Abstract
Serotonin (5-HT) modulates emotional and cognitive functions such as fear conditioning (FC) and decision making. This study investigated the effects of a functional polymorphism in the regulatory region (5-HTTLPR) of the human 5-HT transporter (5-HTT) gene on observational FC, risk taking and susceptibility to framing in decision making under uncertainty, as well as multidimensional anxiety and autonomic control of the heart in healthy volunteers. The present results indicate that in comparison to the homozygotes for the long (l) version of 5-HTTLPR, the carriers of the short (s) version display enhanced observational FC, reduced financial risk taking and increased susceptibility to framing in economic decision making. We also found that s-carriers have increased trait anxiety due to threat in social evaluation, and ambiguous threat perception. In addition, s-carriers also show reduced autonomic control over the heart, and a pattern of reduced vagal tone and increased sympathetic activity in comparison to l-homozygotes. This is the first genetic study that identifies the association of a functional polymorphism in a key neurotransmitter-related gene with complex social–emotional and cognitive processes. The present set of results suggests an endophenotype of anxiety disorders, characterized by enhanced social learning of fear, impaired decision making and dysfunctional autonomic activity.
Keywords: serotonin transporter, genetic association study, fear conditioning, decision making
INTRODUCTION
Studies in developing and adult humans have offered ample evidence that emotional learning and decision making are interconnected and make equally important contributions to our social functioning (Rushworth et al., 2007; Frith and Singer, 2008). For instance, children can rapidly learn emotions by observing the facial expression of their mother (Gerull and Rapee, 2002), and their emotional development is all the more important as it contributes to the elaboration of prosocial fairness, altruism and strategic tolerance to uncertainty and risk taking in decision making (Mischel et al., 1989; Fehr et al., 2008; Heilman et al., 2009). In adults, emotional learning continues to inform decision making under uncertainty and risk, both in individual and social settings (Bechara et al., 1997; Xiao and Houser, 2005; van Dijk et al., 2008). Research in neuroeconomics and social cognitive and affective neuroscience has started to identify the computational and neurobiological mechanisms of the interactions between emotions, decision making and social information processing (Frith and Frith, 2007; Ochsner, 2008; Olsson and Ochsner, 2008; Seymour and Dolan, 2008).
Serotonin (5-hydroxytryptamine, 5-HT) has been shown to play a central role in the neurobiology of emotional learning, decision making and social behavior. Indeed, if one were to look for a neurotransmitter crucial for something as complex as social–emotional and cognitive functions, one would probably focus on 5-HT. 5-HT is evolutionary ancient, its signaling is carried out by a family of functionally differentiated and flexible receptors, its projections are widespread in regions of the forebrain that are involved in sensory processing, motivation and memory, and it plays an important role in neural development (Insel and Winslow, 1998). Dysregulations of 5-HT modulatory effects on emotion, decision making and social behavior have been implicated in anxiety disorders (Stein and Stein, 2008; Miu and Visu-Petra, 2009).
In humans, the acute administration of selective 5-HT reuptake inhibitors (SSRIs) or 5-HT precursor tryptophan, both resulting in increased 5-HT availability in the brain, enhance the recognition of fearful faces (Attenburrow et al., 2003; Browning et al., 2007). In contrast, the dietary tryptophan depletion impairs recognition of fearful faces (Harmer et al., 2003). Other studies have indicated the impact of 5-HT manipulations on fear conditioning (FC), a form of associative emotional learning that has been extensively studied in animals and humans (LeDoux, 2000; Phelps, 2006). The acute administration of SSRI citalopram before or after the learning phase of FC enhances the acquisition and expression of conditioned fear in rats (Burghardt et al., 2004; Burghardt et al., 2007;). The effects of 5-HT on emotion also extend to social behavior. Chronic SSRI paroxetine administration reduces negative affect in healthy volunteers, and this is related to decreases in hostility and increases in social cooperation (Knutson et al., 1998). In social anxiety disorder, SSRIs reduce perceived fear, avoidance and physiological arousal (Connor et al., 2006).
The link between 5-HT and decision making has only recently started to be investigated. Tryptophan depletion impairs financial decision making by the reduced discrimination of the gains or losses associated with options (Rogers et al., 2003; Blair et al., 2008). A new study used a social bargaining game (i.e. Ultimatum Game) to show that the reduced 5-HT availability in the brain increases the rejection of unfair financial offers (i.e. proposers keep 80% of a gain and offer 20% with the responder) (Crockett et al., 2008). Therefore, 5-HT availability in the brain seems important for the regulation of reactions to unfairness in this social–economic game (Emanuele et al., 2008).
In recent years, one of the most prolific approaches in this line has focused on the associations between genetic polymorphisms in 5-HT-related genes and various social–emotional and cognitive phenotypes. For instance, many studies have investigated 5-HT transporter (5-HTT) that controls the reuptake of 5-HT from the synaptic cleft. In humans, a single gene (i.e. SLC6A4) encodes 5-HTT, and has at least two polymorphic regions. The polymorphism in the regulatory region of SLC6A4—i.e. 5-HTT-linked polymorphic region (5-HTTLPR)—modulates the transcriptional activity of 5-HTT, in such a way that the short (s) version is associated with reduced expression and function of 5-HTT compared to the long (l) version. A growing literature has linked 5-HTTLPR with dispositional anxiety, reduced prefrontal control over amygdala activation to fearful faces and response to SSRIs (Hariri et al., 2006; Canli and Lesch, 2007). A longitudinal study has also associated 5-HTTLPR with coping strategies in stress (Wilhelm et al., 2007). Recent studies have started to unravel the effects of 5-HTTLPR on fear-potentiated startle (Lonsdorf et al., 2009), as well as decision making in healthy volunteers and patients with personality disorders (Maurex et al., 2009; Kuhnen and Chiao, 2009). A twin study reported significant heritability of several aspects of social behavior and called for genetic association studies (Jackson, 2009; Fowler et al., 2009). Indeed, 5-HTTLPR influences impulsivity and a polymorphism in 5-HT2A receptor is also associated with the degree of popularity in sociometric ranking tasks (Burt, 2008; Paaver et al., 2008). This is clearly a rapidly developing line of work to which we aim to contribute in this paper by investigating for the first time the influence of 5-HTTLPR on social learning of fear and economic decision making under uncertainty and risk.
Therefore, this study tested the effects of 5-HTTLPR alleles on observational FC, risk taking assessed by self-report and behavioral measures and susceptibility to framing in economic decision making. In addition, the effects of this genotype on the various dimensions of anxiety and the autonomic control of the heart were also investigated. Our hypotheses were that in comparison to l-homozygotes, s-carriers would show enhanced observational FC, general risk aversiveness and increased susceptibility to framing (i.e. reduced rationality) in decision making. We also expected increased trait anxiety (TA), as well as reduced heart rate (HR) variability (HRV) and vagal tone in s-carriers compared to l-homozygotes.
METHODS
Participants
Healthy volunteers (N = 36) were recruited from the Babeş-Bolyai University campus. After completing a questionnaire on their health and sociodemographic status, four were excluded because of chronic medical conditions or current medication. The remaining 32 participants (23 women, mean age ± s.d.: 26.75 ± 6.69 years) had no history of neuropsychiatric or chronic somatic conditions, they were medication free and were instructed to refrain from caffeine, alcohol, smoking and intense effort at least four hours before the experiments. The questionnaires, cognitive tasks and electrophysiological recordings (i.e. HRV) took place in different sessions. The participants signed an informed consent before they enrolled in the study, and all the experimental procedures complied with the Declaration of Helsinki regarding the use of human participants to biomedical research.
Measures
Genotyping
DNA was extracted from leukocytes (EDTA-anticoagulated blood) using Genomic DNA Extraction Kit (Promega) and kept at –20°C. The promoter region (5-HTTLPR) polymorphism was typed using the primers with reported sequences (Gelernter et al., 1997; Melke et al., 2001; Bozina et al., 2006;): forward: 5'-ATG-CCA-GCA-CCT-AAC-CCC-TAA-TGT-3'; reverse: 5'-GGA-CCG-CAA-GGT-GGG-CGG-GA-3'. These primers were used to generate 5-HTTLPR allele-specific fragments—419 base pair (bp) and 375 bp—by polymerase chain reaction (PCR). Alleles of this polymorphism were designated according to their relative size, depending on the insertion or deletion of 44 bp: long l (16 repeats) and short s (14 repeats), respectively.
PCR assay conditions were optimized as follows: each 50 μl reaction volume included 50 ng genomic template, 5 μl buffer with (NH4)2SO4 (Fermentas), dNTPs (200 μmol/L), MgCl2 (1.5 mmol/l), primers (400 nmol/l) and 0.5 μl (2.5 U) Taq DNA Polymerase (Fermentas). Cycling conditions were: 94°C for 2 min, followed by 35 cycles at 94°C for 1 min, 66°C for 2 min, 72°C for 2 min, with the final elongation of 4 min at 72°C. PCR products were separated on a 2% agarose gel in a 1xTAE running buffer, stained with ethidium bromide and visualized by transillumination for size estimation. A 50 bp marker was used to measure the PCR product size for l and s alleles. After genotyping, the compositions of the groups were as follows: nine s-homozygotes (seven women), nine s/l heterozygotes (seven women), and 14 l-homozygotes (nine women). There were no significant sex differences in genotype frequencies, and the genotype frequencies for women (χ2 = 3.44, non-significant) and men (χ2 = 1.16, non-significant) were in Hardy–Weinberg equilibrium. Carriers of one or two copies of the s allele were combined to form the s-allele carrier group that was compared with l-homozygotes.
Self-report measures
All the participants completed several anxiety questionnaires. Endler Multidimensional Anxiety Scales (EMAS) is a self-report instrument including three different scales that assess different types of anxiety (Table 1) (Endler et al., 1991; Miclea et al., 2009). While EMAS measures multidimensional anxiety, State-Trait Anxiety Inventory (STAI) measures global anxiety (Spielberger, 1983; Pitariu and Peleasa, 2007). We used the trait version of STAI (STAI-T) because of its known correlations with psychometric measures of anxiety that have been related to 5-HTTLPR in previous studies (e.g. Cloninger's Temperament and Character Inventory; Lesch et al., 1996). The third questionnaire that the participants had to complete was the revised version of the Domain-Specific Risk-Taking (DOSPERT) (Blais and Weber, 2006). DOSPERT measures conventional risk attitudes (i.e. the reported level of risk taking) and perceived risk attitudes (i.e. the willingness to engage in a risky activity as a function of its perceived riskiness) in five decision making domains: ethical (e.g. items such as passing off somebody else's work as your own), financial (e.g. betting a day's income at the horse races), health/safety (e.g. engaging in unprotected sex), social (e.g. speaking your mind about an unpopular issue) and recreational (e.g. bungee jumping of a tall bridge). EMAS, STAI-T and DOSPERT were administered in independent sessions and balanced between groups.
Table 1.
Self-report anxiety scores on Endler Multidimensional Anxiety Scales (EMAS) and the trait portion of Spielberger's State-Trait Anxiety Inventory (STAI-T)
| Genotype | Measure |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMAS-S-CW | EMAS-S-AE | EMAS-T-SE | EMAS-T-PD | EMAS-T-AM | EMAS-T-DR | EMAS-P-1 | EMAS-P-2 | EMAS-P-3 | EMAS-P-4 | EMAS-P-5 | STAI-T | |
| s/s or s/l | 13.42 ± 4.65 | 14.35 ± 3.27 | 39.07 ± 2.74* | 38.92 ± 4.47 | 41 ± 3.94 | 38.35 ± 3.12 | 3.46 ± 1.05 | 1.46 ± 0.87 | 3.15 ± 0.68* | 3.23 ± 1.16 | 1.35 ± 0.63 | 36.88 ± 11.29 |
| l/l | 13.33 ± 4.58 | 14 ± 4.3 | 35.88 ± 4.64* | 36.55 ± 5.08 | 39.11 ± 5.39 | 35.66 ± 6.16 | 2.88 ± 1.16 | 1.33 ± 1 | 2.55 ± 0.88* | 2.55 ± 1.13 | 1.22 ± 0.66 | 35.28 ± 7.64 |
S, State; T, Trait: P, Perception; CW, cognitive worry; AE, autonomic-emotional; SE, anxiety due to threat in social evaluation or interpersonal situations; PD, anxiety due to threat of physical danger; AM, ambiguous threat anxiety; DR, anxiety while engaged in innocuous activities or daily routines; P-1, social evaluation threat perception; P-2, physical danger threat perception; P-3, ambiguous threat perception; P-4, innocuous or daily routines threat perception; P-5, the degree to which the individual feels threatened in the current situation.
P ≤ 0.05 on t-tests.
Observational FC
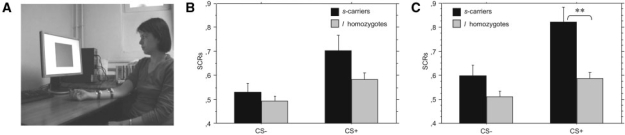
The stimuli and procedure were similar to the ones used by Olsson et al. (Olsson et al., 2007). The movie (3 min, 48 s) displayed a participant (i.e. the learning model who was not familiar to the participants) taking part in a conditioning experiment (Figure 1A). Conditioned stimuli (CSs) were two colored squares (i.e., blue or yellow), presented on a computer screen in front of the learning model. CSs were presented in pseudorandomized order, each for 10 s, with an interstimulus interval ranging between 10 and 14 s, during which the word ‘rest’ was displayed. Each CS was presented five times, out of which one of them (CS+) coterminated with the administration of an uncomfortable shock—unconditioned stimulus (US)—in three of its five presentations. The other CS (CS−) was never associated with US. Each participant first viewed the movie (i.e. the observation stage), after being told that s/he should pay attention to the movie because s/he will be tested in an identical condition; after a break, the participant was actually presented (i.e. the test stage) with CS+ and CS−. However, no USs were presented during the test stage to ensure that learning occurred through indirect, social means. Skin conductance was continuously recorded during the observation and test stages, and skin conductance responses (SCRs) were determined from the 0.5–4.5 s latency window following stimulus onset. At the end of the experiment, the participants were debriefed and asked whether they believed the instructions.
Fig. 1.
A snapshot from the movie presenting the learning model facing a computer screen on which CS+ and CS− were displayed; the electrodes are attached to the right wrist of the model; she displayed signs of distress on each of the three presentations of the CS+ that were associated with shock (A). Mean SCRs of s-carriers and l-homozygotes during the observation (B) and test stages (C) of the observational FC task. **P ≤ 0.01.
Behavioral risk taking
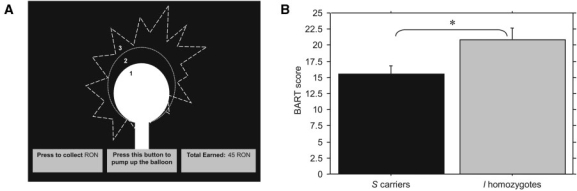
We used a computerized version of the Balloon Analogue Risk Task (BART) (Lejuez et al., 2002). BART is a measure of risk taking, in which participants can earn financial rewards by pumping balloons presented on a screen (Figure 2A); different balloons have variable explosion points, and once a balloon explodes, the money deposited for pumping that balloon is lost. Risk taking is defined in terms of mean pumps per unexploded balloon.
Fig. 2.
Diagram of the Balloon Analogue Risk Taks (A). Mean number of pumps per unexploded balloon in Balloon Risk Analogue Task (BART) (B). *P ≤ 0.05.
Framing bias
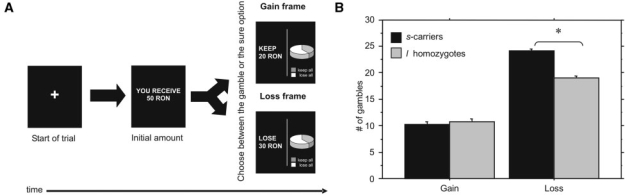
We used a computerized version of a task similar to that of De Martino et al. (De Martino et al., 2006) (Figure 3A). The participants received a message indicating the amount of money that they would initially receive in that trial (e.g. “You receive 50 Romanian New Currency [RON]”). Then, they were told that they would not be able to retain the whole of this initial amount, so they have to choose between a ‘sure’ and a ‘gamble’ option presented in a Gain or a Loss frame. The sure option was formulated as either the amount of money retained or lost from the starting amount (e.g. “Keep 20 RON of the initial 50 RON” in the Gain frame, or “Lose 30 RON of the initial 50 RON” in the Loss frame). The gamble option was identical in the two frames, being represented as a pie chart depicting the probability of winning and losing. The task included 96 trials: 32 Loss frame, 32 Gain frame and 32 Catch trials. While expected outcomes of sure and gamble options were equivalent in each trial, they were unbalanced in the catch trials, with the gamble option being preferable (e.g. 95% probability of winning by taking the gamble vs the sure option of 50% of the initial amount) or not (e.g. 5% probability of winning by taking the gamble vs the sure option of 50% of the initial amount) (De Martino et al., 2006). The number of trials in which the participants chose the gamble option was calculated for each frame. A rationality or sensitivity to framing index was calculated from the difference between the proportions of trials in which a participant chose the gamble option in the Loss frame, as compared to the Gain frame. SCRs were recorded during the behavioral task, and the area under the curve (see below) was quantified in the 0.2–1.2 s following stimulus onset.
Fig. 3.
Diagram of the financial decision making task for framing (A). Mean number of trials in which the participants chose the gamble option in the Gain and Loss frames of the framing task (B). *P ≤ 0.05.
Electrophysiologic recordings
All electrophysiological measures were recorded using a Biopac MP150 system (Biopac Systems). For electrocardiography (ECG), electrodes filled with isotonic gel were placed in a bipolar precordial lead. The analyses were done on 5 min segments from ECG recordings (sample rate of 500 samples) made with unpaced and paced breathing to control for the influence of breathing on certain autonomic indices. These ECG recordings were done several days before the behavioral experiments, with the paced and unpaced conditions balanced between participants. After visual inspection of the recordings and editing to exclude artifacts in AcqKnowledge 3.7.1, all the recordings were analyzed using Nevrokard 7.0.1 (Intellectual Services, Ljubljana, Slovenia). The time domain analysis of ECGs involved generating a time series of interbeat intervals (RR intervals) that reflected the time in milliseconds between consecutive R waves in the ECG waveform. RR intervals are a direct index of HRV reflecting the autonomic control of the heart. Reduced HRV has been associated with anxiety and in addition, it is an important risk factor both for anxiety disorders and coronary diseases (Bleil et al., 2008; Miu et al., 2009). Spectral power analysis was also used to derive the three frequency component power estimates, as follows: high frequency (HF-HRV), also known as respiratory sinus arrhythmia or vagal tone, was defined in the 0.15–0.4 Hz band; low frequency (LF-HRV) in the 0.05–0.15 Hz and very low frequency (VLF-HRV) in the 0.003–0.05 Hz. While HF-HRV is considered to reflect vagal modulation of the heart, LF-HRV reflects a complex interplay between sympathetic and vagal influences (Berntson et al., 1997). All the analyses on HRV indices in the frequency domain were repeated and confirmed on ECGs recorded during paced breathing, to exclude the possible confounding influence of respiration. Breathing was paced by auditory tones cueing participants to inhale and exhale at a mean breathing rate of 11 breaths per minute. Respiration was recorded using the appropriate Biopac modules (RSP100C) and transducers (TSD201).
SCRs were recorded during the observational FC and susceptibility to framing tasks, via two TSD203 electrodermal response electrodes also filled with isotonic gel and attached to the volar surfaces of the index and medius fingers. All the recordings were screened for physiological artifacts (e.g. motion) and analyzed offline using AcqKnowledge. From SCR recordings, we extracted the area under the curve (microSiemens) of SCRs in the intervals of interest, after the downdrift in the SCR waves was eliminated using the ‘difference’ function of AcqKnowledge, as described in Bechara et al., 1999. All the participants included in this study displayed SCRs during the observational FC task.
Data analysis
The data were analyzed using SPSS software, by t-tests, ANOVA or ANCOVA followed by post-hoc comparisons and correlation analyses. The comparisons were Bonferroni corrected for repeated measures where appropriate and unless otherwise specified, the statistically significant effects reported in this study survived the correction for multiple comparisons.
RESULTS
Self-reported anxiety
Table 1 reports the scores of the participants in EMAS and STAI scales. There was a significant effect of the genotype on TA due to threat in social evaluation or interpersonal situations (EMAS-T-SE) (t[30] = 2.42, P = 0.02, Cohen's d = 0.88), as well as ambiguous threat perception (EMAS-P-3) (t[30] = 2.17, P = 0.03, Cohen's d = 0.79). The analyses also indicated marginally significant differences on TA due to threat of physical danger (t[30] = 1.4, P < 0.1, Cohen's d = 0.51), and TA while engaged in innocuous activities or daily routines (t[30] = 1.61, P < 0.1, Cohen's d = 0.58). On all these dimensions, s-carriers have higher scores compared to l-homozygotes. In addition, sex had significant effects on EMAS-T-SE (t[30] = 4.49, P < 0.0001, Cohen's d = 1.63) and ambiguous threat anxiety (t[30] = 3.38, P = 0.002, Cohen's d = 1.23).
Observational FC
The participants showed significantly greater SCRs to CS+ compared to CS− in the test stage (t[30] = 6.06, P < 0.0001, Cohen's d = 2.21), which indicated that they successfully learned about the CS−US contingency. This effect was separately replicated in women (t[21] = 5.44, P < 0.0001, Cohen's d = 2.37) and men (t[7] = 2.71, P < 0.009, Cohen's d = 2.04). In addition, SCRs were greater when the participants watched the learning model being presented with the US compared to CS− (t[30] = 4.16, P = 0.0001, Cohen's d = 1.51). Again, this effect was separately replicated in women (t[21] = 4.53, P < 0.0001, Cohen's d = 1.97) and men (t[21] = 2.36, P < 0.02, Cohen's d = 1.78). These two findings confirmed that the participants displayed increased autonomic arousal when they perceived emotional distress in the learning model, and the model's facial expression of distress successfully served as an aversive US.
The effects of 5-HTTLPR genotype on SCRs during the observation and test stages were tested by ANCOVA analyses, with EMAS-T-SE included as covariate. We found a significant effect of the genotype on SCRs to CS+(F[2, 29] = 10.59, P < 0.0001, partial η2 = 0.13), but not CS− in the test stage (Figure 1C). Post-hoc comparisons indicated that s-carriers displayed significantly greater SCRs to CS+ compared to l-homozygotes. There was also a marginally significant effect of the genotype on SCRs to CS+ and US (F[2, 29] = 2.61, P = 0.07, partial η2 = 0.03), but not CS− in the observation stage (Figure 1B). s-carriers had a tendency to display increased SCRs to CS+ and US compared to l-homozygotes. Sex had a significant effect on SCRs to CS+ (F[1, 29] = 11.9, P = 0.0008, partial η2 = 0.04), and its interaction with genotype was also significant (F[3, 28] = 23.67, P < 0.0001, partial η2 = 0.18). Post-hoc comparisons indicated that men displayed decreased SCRs to CS+ , in comparison to women.
Risk perception and risk taking
ANOVA analyses tested the effects of the genotype on self-reported scores of risk perception and risk taking in DOSPERT (Table 2), as well as BART performance. In the questionnaire, s-carriers had significantly lower scores of financial risk taking compared to l-homozygotes (t[30] = 2.76, P = 0.009, Cohen's d = 1). There was also a marginally significant difference on the ethical risk perception scale of DOSPERT (t[30] = 1.9, P = 0.06, Cohen's d = 0.69), with s-carriers reporting lower scores than l-homozygotes. There was no main effect or interaction of sex × genotype on DOSPERT scores.
Table 2.
Self-report scores on the Domain-Specific Risk-Taking (DOSPERT) scales
| Genotype | Measure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk perception |
Risk taking |
|||||||||
| Ethical | Financial | Health/Safety | Recreational | Social | Ethical | Financial | Health/Safety | Recreational | Social | |
| s/s or s/l | 11.33 ± 5.8 | 15.22 ± 3.86 | 20.92 ± 8.23 | 25.07 ± 8.66 | 29.07 ± 3.93 | 27.5 ± 5.77 | 28.07 ± 3.93** | 28.44 ± 5.63 | 25.44 ± 8.66 | 16.77 ± 5.76 |
| l/l | 15.21 ± 5.57 | 15.71 ± 3.31 | 21.33 ± 9.53 | 26.22 ± 10.97 | 31.77 ± 5.51 | 28.55 ± 8.66 | 31.77 ± 3.51** | 30.21 ± 7.07 | 26 ± 7.78 | 17.14 ± 4.55 |
P ≤ 0.01 on t-tests.
This effect of the genotype on risk taking was confirmed on BART performance. Performance in this task was indexed by the mean pumps per unexploded balloons, and analyzed by an ANCOVA with DOSPERT financial risk taking score included as a covariate. In BART, s-carriers had significantly lower mean number of pumps per unexploded balloons compared to l-homozygotes (F[2, 29] = 5.5, P < 0.05, partial η2 = 0.27), which indicated their general risk aversiveness in economic decision making (Figure 2B). There were no significant effects of sex on BART performance.
Rationality and susceptibility to framing
The gambling task allowed us to investigate the susceptibility to framing of the participants, which inversely reflected their rationality in economic decision making. A comparison between the number of gamble options in the Gain and Loss frames indicated that the framing manipulation significantly influenced the decisions of the participants (t[30] = –6.27, P < 0.0001, Cohen's d = 2.28). The participants were generally risk averse in the Gain frame, and risk seeking in the Loss frame. There was no significant effect of sex in this task.
The genotype had a significant effect on the latter tendency, with s-carriers choosing the gamble over the sure option more often than l-homozygotes in the Loss frame (F[2, 29] = 3.35, P < 0.05, partial η2 = 0.18) (Figure 3B). This was corroborated by the marginally significant effect of the genotype on the rationality index, according to which the decisions of s-carriers were less rational than those of l-homozygotes (F[2, 29] = 2.65, P = 0.08, partial η2 = 0.01). The effects of sex or the sex × genotype interaction were not significant. There was no significant effect of the genotype on gamble options in the Gain frame. Genotype also had significant effects on SCRs during the Gain (F[2, 29] = 4.37, P = 0.03, partial η2 = 0.008) and Loss trials (F[2, 29] = 12.15, P = 0.0005 partial η2 = 0.02), with s-carriers displaying greater areas under the curve in comparison to l-homozygotes.
HRV and vagal tone
We investigated possible differences in HR and the autonomic control of cardiovascular activity. Table 3 describes the time and frequency domain indices of HRV, as well as HR. RR is a general index of the autonomic control of the heart, and HF-HRV reflects the vagal control of the heart or the vagal tone.
Table 3.
Heart rate and heart rate variability indices in the time and frequency domain in conditions of paced and unpaced breathing
| Genotype | Measure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unpaced breathing |
Paced breathing |
|||||||||
| HR | RR | VLF | LF | HF | HR | RR | VLF | LF | HF | |
| s/s or s/l | 83.32 ± 15.11* | 738.65 ± 117.31* | 33.22 ± 19.4 | 73.3 ± 32.14 | 44.04 ± 9.14* | 85.72 ± 15.94* | 718.31 ± 113.28* | 28.86 ± 28.52 | 44.31 ± 48.83 | 76.26 ± 19.28* |
| l/l | 74.16 ± 7.46* | 816.99 ± 86.19* | 35.44 ± 17.86 | 71.55 ± 35.52 | 51.97 ± 10.13* | 76.58 ± 5.29* | 786.83 ± 52.29* | 21.38 ± 20.69 | 26.89 ± 14.73 | 84.69 ± 8.3* |
HR, heart rate; RR, R-R intervals; VLF, very low frequency; LF, low frequency; HF, high frequency.
P ≤ 0.05 on post-hoc comparisons.
We included sex in our analyses because it has been known to influence cardiovascular activity. 2 (genotype: s-carriers vs l-homozygotes) × 2 (sex: males vs females) ANOVAs on HR and HRV measures indicated several significant effects. There was a main effect of the genotype (F[2, 29] = 4.41, P = 0.02, partial η2 = 0.17) and a significant interaction of genotype × sex (F[1, 28] = 4.08, P = 0.03, partial η2 = 0.13) on vagal tone, with s-carriers showing reduced vagal tone compared to l-homozygotes. There were also marginally significant effects of the genotype (F[2, 29] = 2.46, P = 0.1, partial η2 = 0.08) and genotype × sex (F[1, 28] = 3.23, P = 0.06, partial η2 = 0.17) on LF-HRV. Overall, s-carriers were characterized by reduced vagal tone and a tendency of increased sympathetic tone compared to l-homozygotes. Table 3 indicates that in comparison to l-homozygotes, s-carriers also had increased HR and reduced HRV. The correlation analyses of HRV and anxiety indicated significant negative correlations of both LF- and HF-HRV with the social TA scores (r = –0.38, P = 0.04).
DISCUSSION
This study yielded several important findings that were consistent with our hypotheses. First, it provided evidence of enhanced social learning of fear in 5-HTTLPR s-carriers compared to l-homozygotes. Second, it found that s-carriers display increased risk aversiveness and susceptibility to framing in financial decision making. Third, it identified social evaluation, physical threat and daily routines as the dimensions of TA that are specifically influenced by 5-HTTLPR. Fourth, it indicated that s-carriers have reduced autonomic control over the heart. These effects of 5-HTTLPR support the developing view that complex social–emotional and cognitive functions are significantly influenced by genetic variations, and identify social learning of fear and decision making biases as candidate mechanisms by which 5-HTTLPR contributes to risk for and pathogenesis of anxiety disorders.
Observational FC is a model of emotional learning by social means (Phelps, 2006). Humans and other primates can vicariously learn to display fear to initially neutral stimuli after observing the emotional expression of a conspecific in which those neutral stimuli were paired with shocks (Berger, 1962; Hygge and Ohman, 1978; Mineka and Cook, 1993). Similar to FC, observational FC involves amygdala activation and does not depend on awareness (Olsson and Phelps, 2004; Olsson et al., 2007). We hypothesized that s-carriers of 5-HTTLPR polymorphism would show enhanced observational FC considering that 5-HT manipulations affect FC (Burghardt et al., 2004; Burghardt et al., 2007), and 5-HTTLPR variation modulates amygdala activation to fearful stimuli (Hariri et al., 2006; Canli and Lesch, 2007). In addition, a twin study reported that over 45% of social behavior (i.e. ‘in-degree’ or how many times a person is named as a friend, and ‘node transivity’ or if A and B are friends, and B and C are friends, what is the likelihood that A and C are friends) is heritable and called for the identification of genetic associations (Fowler et al., 2009). The present results supported our hypotheses as s-carriers tended to display enhanced autonomic responses when they observed a model being submitted to an aversive event, knowing that the same treatment awaits themselves, and showed increased autonomic responses to CS+ when they were subsequently placed in an analogous situation. The association between 5-HTTLPR and enhanced observational FC identifies an important candidate endophenotype of anxiety disorders. Indeed, since s-carriers show enhanced social learning of fear, they could be at increased risk for anxiety problems. This view is supported by our findings of increased TA due to threat in social evaluation or interpersonal situations, and ambiguous threat perception—as well as TA due to threat of physical danger, and TA while engaged in innocuous activities or daily routines—in s-carriers compared to l-homozygotes. Increased TA is a risk factor for some anxiety disorders and it is associated with cognitive biases toward threatening information processing (Mathews and MacLeod, 2005; Miu and Visu-Petra, 2009).
Decision-making biases are also central to anxiety disorders and they have been extensively studied in behavioral economics and neuroeconomics (Paulus, 2007; Miu, Miclea, and Houser, 2008). We investigated the effect of 5-HTTLPR variations on risk taking and susceptibility to framing in decision making under uncertainty. The present results indicated that s-carriers display increased risk aversiveness, for they had lower scores in the financial risk taking scale of DOSPERT, and lower number of pumps per unexploded balloons in BART in comparison to l-homozygotes. Using other economic games, similar studies also found increased risk aversiveness in 5-HTTLPR s-carriers (Maurex et al., 2009; Kuhnen and Chiao, 2009). Risk aversiveness may result from a hypervigilant decision making style characterized by a non-systematic or selective information search, limited consideration of alternatives, rapid evaluation of data and selection of a solution without extensive review or reappraisal (Johnston et al., 1997). Another mechanism underlying risk aversiveness is related to dysregulations of autonomic signals during the processing of behavioral outcomes—i.e. rewards and punishments—in decision making (Preston et al., 2007; Miu et al., 2008). In addition, s-carriers may be especially prone to decision making biases. Indeed, the present results provided direct evidence that s-carriers have increased susceptibility to framing, for they chose the gamble option more often than l-homozygotes in the Loss frame. The fact that the genotype effect was limited to the condition in which options were presented as losses suggests that the processing of potential losses of different magnitudes is altered in s-carriers (Blair et al., 2008). Alternatively, losses may have been perceived as more emotionally arousing and biased decision making. This explanation is supported by the higher magnitude of the effect of the genotype on GSRs during the trials in the Loss frame compared to those in the Gain frame. Increased amygdala activation may underlie the augmented framing bias in s-carriers (De Martino et al., 2006). It is worth mentioning that the performance in this task should be viewed as the difference between decisions made in two objectively equivalent conditions that differed only in terms of phrasing of the alternatives. Therefore, the increased risk seeking in the Loss trials does not indicate risk seeking per se, but rather a shift of the decision making style in comparison to the Gain trials. Considering that the alternatives in the two conditions were objectively equivalent, this shift thus indicates the susceptibility to making systematic errors of judgment in decision making (Tversky and Kahneman, 1981). Like the s-carriers in the present study, anxious participants are generally risk aversive in BART, but they take more risks compared to non-anxious participants when they have to avoid suffering a loss (Lauriola and Levin, 2001; Maner et al., 2007).
This study also found decreased autonomic control over the heart in s-carriers. In comparison to l-homozygotes, s-carriers are characterized by reduced vagal tone and a tendency for increased sympathetic activity. In addition, there was a negative correlation of HRV with social TA in the present study. Reduced vagal tone has been reliably supported in high TA and anxiety disorders (Bleil et al., 2008; Miu et al., 2009), and its association with increased sympathetic activity may be explained by the fact that a reduction in the parasympathetic innervation leaves the heart exposed to unopposed stimulation by the sympathetic nervous system (Gorman and Sloan, 2000). Reduced autonomic control and vagal tone in particular are likely to play an important role in the risk and pathogenesis of anxiety disorders (Friedman, 2007). Therefore, our finding of the association of 5-HTTLPR with reduced HRV and vagal tone suggests potential psychophysiological mechanisms underlying an endophenotype of anxiety.
The main limits of the present study are related to not having genotyped the single nucleotide polymorphism in the l allele of 5-HTTLPR itself (Zalsman et al., 2006), and not having included other polymorphisms that are known to influence social–emotional and cognitive functions (e.g. 5-HT2A receptor, tryptophan hydroxylase-1, catechol-O-methyltransferase genes) (Burt, 2008; Lonsdorf et al., 2009; Maurex et al., 2009). Future studies could thus use the triallelic approach to 5-HTTLPR and also investigate its epistatic interaction with other functional genetic polymorphisms on social–emotional and cognitive functions. The present sample was also rather small and these effects would certainly deserve replication in larger samples. Nonetheless, it is noteworthy that other recent studies (Strange et al., 2008; Lonsdorf et al., 2009; Maurex et al., 2009; Kuhnen and Chiao, 2009) that also uncovered significant effects of 5-HTTLPR on emotion and cognition have relied on similar samples. This suggests that the effects of this genetic polymorphism on cognitive and biological measures related to harm avoidance are particularly strong. Also, the use of multidimensional instead of global anxiety scales may have allowed us to identify the effects of the genotype on self-report anxiety, despite the small sample. The computational and neurobiological mechanisms underlying social learning of fear (e.g. empathy, contingency awareness) (Phelps et al., 2001; Carter et al., 2006; Ochsner et al., 2008) that are specifically modulated by 5-HTT may also be investigated in the future. The 5-HTTLPR-related endophenotype characterized by enhanced social learning of fear, increased risk aversiveness and susceptibility to decision making biases, high TA and reduced autonomic control would worth being followed-up in clinical populations—e.g. anxiety disorders.
In conclusion, this study found significant effects of 5-HTTLPR on social learning of fear, risk taking and the framing bias in decision making, as well as on autonomic activity. To our knowledge, this is the first study that identifies the genetic contribution of a key regulator of 5-HT signaling on observational FC, decision making under uncertainty and risk and autonomic control over the heart. These findings suggest candidate mechanisms underlying an endophenotype of anxiety disorders.
REFERENCES
- Attenburrow MJ, Williams C, Odontiadis J, et al. Acute administration of nutritionally sourced tryptophan increases fear recognition. Psychopharmacology (Berl) 2003;169:104–107. doi: 10.1007/s00213-003-1479-x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Berger SM. Conditioning through vicarious instigation. Psychological Review. 1962;69:450–466. [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blair KS, Finger E, Marsh AA, et al. The role of 5-HTTLPR in choosing the lesser of two evils, the better of two goods: examining the impact of 5-HTTLPR genotype and tryptophan depletion in object choice. Psychopharmacology (Berl) 2008;196:29–38. doi: 10.1007/s00213-007-0920-y. [DOI] [PubMed] [Google Scholar]
- Blais AR, Weber EU. A Domain-Specific Risk-Taking (DOSPERT) scale for adult population. Judgement and Decision Making. 2006;1:33–47. [Google Scholar]
- Bleil ME, Gianaros PJ, Jennings JR, Flory JD, Manuck SB. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosomatic Medicine. 2008;70:328–337. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- Bozina N, Mihaljevic-Peles A, Sagud M, Jakovljevic M, Sertic J. Serotonin transporter polymorphism in Croatian patients with major depressive disorder. Psychiatr Danub. 2006;18:83–89. [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. Journal of Psychopharmacology. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Burt SA. Genes and popularity: evidence of an evocative gene-environment correlation. Psychological Science. 2008;19:112–113. doi: 10.1111/j.1467-9280.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR, Chung H, Yang R, Clary CM. Multidimensional effects of sertraline in social anxiety disorder. Depress Anxiety. 2006;23:6–10. doi: 10.1002/da.20086. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Brondino N, Bertona M, Re S, Geroldi D. Relationship between platelet serotonin content and rejections of unfair offers in the ultimatum game. Neuroscience Letters. 2008;437:158–161. doi: 10.1016/j.neulet.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Endler NS, Edwards JM, Vitelli R. Endler Multidimensional Anxiety Scales (EMAS): Manual. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- Fehr E, Bernhard H, Rockenbach B. Egalitarianism in young children. Nature. 2008;454:1079–1083. doi: 10.1038/nature07155. [DOI] [PubMed] [Google Scholar]
- Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proceedings of the National Academy of Sciences of USA. 2009;106:1720–1724. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility—neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Current Biology. 2007;17:R724–732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Frith CD, Singer T. The role of social cognition in decision making. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2008;363:3875–3886. doi: 10.1098/rstb.2008.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African– and European–American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gerull FC, Rapee RM. Mother knows best: Effects of maternal modelling on the acquisition of fear and avoidance behaviour in toddlers. Behaviour Research and Therapy. 2002;40:279–287. doi: 10.1016/s0005-7967(01)00013-4. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. American Heart Journal. 2000;140(Suppl 4):77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- Heilman RM, Miu AC, Benga O. Developmental and sex-related differences in preschoolers' affective decision making. Child Neuropsychology. 2009;15:73–84. doi: 10.1080/09297040802266436. [DOI] [PubMed] [Google Scholar]
- Hygge S, Ohman A. Modeling processes in the acquisition of fears: vicarious electrodermal conditioning to fear-relevant stimuli. Journal of Personality and Social Psychology. 1978;36:271–279. doi: 10.1037//0022-3514.36.3.271. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Serotonin and neuropeptides in affiliative behaviors. Biological Psychiatry. 1998;44:207–219. doi: 10.1016/s0006-3223(98)00094-8. [DOI] [PubMed] [Google Scholar]
- Jackson MO. Genetic influences on social network characteristics. Proceedings of the National Academy of Sciences of USA. 2009;106:1687–1688. doi: 10.1073/pnas.0813169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JH, Driskell JE, Salas E. Vigilant and hypervigilant decision making. Journal of Applied Psychology. 1997;82:614–622. doi: 10.1037/0021-9010.82.4.614. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. American Journal of Psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Chiao JY. Genetic determinants of financial risk taking. PLoS ONE. 2009;4:e4362. doi: 10.1371/journal.pone.0004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriola M, Levin IP. Personality traits and risky decision-making in a controlled experimental task: an exploratory study. Personality and Individual Differences. 2001;31:215–226. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene–environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, et al. Dispositional anxiety and risk-avoidant decision-making. Personality and Individual Differences. 2007;42:665–675. [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Maurex L, Zaboli G, Wiens S, Asberg M, Leopardi R, Ohman A. Emotionally controlled decision-making and a gene variant related to serotonin synthesis in women with borderline personality disorder. Scandinavian Journal of Psychology. 2009;50:5–10. doi: 10.1111/j.1467-9450.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- Melke J, Landen M, Baghei F, et al. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. American Journal of Medical Genetics. 2001;105:458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- Miclea S, Albu M, Ciuca A. The Romanian adaptation of Endler Multidimensional Anxiety Scales. Cognition, Brain, Behavior: An Interdisciplinary Journal. 2009;13:59–77. [Google Scholar]
- Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. Journal of Experimental Psychology: General. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biological Psychology. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Autonomic Neuroscience. 2009;145:99–103. doi: 10.1016/j.autneu.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Miu AC, Miclea M, Houser D. Anxiety and decision making: toward a neuroeconomics perspective. In: Houser D, McCabe K, editors. Neuroeconomics. 55–84. London: Emerald Group Publishing Limited; 2008. [PubMed] [Google Scholar]
- Miu AC, Visu-Petra L. Anxiety disorders in children and adults: a cognitive, neurophysiological and genetic characterization. In: Carlstedt R, editor. Integrative Clinical Psychology, Psychiatry, and Behavioral Medicine: Perspectives, Practices, and Research. New York: Springer; 2009. (in press) [Google Scholar]
- Ochsner KN. The social–emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biological Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Social Cognitive and Affective Neuroscience. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Social Cognitive and Affective Neuroscience. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychological Science. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Paaver M, Kurrikoff T, Nordquist N, Oreland L, Harro J. The effect of 5-HTT gene promoter polymorphism on impulsivity depends on family relations in girls. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2008;32:1263–1268. doi: 10.1016/j.pnpbp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry-altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitariu H, Peleasa C. Manual for the Romanian version of State-Trait Anxiety Inventory form Y. Cluj-Napoca: Sinapsis; 2007. [Google Scholar]
- Preston SD, Buchanan TW, Stansfield RB, Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience. 2007;121:257–263. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Science. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State–Trait Anxiety Inventory (form V) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Strange BA, Kroes MC, Roiser JP, Tan GC, Dolan RJ. Emotion-induced retrograde amnesia is determined by a 5-HTT genetic polymorphism. Journal of Neuroscience. 2008;28:7036–7039. doi: 10.1523/JNEUROSCI.0834-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- van Dijk E, van Kleef GA, Steinel W, van Beest I. A social functional approach to emotions in bargaining: when communicating anger pays and when it backfires. Journal of Personality and Social Psychology. 2008;94:600–614. doi: 10.1037/0022-3514.94.4.600. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Siegel JE, Finch AW, et al. The long and the short of it: associations between 5-HTT genotypes and coping with stress. Psychosomatic Medicine. 2007;69:614–620. doi: 10.1097/PSY.0b013e31814cec64. [DOI] [PubMed] [Google Scholar]
- Xiao E, Houser D. Emotion expression in human punishment behavior. Proceedings of the National Academy of Sciences of USA. 2005;102:7398–7401. doi: 10.1073/pnas.0502399102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]