Abstract
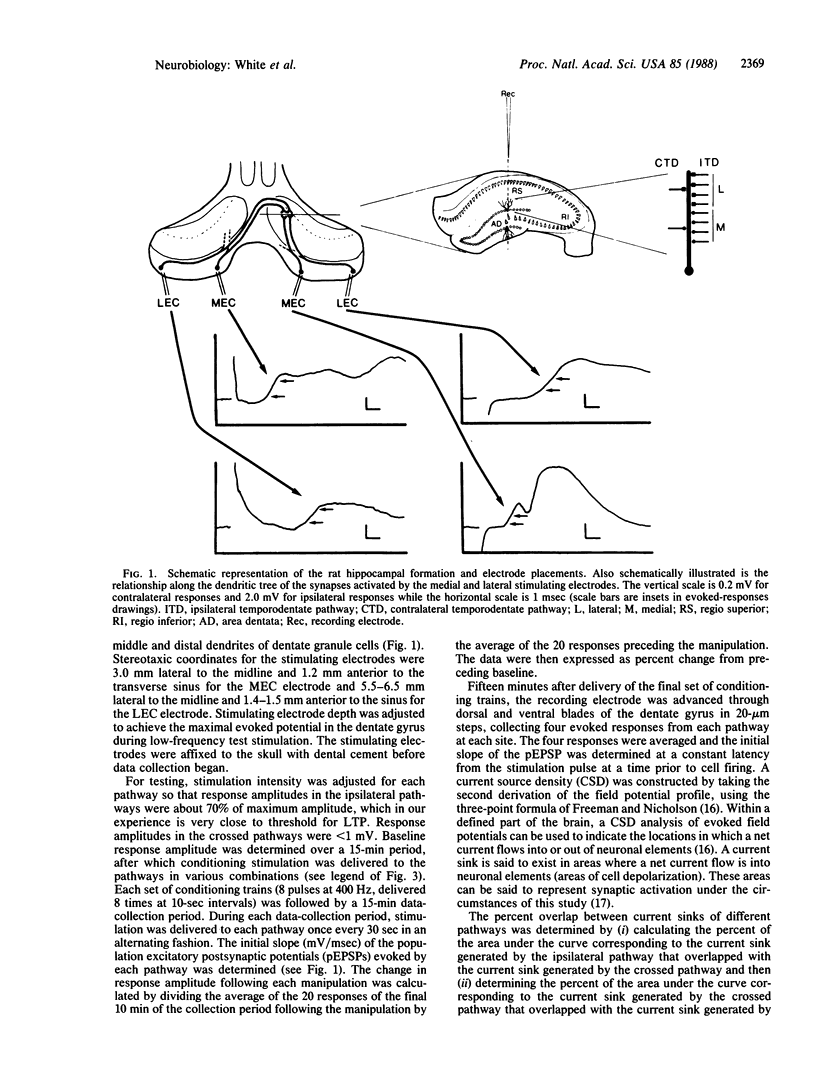
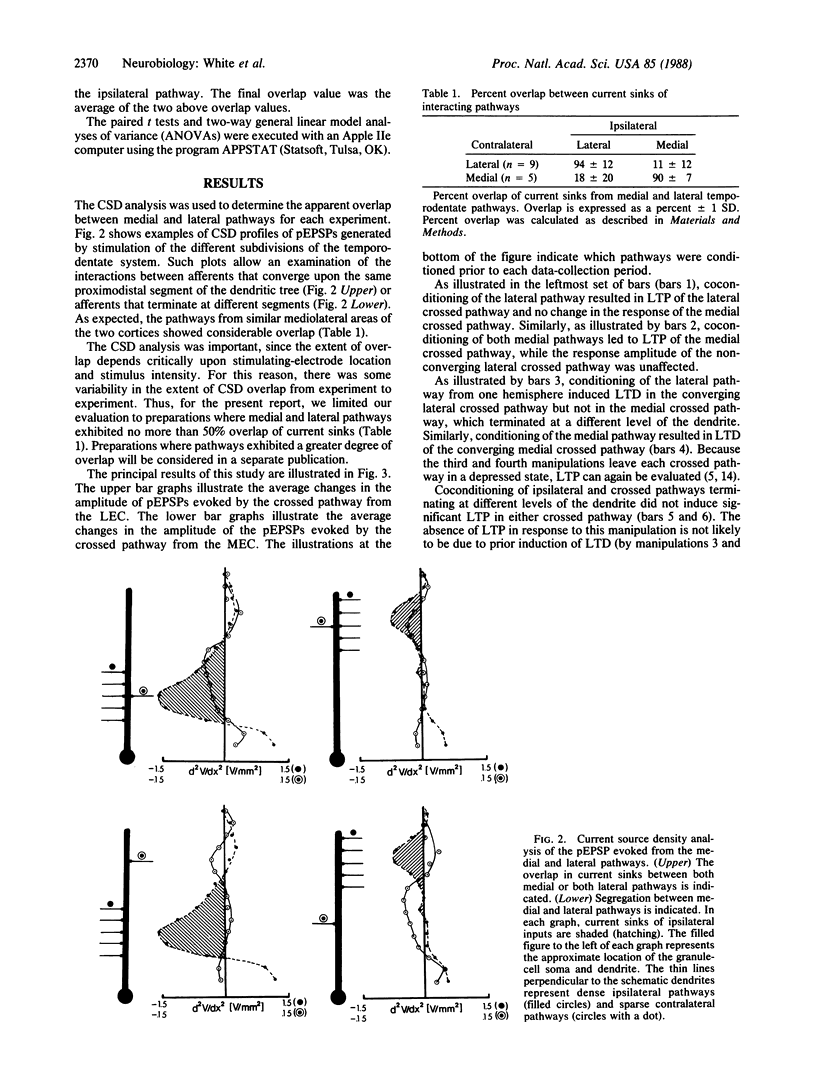
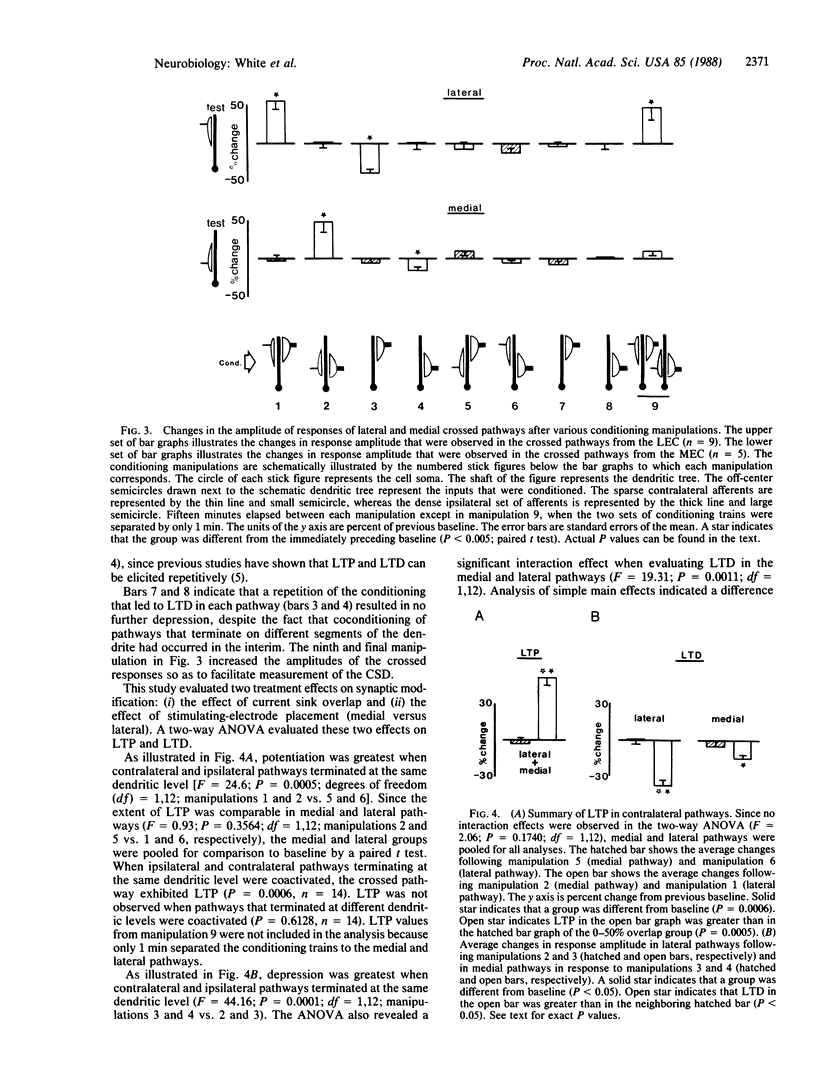
The present study evaluates whether the associative interactions between synapses that lead to long-term potentiation and depression (LTP and LTD) can occur between spatially segregated synapses of the medial and lateral temporodentate pathway of the rat. Coconditioning of crossed and ipsilateral pathways resulted in LTP of the crossed system only when the current sinks of the two conditioned pathways overlapped sufficiently. Likewise, conditioning of an ipsilateral pathway alone resulted in LTD of the crossed pathway only when those current sinks overlapped sufficiently. These observations support the idea that associative events that lead to LTP or LTD can be restricted to a local dendritic domain. The postsynaptic cell can therefore serve as more than one unit of integration for synaptic modification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrionuevo G., Brown T. H. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Lynch G. Hypothesis regarding the cellular mechanisms responsible for long-term synaptic potentiation in the hippocampus. Exp Neurol. 1980 Apr;68(1):202–204. doi: 10.1016/0014-4886(80)90078-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22(2):131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984 Aug 20;309(1):35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. Synaptic correlates of associative potentiation/depression: an ultrastructural study in the hippocampus. Brain Res. 1983 Apr 11;265(1):21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975 Mar 21;86(2):205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Freeman J. A., Nicholson C. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol. 1975 Mar;38(2):369–382. doi: 10.1152/jn.1975.38.2.369. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Hippocampal long-lasting potentiation produced by pairing single volleys and brief conditioning tetani evoked in separate afferents. J Neurosci. 1986 Jun;6(6):1575–1582. doi: 10.1523/JNEUROSCI.06-06-01575.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Schottler F., Oliver M., Lynch G. Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J Neurophysiol. 1980 Aug;44(2):247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Brassel S. E., Moore S. D. Partial quantification of the associative synaptic learning rule of the dentate gyrus. Neuroscience. 1983 Apr;8(4):799–808. doi: 10.1016/0306-4522(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983 Apr;8(4):791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Steward O., Vinsant S. L. Identification of the cells of origin of a central pathway which sprouts following lesions in mature rats. Brain Res. 1978 May 26;147(2):223–243. doi: 10.1016/0006-8993(78)90837-5. [DOI] [PubMed] [Google Scholar]
- Wigström H., McNaughton B. L., Barnes C. A. Long-term synaptic enhancement in hippocampus is not regulated by postsynaptic membrane potential. Brain Res. 1982 Feb 4;233(1):195–199. doi: 10.1016/0006-8993(82)90941-6. [DOI] [PubMed] [Google Scholar]


