Abstract
Previous research has implicated regions of anterior insula/frontal operculum in processing conspecific facial expressions of disgust. It has been suggested however that there are a variety of disgust facial expression components which relate to the disgust-eliciting stimulus. The nose wrinkle is predominantly associated with irritating or offensive smells, the mouth gape and tongue extrusion with distaste and oral irritation, while a broader range of disgust elicitors including aversive interpersonal contacts and certain moral offenses are associated primarily with the upper lip curl. Using functional magnetic resonance imaging, we show that activity in the anterior insula/frontal operculum is seen only in response to canonical disgust faces, exhibiting the nose wrinkle and upper lip curl, and not in response to distaste facial expressions, exhibiting a mouth gape and tongue protrusion. Canonical disgust expressions also result in activity in brain regions linked to social cognition more broadly, including dorsal medial prefrontal cortex, posterior cingulate cortex, temporo-parietal junction and superior temporal sulcus. We interpret these differences in relation to the relative functional and communicative roles of the different disgust expressions and suggest a significant role for appraisal processes in the insula activation to facial expressions of disgust.
Keywords: disgust, fMRI, emotion, insula, distaste
INTRODUCTION
Facial expressions have been at the centre of the study of emotions since Ekman and Friesen (1971) and Izard (1971) noted a strong cross-cultural agreement in the facial signals of certain emotions. In recent years, affective neuroscience research has identified brain systems that play a disproportionate role in coding specific facial expressions of emotion. Disgust is one emotion that has been studied in this context. Functional imaging studies have reported consistent involvement of the insula and adjacent frontal opercula in response to viewing facial expressions of disgust (Phillips et al., 1997; Sprengelmeyer et al., 1998; Anderson et al., 2003; Wicker et al., 2003; Schroeder et al., 2004; Sambataro et al., 2006; Jabbi et al., 2007; Thielscher and Pessoa, 2007; van der Gaag et al., 2007). Similarly, intracerebral recording in the human insula has shown a selective response to expressions of disgust but not other emotions (Krolak-Salmon et al., 2003). Further evidence for the disproportionate role of the insula in disgust processing has come from lesion studies and patients with neurodegenerative disorders. Calder et al. (2000) reported a patient with damage to the left insula and basal ganglia who displayed a selective impairment in the recognition of facial and vocal signals of disgust (see also Adolphs et al., 2003). More recently, a study of asymptomatic Huntington's disease gene-mutation carriers demonstrated a significant positive correlation between insula volume and the recognition of disgust facial expressions (Kipps et al., 2007).
There are three principal facial movements associated with the disgust expression: the mouth gape, with or without tongue extrusion, the nose wrinkle and the upper lip curl (Darwin, 1872/1965; Izard, 1971; Ekman and Friesen, 1975), and Rozin and colleagues (1994) suggest that these map onto eliciting situations in an orderly way, both functionally and in terms of communication (see also Smith and Scott, 1997).
According to Darwin (1872/1965) and Rozin et al. (2000), disgust has its origins in the more basic distaste/oral rejection response that is likely to have evolved as a defensive mechanism against the ingestion of potentially harmful substances. The combination of mouth gape and tongue protrusion constitutes the basic facial distaste reaction to unpleasant tastes or oral irritation intended to expel contents from the mouth, homologues of which are displayed throughout mammalian species (Steiner et al., 2001). In contrast, the nose wrinkle is associated primarily with bad smells, while the upper lip curl is most often seen to communicate the presence of expanded disgust elicitors, such as interpersonal disgust, related to contact with undesirable persons and violations of moral ‘purity’ (Rozin et al., 1994).
To date, research investigating the neural response to disgust facial expressions has used expressions displaying a nose wrinkle and upper lip curl, what we will term ‘canonical disgust’ expressions. This is perhaps largely because the most widely used stimulus set of facial expressions (Ekman and Friesen's 1976 Pictures of Facial Affect series) contains examples of the canonical disgust but not the distaste (mouth gape, tongue extrusion) expression. Given that the distaste expression conveys information about an unpleasant stimulus in the mouth of the expresser (e.g. oral irritation, bad taste) whereas the canonical disgust expression primarily conveys a disgust reaction to a stimulus in the surrounding environment (e.g. bad smell, offensive behaviour), we were interested in determining whether the processing of these disgust expressions which convey different information is also mirrored by distinct neural correlates. We addressed this in the context of a functional magnetic resonance imaging (fMRI) study, by contrasting the neural response to the viewing of facial expressions of distaste and canonical disgust.
A popular account of emotion theory posits that the recognition of emotion from another's facial expression is achieved by simulating the seen emotion in brain regions that subserve one's own experience of the emotion (Gallese et al., 2004). Indeed, previous research has implicated the insula/frontal operculum in both the experience of disgust and processing other agents’ expressions of disgust (Calder et al., 2000; Wicker et al., 2003; Jabbi et al., 2007). Since unpleasant tastes (Zald et al., 2002; Small et al., 2005; Jabbi et al., 2007) and even direct stimulation of the tongue (Barry et al., 2001) produce insula activation, simulation theories predict that, like canonical disgust expressions, distaste expressions should also engage the insula. However, since distaste expressions are less intimately linked to social and moral cognition than canonical disgust expressions which convey information of greater interpersonal significance, we anticipated that distaste would be less likely to engage brain regions linked to social cognitive processes more generally.
METHODS
Participants
Twenty-seven right-handed volunteers (14 females, mean age = 27 ± 8 years) with normal or corrected-to-normal vision and no known neurological or psychiatric history participated in the study for payment. The study was approved by the NHS Local Research Ethics Committee for Cambridge and participants gave their informed and written consent prior to taking part. Participants were paid for taking part in the study.
Stimuli and design
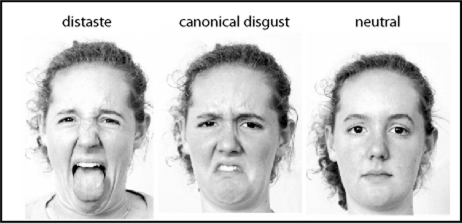
Participants performed a gender discrimination task while viewing pictures of faces in the scanner. Reaction times and accuracy were recorded via button press. Three types of facial expressions were presented: ‘distaste’ (expressed as mouth gape and tongue protrusion plus nose wrinkle), ‘canonical disgust’ (expressed by curling the upper lip and wrinkling the nose), and neutral faces (Figure 1). Blocks of fixation were also included as a baseline. Faces were obtained from the NimStim face stimulus set (Tottenham et al., in press) and supplemented by a selection from our own facial expression database.
Fig. 1.
Distaste, canonical disgust and neutral facial expressions.
All faces were rated on a Likert scale from one to seven by 10 individuals who were not part of the fMRI study. Participants were asked to indicate the degree to which each facial expression was angry, disgusted, sad, pleasant and arousing. A subset of 16 identities with the highest disgust ratings were chosen (half of which were female). Mean ratings for these identities’ facial expressions are shown in Table 1. Wilcoxon's signed rank tests showed that facial expressions of distaste and canonical disgust were rated as being significantly more disgusted than neutral facial expressions (P < 0.001), as well as being significantly more arousing (P < 0.001) and significantly less pleasant than neutral facial expressions (P < 0.001). Critically, distaste and canonical disgust facial expressions did not differ significantly on any of the ratings (all P > 0.1); hence, any differences we observe between the two types of disgust expressions cannot be attributed to differences in the intensity of disgust or related negative emotions, or differences in their arousal and valence. A separate group of 10 participants were also asked to rate the distaste and canonical disgust expressions according to the degree to which each expression was likely to signal a response to an unpleasant taste, an unpleasant smell, or a response to another person's behaviour (all were rated on a Likert scale from one to seven as above, with one being the least likely and seven being the most likely). Distaste facial expressions were rated as significantly more likely to express a response to an unpleasant taste than canonical disgust and neutral expressions (P < 0.001). Conversely, canonical disgust expressions were rated as significantly more likely than distaste or neutral expressions to express a response to a bad smell or to another person's behaviour (P < 0.001) (see Table 1 for list of ratings).
Table 1.
Mean ratings of facial expressions
| Facial expressions | Anger | Disgust | Sadness | Arousal | Pleasantness | Taste | Smell | Behaviour |
|---|---|---|---|---|---|---|---|---|
| Canonical Disgust | 3.18 ± 0.44 | 5.20 ± 0.48 | 2.85 ± 0.76 | 4.94 ± 0.41 | 2.34 ± 0.44 | 4.38 ± 0.54 | 5.90 ± 0.46 | 4.41 ± 0.55 |
| Distaste | 3.13 ± 0.43 | 5.47 ± 0.53 | 2.55 ± 0.77 | 5.22 ± 0.69 | 2.13 ± 0.31 | 5.58 ± 0.54 | 3.98 ± 0.46 | 3.43 ± 0.49 |
| Neutral | 1.59 ± 0.43 | 1.22 ± 0.19 | 1.88 ± 0.76 | 4.04 ± 0.43 | 4.69 ± 0.43 | 1.03 ± 0.05 | 1.08 ± 0.11 | 1.72 ± 0.51 |
Each facial expression category comprised 16 images (half female). The same identities were used in all categories. Stimuli were presented in blocks of 17.6 s. Each block comprised four images from the same category (distaste, canonical disgust, neutral) with each image presented for 4000 ms followed by a 400 ms interstimulus interval. Eight blocks of each type were presented in one of two pseudorandom orders. Eight blocks of fixation were also included resulting in a total acquisition time of ∼9 min.
Imaging and statistical analysis
Participants underwent whole-brain T2*-weighted echo-planar imaging (EPI) scanning on a 3T Medspec scanner (Bruker, Ettlingen, Germany) with a head coil gradient set. Twenty-one interleaved 4-mm thick slices were acquired in the transverse oblique plane, angled to avoid the eyeballs (1 mm interslice gap; TR = 1100 ms; TE = 30 ms; 65° flip angle; 24 × 24 cm FOV; 64 × 64 matrix; 144-kHz voxel bandwidth). The first 14 volumes were dummy scans and were discarded to allow for equilibration effects.
Data were analysed using SPM2 software (Wellcome Trust Centre for NeuroImaging, London, UK). EPI images were corrected for slice timing and head movement, and undistorted based on field strength derived from a phase map (Cusack et al., 2003). Three participants had sudden head movements of greater than 2 mm between successive acquisition volumes and these volumes were removed from subsequent analyses. EPI and structural images were co-registered and normalised to a standard template (SPM2's MNI 152avg 2 × 2 × 2 mm template) and smoothed with a Gaussian kernel of 10 mm full-width half-maximum. Condition effects were estimated using boxcar regressors convolved with a canonical hemodynamic response function in a general linear model. Spatial realignment parameters were included as regressors in the model to account for any residual movement-related variance. Data were high-pass filtered at 128 s to remove low-frequency signal drift, and low-pass filtered with the canonical hemodynamic response function. Statistical parametric maps were generated for each individual by estimating activation contrasts between conditions (i.e. canonical disgust vs neutral, canonical disgust vs distaste and distaste vs neutral). Data were analysed at the group level by conducting a random-effects analysis and thresholded at P < 0.001, uncorrected for multiple comparisons, in the first instance. Activations surviving False Discovery Rate (FDR) (Genovese et al., 2002) correction for multiple comparisons are also indicated. A minimum cluster extent threshold of 10 voxels was used.
Based on previous functional imaging studies of disgust processing, an a priori region-of-interest (ROI) was defined as the anterior insula in both hemispheres. For these ROIs, a small-volume-correction (SVC) was applied using a sphere of 15 mm diameter, centred on activation cluster maxima determined by Phillips et al.'s (1997) functional imaging study of disgust faces. The Phillips et al. (1997) Talairach coordinates were converted into MNI coordinate space using the tal2mni conversion tool (M. Brett, http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). All coordinates here are listed in MNI coordinate space.
RESULTS
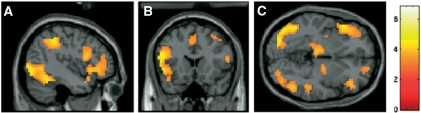
For the contrast canonical disgust faces compared with neutral faces, increased activation was found in the a priori left and right anterior insula ROIs. This activation was part of a larger cluster of activation extending into the frontal operculum in the left hemisphere. In addition, there was significantly increased activation in dorsal medial prefrontal cortex (mPFC), right superior temporal sulcus (STS), left and right intraparietal sulcus (IPS), posterior cingulate cortex (PCC), visual cortex, thalamus, globus pallidus and fusiform gyrus, all P < 0.05 FDR (Figure 2 and Table 2). Canonical disgust facial expressions therefore engaged a network of brain regions implicated in facial expression perception more generally (Haxby et al., 2000) and in mental state attribution or theory-of-mind (ToM) (Frith and Frith, 1999; Saxe, 2006).
Fig. 2.
Regions of significant activation for the contrast canonical disgust vs neutral facial expressions at x = –42 (A), y = 18 (B) and z = –3 (C). Activations are thresholded at P < 0.003, uncorrected, for visualisation.
Table 2.
Brain regions exhibiting significantly increased activation to disgust facial expressions.
| Brain region | Hemisphere | MNI coordinates |
Size | Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Canonical Disgust > Neutral | ||||||
| Occipital gyrus | L | −21 | −93 | 9 | 871 | 5.13 |
| Occipital gyrus | R | 30 | −87 | 9 | 372 | 4.99 |
| STS | R | 54 | −33 | 3 | − | 3.60 |
| Posterior cingulate cortex | L | −6 | −39 | 21 | 59 | 4.30 |
| Fusiform gyrus | R | 24 | −48 | −27 | 102 | 4.01 |
| Intraparietal sulcus | L | −45 | −39 | 45 | 317 | 3.99 |
| Intraparietal sulcus | R | 39 | −30 | 45 | 137 | 3.95 |
| Frontal Operculum | L | −57 | 15 | 9 | 345 | 3.98 |
| Insula | L | −45 | 15 | 21 | 48 | 3.72* |
| Frontal Operculum | R | 42 | 33 | −3 | 10 | 3.35* |
| Thalamus | L | −15 | −27 | 9 | 68 | 3.55 |
| Thalamus | R | 15 | −21 | 12 | 72 | 3.64 |
| Globus pallidus | R | 21 | −12 | 0 | − | 3.26 |
| Posterior midcingulate cortex | L | −6 | −24 | 51 | 19 | 3.50 |
| mPFC | R | 6 | 27 | 36 | 33 | 3.38 |
| Anterior orbitofrontal cortex | R | 33 | 54 | −12 | 18 | 3.31 |
| Distaste > Neutral | ||||||
| Occipital gyrus | L | −21 | −93 | 9 | 219 | 5.02 |
| Occipital gyrus | R | 30 | −84 | 12 | 107 | 4.97 |
| Occipitotemporal jcn | R | 51 | −72 | −6 | 41 | 3.50† |
| Fusiform gyrus | L | −24 | −69 | −12 | 22 | 3.42† |
| Canonical Disgust > Distaste | ||||||
| Frontal operculum | R | 48 | 36 | −6 | 64 | 4.43 |
| Insula | R | 42 | 33 | −3 | 18 | 3.74* |
| STS | L | −54 | −9 | −12 | 149 | 3.90 |
| STS | R | 57 | −24 | −3 | 341 | 4.28 |
| Thalamus | L | −21 | −27 | 15 | 44 | 4.06 |
| Thalamus | R | 18 | −24 | 12 | 384 | 3.94 |
| Caudate/putamen/globus pallidus | R | 21 | 15 | 6 | 3.62 | |
| mPFC | L | −3 | 45 | 30 | 230 | 4.01 |
| Inferior frontal gyrus | R | 57 | 21 | 21 | 60 | 3.86 |
| Temporo-parietal junction | L | −48 | −45 | 24 | 83 | 3.80 |
| Middle frontal gyrus | L | −33 | 57 | −3 | 22 | 3.56 |
| Visual cortex | L | −15 | −81 | 18 | 16 | 3.53 |
| Putamen/caudate | L | −18 | 9 | 12 | 40 | 3.52 |
| Posterior cingulate cortex | L | −9 | −15 | 39 | 27 | 3.52 |
| Orbitofrontal cortex | R | 15 | 60 | −9 | 34 | 3.46 |
*P < 0.05 SVC; †P < 0.001 uncorrected; all other clusters are P < 0.05 FDR corrected.
In contrast, the group-level one-sample t-test for the contrast distaste vs neutral faces revealed very little significant response and there was no activation within the insula even at reduced thresholds (P < 0.01, uncorrected). In fact, clusters of increased activation for viewing of distaste faces were found only in the occipital lobes in left and right visual cortex (P < 0.05 FDR correction). At a lower threshold of P < 0.001 uncorrected, left fusiform gyrus and right occipito-temporal junction were also activated. A full list of cluster coordinates and Z scores is given in Table 2.
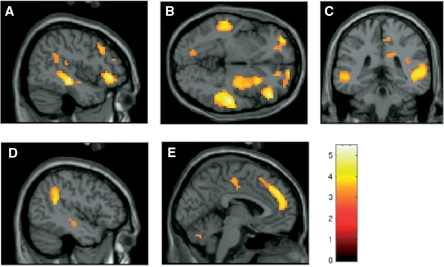
An analysis of the response to canonical disgust facial expressions directly compared with distaste facial expressions again revealed significant activation in the a priori right insula ROI. This activation formed a sub-cluster within a larger cluster extending into frontal operculum. Increased activity was also found bilaterally in the posterior STS, dorsal mPFC, PCC and temporo-parietal junction (TPJ) (Figure 3). The activation of these regions for the contrast canonical disgust compared with distaste facial expressions is consistent with the proposal that a key difference between these two forms of disgust expression is the greater interpersonal nature of the canonical disgust expressions. A full list of clusters and their significance levels for the contrast canonical disgust compared with distaste expressions is listed in Table 2.
Fig. 3.
Regions of significant activation for the contrast canonical disgust facial expressions vs distaste facial expressions in frontal operculum/insula and STS at x = 48 (A), z = –6 (B), and y = –24 (C), in TPJ at x = –48 (D) and mPFC at x = –3 (E). Activations are thresholded at P < 0.003, uncorrected, for visualization.
No significant activation was found when the viewing of distaste facial expressions was contrasted with canonical disgust even at a reduced threshold (P < 0.01, uncorrected). In addition, all results remained significant after factoring out sex differences by including gender as a nuisance covariate.
DISCUSSION
We present the first comparison of the neural mechanisms involved in processing facial expressions of canonical disgust, comprising a nose wrinkle and upper lip curl, and the more basic distaste face, consisting of mouth gape, tongue protrusion and nose wrinkle. Relative to neutral or distaste expressions, canonical disgust facial expressions engaged the anterior insula and frontal operculum, together with a network of key regions involved in mental state attribution—the mPFC, PCC, TPJ and STS. In contrast, activation to distaste relative to neutral expressions was restricted to striate and extrastriate regions, and a comparison of distaste and canonical disgust produced no significant activation, even at reduced statistical threshold (P < 0.01, uncorrected). Thus, the insula's involvement in processing static facial expressions of disgust may be particular to canonical disgust expressions involving the nose wrinkle and upper lip curl.
The involvement of insula and frontal operculum in disgust processing has been well documented in previous research. Functional MRI studies have shown that these regions are activated when viewing facial expressions of disgust, pictures of disgusting food or exposure to disgusting tastes and smells (Kinomura et al., 1994; Phillips et al., 1997; Anderson et al., 2003; Heining et al., 2003; Wicker et al., 2003; Sambataro et al., 2006; Schroeder et al., 2004; Calder et al., 2007; Jabbi et al.; 2007; Thielscher and Pessoa, 2007). Given this region's involvement in coding disgusting tastes, and indeed taste and smell more generally (Small et al., 2005), the absence of activation to expressions of distaste is perhaps surprising. However this result is not as unexpected as it first appears and may be explained by the different functional and communicative roles of canonical disgust and distaste expressions.
Besides their different physical properties, canonical disgust and distaste facial expressions differ in the situations in which they are elicited and the information they convey (Rozin et al., 1994). Rozin et al. (1994) found that viewers take different meanings from different components of the disgust expression. Gape and tongue extrusion, sometimes together with nose wrinkle, communicate both oral irritation and distaste. Since distaste expressions inform the observer about an unpleasant taste in the mouth of the sender, the information conveyed relates to a stimulus internal to the expresser which is less likely to directly affect the observer, suggesting that distaste has a greater functional than communicative value (i.e. to expel food).
In contrast, expressions of canonical disgust consisting of a nose wrinkle and upper lip curl serve a greater communicative role by informing the observer that the expresser finds an external stimulus or the observer himself aversive. While the nose wrinkle, a prominent feature of the canonical disgust expression, is most often associated with bad smell, the precise functional value of the upper lip curl is less clear, and the type of offense or threat that it indicates is much less well defined. However, Rozin et al. (1994) suggest that the upper lip curl predominantly expresses disgust in its expanded form, in particular communicating the presence of elicitors that fit under elaborated disgust, e.g. interpersonal contamination, and moral offense. Thus, Rozin et al. (1994) suggest that because the upper lip curl indicates an offense that is likely to be external to the body, it may recruit more action from the observer (because the observer is likely to encounter the elicitor) than would a mouth gape, which informs about something in the mouth of the sender (see also Miller, 1997).
The facial expression ratings we collected further support the distinction between distaste and canonical disgust expressions since distaste expressions were rated as significantly more likely to express a reaction to a bad taste (internal stimulus), whereas canonical disgust expressions were rated as significantly more likely to reflect the response to a bad smell or another person's behaviour (external stimuli; Table 1). Another key difference in communicative value of distaste expressions and expressions of canonical disgust is that canonical disgust expressions may be directed at another person, whereas a gape and tongue protrusion in the context of the distaste expression is unlikely to be directed at another person. However given the nature of our experiment, it is impossible to disambiguate whether the differential brain activation we observed in response to canonical disgust and distaste expressions is primarily due to differences in the location of the stimulus in the environment (internal vs external) or because canonical disgust expressions may be interpreted as being directed at the observer (‘you disgust me’).
Taken together these differences suggest that an important distinction between distaste and canonical disgust expressions is the relevance to the observer. The presence of insula/frontal opercula activation in response to canonical disgust expressions and the absence of its activation in response to distaste expressions, even at reduced statistical thresholds, may therefore have implications for simulation theories of emotion. Such accounts propose that recognition is achieved by simulating the seen emotional expression in circuitry that subserves one's own experience of the emotion (Gallese et al., 2004). A simulation theory of emotion understanding would predict insula activation to both forms of disgust expression used here. However, our results instead suggest that the insula response may be determined, at least in part, by the personal and interpersonal relevance or significance of the disgust expression for the observer. Given that this implies a significant role for appraisal, it is of interest that similar observations have been made regarding the amygdala's response to emotional stimuli (Sander et al., 2003, 2005; Ewbank et al., 2009).
While we have emphasised the insula's role in response to facial expressions of disgust, the anterior insula/frontal operculum has not only been implicated in disgust processing, but has been accorded a more general role in interoceptive processing and awareness of the self (Craig, 2002). While such processes necessarily carry a high personal relevance (e.g. awareness of one's own heartbeat, or experiencing pain), it is interesting to note that viewing another's pain also engages the insula and is strongly influenced by the interpersonal relationship between observer and expresser (Singer et al., 2004, 2006). Specifically, Singer et al (2006) showed that the insula response to observing another person in pain was modulated by the observer's rating of the person's likeability, consistent with the idea that personal emotional significance or relevance plays an important role in insula activation. Strikingly, individuals with congenital insensitivity to pain show a normal insula response to the sight of others in pain (Danziger et al., 2009), again suggesting that emotion simulation cannot fully explain insula activation.
It is important to discuss the lack of insula activation in response to distaste expressions in light of a previous study by Jabbi et al. (2007) that showed apparently contradictory results. Jabbi et al. (2007) found activation in anterior insula/frontal operculum in individuals watching video clips of actors sipping from a glass and then expressing their pleasure or disgust. Their results appear contradictory to our lack of insula activation to viewing of distaste facial expressions; however, the examples of disgust expressions they used did not consist of mouth gape and tongue protrusion characteristic of the distaste response, and instead comprised nose wrinkle and upper lip curl, meaning that they are more similar to our canonical disgust expressions. Hence, their insula activation may have resulted as a response to the canonical disgust expressions. Further, activity in insula/frontal operculum to the sight of another's disgust was strongly correlated with both the personal distress (e.g. ‘being in a tense emotional situation scares me’) and fantasy (e.g. ‘I get really involved with the feelings of the characters in a novel’) scales of the Davis Interpersonal Reactivity Index (Davis, 1980), consistent with our suggestion that relevance to the observer is a key modulator of insular activity to disgust.
In addition to anterior insula/frontal operculum, a large number of other brain regions implicated in mental state attribution were activated while viewing expressions of canonical disgust, but not distaste. Contrasts comparing canonical disgust with neutral faces or distaste expressions showed a significant increase in activation in the dorsal mPFC, TPJ, PCC and posterior STS. These regions have been implicated in moral cognition (Moll et al., 2008) and inferring the mental states of others (Frith and Frith, 1999; Saxe, 2006), and can be activated in the absence of explicit mentalising tasks when the observed behaviour of others is highly relevant to one's own intentions and actions (Kampe et al., 2003; Schulte-Ruther et al., 2007). Consistent with our findings, Benuzzi et al. (2008) found activation in these regions during viewing of videos consisting of human contact with disgusting animals (i.e. canonical, non-food disgust elicitors) when participants had to imagine how disgusting the contact would be. Similarly, Zaki et al. (2007) found that anterior insula was functionally connected with the mentalising network while watching others in pain (see also Lamm et al., 2007); and Olsson and Phelps (2007) argue that dorsal mPFC activity, together with emotion-related activity, is involved in social learning via facial expression (which can occur for canonical disgust faces with the upper lip curl (Baeyens et al., 1996)).
These findings appear consistent with the work of Rozin et al. (1994) who found that the upper lip curl was associated with disgust elicited in the context of interpersonal contamination and moral offense—to this extent, activations in dorsal mPFC, TPJ, PCC and STS may reflect ‘on-line’ mentalising related to the associations of the canonical disgust face. In addition, being the target of someone else's disgust has great interpersonal significance for the viewer, and the self-referential decoding of emotional expressions is known to activate these brain regions (Schulte-Ruther et al., 2007).
In conclusion, we have shown that differences in disgust expressions in humans are mirrored by differences in the underlying neural substrates involved in processing these facial expressions, consistent with their different functional and communicative roles. Facial expressions of canonical disgust engaged a wide range of brain regions, including the insula/frontal opercula and the classic social cognitive network—medial prefrontal cortex, temporo-parietal junction, posterior cingulate and posterior STS. In contrast, the distaste expression did not engage these regions. We propose that our findings can be explained by the difference in relevance or significance of these two expressions for the observer. While canonical disgust facial expressions contain a strong affective social and interpersonal component, and are likely to reflect the expresser's attitude towards the observer or to an external stimulus which has a higher relevance to the observer, signals of distaste are less relevant in an interpersonal context and relate to a stimulus internal to the expresser. The relative importance of significance appraisal or relevance-to-self in the activation of insular cortex has important implications for theories of emotion expression processing and is a key topic for future investigations.
Acknowledgments
We would like to thank the radiographers at the Wolfson Brain Imaging Centre (WBIC) for their help in data acquisition. Development of the MacBrain Face Stimulus Set (NimStim) was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. This work was supported by the Medical Research Council (MRC) under project code U.1055.02.001.0001.01 (to A.J.C.).
REFERENCES
- Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain and Cognition. 2003;52:61–9. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23:5627–33. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens F, Vansteenwegen D, De Houwer J, Crombez G. Observational conditioning of food valence in humans. Appetite. 1996;27:235–50. doi: 10.1006/appe.1996.0049. [DOI] [PubMed] [Google Scholar]
- Barry MA, Gatenby JC, Zeiger JD, Gore JC. Hemispheric dominance of cortical activity evoked by focal electrogustatory stimuli. Chemical Senses. 2001;26:471–82. doi: 10.1093/chemse/26.5.471. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. Journal of Neuroscience. 2008;28:923–31. doi: 10.1523/JNEUROSCI.4012-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. European Journal of Neuroscience. 2007;25:3422–8. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cusack R, Brett M, Osswald K. An evaluation of the use of magnetic field maps to undistort echo-planar images. Neuroimage. 2003;18:127–42. doi: 10.1006/nimg.2002.1281. [DOI] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–12. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The Expression of the Emotions in Man and Animals. Chicago: University of Chicago Press; 1872/1965. [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17:124–9. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Unmasking the Face. Englewood Cliff, NJ: Prentice Hall; 1975. [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heining M, Young AW, Ioannou G, et al. Disgusting smells activate human anterior insula and ventral striatum. Annals of the New York Academy of Sciences. 2003;1000:380–4. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Izard CE. The Face of Emotion. New York: Appleton-century-Crofts; 1971. [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Frith U. “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. Journal of Neuroscience. 2003;23:5258–63. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Kawashima R, Yamada K, et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Research. 1994;659:263–6. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. Journal of Cognitive Neuroscience. 2007;19:1206–17. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Isnard J, et al. An attention modulated response to disgust in human ventral anterior insula. Annals of Neurology. 2003;53:446–53. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Miller WI. The Anatomy of Disgust. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- Moll J, de Oliveira-Souza R, Zahn R. The neural basis of moral cognition: sentiments, concepts, and values. Ann N Y Acad Sci 2008. 2008;1124:161–80. doi: 10.1196/annals.1440.005. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience. 2007;10:1095–102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley CR. Disgust. New York: Guildford Press; 2000. pp. 637–53. [Google Scholar]
- Rozin P, Lowery L, Ebert R. Varieties of disgust faces and the structure of disgust. Journal of Personality and Social Psychology. 1994;66:870–81. doi: 10.1037//0022-3514.66.5.870. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Dimalta S, Di Giorgio A, et al. Preferential responses in amygdala and insula during presentation of facial contempt and disgust. European Journal of Neuroscience. 2006;24:2355–62. doi: 10.1111/j.1460-9568.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Networks. 2005;18:317–52. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Hennenlotter A, Erhard P, et al. Functional neuroanatomy of perceiving surprised faces. Human Brain Mapping. 2004;23:181–7. doi: 10.1002/hbm.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19:1354–72. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Smith CA, Scott HS. The Psychology of Facial Expression. New York: Cambridge University Press; 1997. [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society B-Biological Sciences. 1998;265:1927–31. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neuroscience and Biobehavioral Reviews. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. Journal of Neuroscience. 2007;27:2908–17. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Social Neuroscience. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanellin J, Wager TD, Mackey SC. Different circuits for different pain: Patterns of functional connectivity reveal distinct networks for processing pain in self and others. Social Neuroscience. 2007;2:276–91. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. Journal of Neurophysiology. 2002;87:1068–75. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]