0.9) and modest with the Preschool Randot® test (rs=0.4, p=0.04).
In an analogous secondary analysis described above, including 8 additional patients with constant XT (control score 5) and “nil” stereoacuity, correlations improved (rs=0.5, p=0.004 for near Frisby, rs=0.6, p=0.0002 for Preschool Randot ®). Similar care should be taken in interpreting this finding because the constant XT patients (control score 5) are driving the positive correlation and therefore the results of this secondary analysis are not applicable to intermittent exotropia.
Discussion
We found that measurable distance stereoacuity thresholds in IXT are highly dependent on the type of test used. A real world contour-based test, the Frisby Davis Distance (FD2), yielded excellent distance stereoacuity thresholds close to the normal range, whereas a Polaroid vectograph-based Randot® test yielded much poorer thresholds.
Our conclusion of poorer distance stereoacuity in IXT than in normals, using the Distance Randot® test, was based on published normal values for the Distance Randot® test,11 and is similar to the difference between IXT and normals found by Stathacopoulos et al7 using the Baylor Visual Acuity Tester Binocular Vision System (BVAT BVS Mentor II) system. Normal values for the distance FD2 have also been published by Adams et al10 and, in contrast to the Distance Randot® test, FD2 values in IXT appear close to normal values.
Our findings of a disparity between performance on several tests of stereoacuity in patients with IXT is analogous to the disparities found between other stereotests reported by Stathacopoulos et al7 and O’Neal et al,20 who compared the BVAT circles to the BVAT random dot E (BVDRE). BVAT circle performance was better than BVRDE performance. O’Neal et al20 speculated that elevated thresholds in Randot® tests may be “due to lack of monocularly visible contours.” In addition to the “fusability” of the different targets, we suggest that real world contour-based tests and Polaroid vectograph Randot® tests measure different aspects of stereoacuity.12 This difference between underlying mechanisms of different stereotests has also been suggested for near tests.21
In the context of IXT, the relative ease of fusing FD2 targets was previously described by Hatt et al 22. It is notable that the median threshold for the FD2 in our patients with IXT, was at least as good as the near thresholds with the near Frisby test and the Preschool Randot® test. These results suggest the FD2 is not particularly suited for evaluating patients with IXT, with the exception that when a patient becomes constantly tropic they will have no measurable threshold on the FD2. Since we did not use the FD2 test at a 6 meter test distance, where it yields thresholds from 5 to 50 secarc, we did not completely rule out a possible subtle deficit in FD2 thresholds in IXT patients. Nevertheless our median measurable threshold of 40” in IXT patients was very similar to the mean threshold of 30” reported in normal children over 4 years reported by Adams et al10 when they used the 6 meter test distance.
In contrast, the Distance Randot® test may be very sensitive to the disturbance in binocular fusion that appears to be an intrinsic component of IXT. Despite no patient having a constant tropia, only 10 (40%) of our 25 patients had a measurable stereoacuity threshold on this test, and only 2 (8%) patients passed the finest level of 60 sec of arc. Our results in IXT patients are consistent with the results of Mireskandari et al23 in macular hole patients, where they suggested that motor fusion deficits might explain poorer improvement in stereoacuity measured using random dot tests versus contour tests following surgery. Fine motor fusion may be more critical for optimum performance in random dot tests than for contour or real world tests.
We expected that distance control would be correlated to distance stereoacuity, but for neither test did we find such a correlation. This finding is consistent with Stathacopoulos et al7 who found no relationship between a 3-level control grade and distance stereoacuity. We had a broader range of possible control scores than Stathacopoulos et al,7 perhaps enhancing our ability to find a relationship between control and distance stereoacuity, if one existed. One reason for the lack of correlation with FD2 thresholds was that too many of these patients had normal or near-normal stereoacuity thresholds, which might create a “ceiling effect.” Conversely, there was a “floor effect” with the Distance Randot® test, where the majority of cases had no measurable threshold, reducing our ability to detect a significant correlation between stereoacuity and control. For future tests of distance stereoacuity to be useful in evaluating patients with IXT, they would need to have 1) a broad range of presentable thresholds and 2) a range of responses in patients with IXT.
We also explored the possibility that patients with poorer distance control than near control were different from those with similar distance and near control. But, even when analysis was limited to those patients who had worse distance control than near control (data not shown), there was no significant correlation between distance control score and distance stereoacuity either with the FD2 test or the Distance Randot® test.
Although our control scale has proven useful in characterizing the whole spectrum of IXT,14 and we now provide evidence that it correlates to the magnitude of the deviation, it is premature to consider the scale a standard test in this condition. Further studies of test-retest reliability and longitudinal studies are underway. Alternative scales have been suggested, but each has potential problems; Petrunak et al24 described a scale that grades time to re-fusion following dissociation, but their method is unable to assign a score to patients who are tropic during their examination. The Newcastle scale9 combines a parental report of home control (0 to 3) with office control (0 to 2 for both distance and near fixation), yielding a total score from 0 to 7. It is possible that a single assessment of control in the office may be insufficient to fully characterize IXT in one individual. It would be of interest to compare a single assessment of office control, multiple office assessments of control, and parental assessment of frequency of the deviation.
We are unaware of any sources of bias that might explain our results. Since the FD2 and Distance Randot® tests have different minimum and maximum measurable thresholds, with the FD2 able to measure 20 to 200 secarc and Distance Randot® 60 to 800 secarc, one might expect a higher proportion of IXT patients to pass “any” measurable threshold on the Distance Randot® test. Nevertheless, our findings were opposite of this potential bias.
The real world contour-based targets of the new Distance FD2 test appear to stimulate fusion in IXT, even when distance control is poor. In contrast, the new Polaroid vectograph-based Distance Randot® test appears very sensitive to disturbances of binocularity. These two new distance stereoacuity tests appear sensitive to opposite ends of the IXT spectrum; FD2 performance only deteriorates when the patient is constantly tropic whereas DR performance deteriorates at the earliest stages of intermittency. Clinical decisions based on measured stereoacuity thresholds should account for the test method used.
Acknowledgments
Supported by National Institutes of Health Grants EY015799 and EY011751 (JMH) and EY005236 (EEB), Research to Prevent Blindness, Inc., New York, NY (JMH as Olga Keith Weiss Scholar and an unrestricted grant to the Department of Ophthalmology, Mayo Clinic), and Mayo Foundation, Rochester, MN.
Footnotes
Presented in part as a poster at the Annual Meeting of the American Association of Pediatric Ophthalmology and Strabismus, March 16, 2006.
No conflicting relationships exist
References
- 1.Mohney BG, Huffaker RK. Common forms of childhood exotropia. Ophthalmology. 2003;110:2093–2096. doi: 10.1016/j.ophtha.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia. A population-based study. Ophthalmology. 2005;112:104–108. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Gnanaraj L, Richardson SR. Interventions for intermittent distance exotropia: review. Eye. 2005;19:617–621. doi: 10.1038/sj.eye.6701617. [DOI] [PubMed] [Google Scholar]
- 4.Rutstein RP, Corliss DA. The clinical course of intermittent exotropia. Optom Vis Sci. 2003;80:644–649. doi: 10.1097/00006324-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Abroms AD, Mohney BG, Rush DP, et al. Timely surgery in intermittent and constant exotropia for superior sensory outcome. Am J Ophthalmol. 2001;131:111–116. doi: 10.1016/s0002-9394(00)00623-1. [DOI] [PubMed] [Google Scholar]
- 6.Pratt-Johnson JA, Barlow JM, Tillson G. Early surgery in intermittent exotropia. Am J Ophthalmol. 1977;84:689–694. doi: 10.1016/0002-9394(77)90385-3. [DOI] [PubMed] [Google Scholar]
- 7.Stathacopoulos RA, Rosenbaum AL, Zanoni D, et al. Distance stereoacuity. Assessing control in intermittent exotropia. Ophthalmology. 1993;100:495–500. doi: 10.1016/s0161-6420(93)31616-7. [DOI] [PubMed] [Google Scholar]
- 8.Chia A, Seenyen L, Long QB. A retrospective review of 287 consecutive children in Singapore presenting with intermittent exotropia. J AAPOS. 2005;9:257–263. doi: 10.1016/j.jaapos.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Haggerty H, Richardson S, Hrisos S, et al. The Newcastle Control Score: a new method of grading the severity of intermittent distance exotropia. Br J Ophthalmol. 2004;88:233–235. doi: 10.1136/bjo.2003.027615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams WE, Hrisos S, Richardson S, et al. Frisby Davis distance stereoacuity values in visually normal children. Br J Ophthalmol. 2005;89:1438–1441. doi: 10.1136/bjo.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu VLN, Birch EE, Holmes JM. Assessment of new distance Randot test in normal and strabismic patients. J AAPOS. 2006;10:419–423. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Leske DA, Birch EE, Holmes JM. Real depth versus randot stereotests. Am J Ophthalmol. doi: 10.1016/j.ajo.2006.04.065. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes JM, Fawcett SL. Testing distance stereoacuity with the Frisby-Davis 2 (FD2) test. Am J Ophthalmol. 2005;139:193–195. doi: 10.1016/j.ajo.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus. doi: 10.1080/09273970600894716. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisby JP, Davis H, McMorrow K. An improved training procedure as a precursor to testing young children with the Frisby Stereotest. Eye. 1996;10:286–290. doi: 10.1038/eye.1996.60. [DOI] [PubMed] [Google Scholar]
- 16.Birch E, Williams C, Hunter J, et al. Random dot stereoacuity of preschool children. J Pediatr Ophthalmol Strabismus. 1997;34:217–222. doi: 10.3928/0191-3913-19970701-08. [DOI] [PubMed] [Google Scholar]
- 17.Lal G, Holmes JM. Postoperative stereoacuity following realignment for chronic acquired strabismus in adults. J AAPOS. 2002;6:233–237. doi: 10.1067/mpa.2002.123399. [DOI] [PubMed] [Google Scholar]
- 18.Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–1353. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 19.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 20.O'Neal TD, Rosenbaum AL, Stathacopoulos RA. Distance stereo acuity improvement in intermittent exotropic patients following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1995;32:353–357. doi: 10.3928/0191-3913-19951101-06. [DOI] [PubMed] [Google Scholar]
- 21.Fawcett SL. An evaluation of the agreement between contour-based circles and random dot-based near stereoacuity tests. J AAPOS. 2005;9:572–578. doi: 10.1016/j.jaapos.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Hatt SR, Haggerty H, Buck D, et al. Distance Stereoacuity in Intermittent exotropia. British Journal of Ophthalmology. 2007;91:219–221. doi: 10.1136/bjo.2006.099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mireskandari K, Garnham L, Sheard R, et al. A prospective study of the effect of a unilateral macular hole on sensory and motor binocular function and recovery following successful surgery. Br J Ophthalmol. 2004;88:1320–1324. doi: 10.1136/bjo.2004.042093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrunak JL, Rao RC, Baker JD. The evaluation of office control in X(T): A systemic approach. In: de Faber JT, editor. Transactions of the 28th European Strabismological Association Meeting. London: Taylor and Francis; 2004. [Google Scholar]
- 25.Baker JD, Davies GT. Monofixational intermittent exotropia. Arch Ophthalmol. 1979;97:93–95. doi: 10.1001/archopht.1979.01020010033007. [DOI] [PubMed] [Google Scholar]