Abstract
Using functional magnetic resonance imaging, we investigated neural activity associated with self-reflection in depressed [current major depressive episode (MDE)] and healthy control participants, focusing on medial cortex areas previously shown to be associated with self-reflection. Both the MDE and healthy control groups showed greater activity in anterior medial cortex (medial frontal gyrus, anterior cingulate gyrus) when cued to think about hopes and aspirations compared with duties and obligations, and greater activity in posterior medial cortex (precuneus, posterior cingulate) when cued to think about duties and obligations (Experiment 1). However, the MDE group showed less activity than controls in the same area of medial frontal cortex when self-referential cues were more ambiguous with respect to valence (Experiment 2), and less deactivation in a non-self-referential condition in both experiments. Furthermore, individual differences in rumination were positively correlated with activity in both anterior and posterior medial cortex during non-self-referential conditions. These results provide converging evidence for a dissociation of anterior and posterior medial cortex depending on the focus of self-relevant thought. They also provide neural evidence consistent with behavioral findings that depression is associated with disruption of positively valenced thoughts in response to ambiguous cues, and difficulty disengaging from self-reflection when it is appropriate to do so.
Keywords: self-reflection, medial frontal cortex, posterior medial cortex, rumination, depression
Anterior medial cortex (e.g., medial frontal gyrus, anterior cingulate cortex) and posterior medial cortex (e.g., posterior cingulate cortex, precuneus) show increased activity during self-referential processing (Johnson et al., 2002; Fossati et al., 2003; Macrae et al., 2004; Ochsner et al., 2005; Vogt and Laureys, 2005; Amodio and Frith, 2006; Johnson et al., 2006; Northoff et al., 2006). Furthermore, activity in these two areas may be associated with different types of self-referential processing. Based on the idea that different types of self-relevant agendas (e.g. goals; Johnson and Reeder, 1997) are an important part of one's self-definition and play a key role in determining one's emotions and regulating interactions with others (Markus and Nurius, 1986; Cantor and Kihlstrom, 1987; Higgins, 1997), Johnson et al. (2006) investigated two broad classes of agendas—hopes and aspirations, and duties and obligations. Replicating others, they observed greater activity in anterior and posterior medial areas for self-referential than for non-self-referential processing [e.g. thinking about concrete things such as polar bears fishing (Nolen-Hoeksema, 2004)]. They also found a dissociation: there was greater activity in anterior medial areas when participants thought about hopes and aspirations, but greater activity in posterior medial areas when participants thought about duties and obligations. In addition, individual differences in chronic promotion orientation (i.e. a focus towards approach goals; Higgins, 1997, 1998) were positively correlated with activity in medial prefrontal cortex (PFC) during hopes/aspirations trials. Similarly, in a medial frontal area near the location that Johnson et al. (2006) found correlated with promotion focus, Sharot et al. (2007) found that more optimistic people showed greater activity when thinking about positive events, relative to negative events. Together, these findings suggest that anterior medial PFC may be recruited during positive, approach-oriented self-relevant thought (see also, Mitchell et al., 2009).
Differences in medial cortex activity have been observed in comparisons of depressed individuals with healthy controls, both in resting state and active tasks (Mayberg et al., 1999; Drevets, 2000; Charney and Manji, 2004; see Drevets et al., 2008; Gotlib and Hamilton, 2008, for reviews). In addition, depression is associated with rumination about negative self-relevant information (Nolen-Hoeksema et al., 2008), and the tendency to ruminate may be related to differences in anterior medial cortex activity (e.g. Ray et al., 2005; Greicius et al., 2007). However, the specific factors (e.g. types of self-focus) that affect whether depressed individuals show elevated or reduced activity in medial cortical regions (and in which subregions) remain to be specified (Drevets, 2000; Davidson et al., 2002; Mayberg, 2003a; Phillips et al., 2003; Mayberg et al., 2005).
The present experiments begin to address this issue by comparing medial cortex activity of individuals experiencing a major depressive episode (MDE) and control participants as they engaged in different types of self-reflective thought compared with non-self-reflective thought. On self-reflection trials in Experiment 1, we cued participants either to think about hopes and aspirations or duties and obligations, conditions shown previously to differentially engage anterior and posterior medial cortex in healthy controls. In Experiment 2, we cued participants either to engage in self-evaluation or to think about their current state, activities that previously have been shown to differentially induce rumination in depressed participants. Thus, Experiment 1 assessed whether depressed participants differed from controls in engagement of anterior medial cortex associated with positive thoughts such as hopes and aspirations when specifically cued to do so; and Experiment 2 assessed whether depressed participants differed from controls in the engagement of this area under conditions that did not specifically cue positive thoughts.
EXPERIMENT 1
Emotional distress (depression and anxiety) has been associated with reductions in promotion or approach goals and increases in avoidance goals (Gray, 1982; Fowles, 1988; Davidson, 1998; Dickson and MacLeod, 2006). Building on the finding that anterior medial PFC is especially associated with thinking about promotion or approach goals (e.g. hopes and aspirations; Johnson et al., 2006), we predicted that, when asked to think about hopes and aspirations, individuals experiencing a MDE would show less activity in anterior medial cortex than controls. The prediction was less clear about the relative activity in the two groups in posterior medial cortex in the hopes and aspirations vs duties and obligations conditions. Activity in this region was not found to correlate with either promotion or prevention orientation (Johnson et al., 2006) or with optimism (Sharot et al., 2007). In addition, although duties and obligations may be associated with avoidance goals (Higgins, 1998), such thoughts are not necessarily negatively valenced.
Depression can be associated with difficulty disengaging from self-focus, particularly from negative self-relevant thoughts, when the task demands it. For example, people who tend to ruminate when distressed (i.e. who passively focus on feelings of distress and their meanings and consequences; Nolen-Hoeksema, 1991) show greater difficulty compared with non-ruminators in disengaging attention from or inhibiting irrelevant information (Siegle et al., 2002; Joormann, 2004, 2005, 2006; Donaldson et al., 2007). Thus, we predicted that individuals experiencing a MDE, and individuals prone to ruminate, would show greater neural activity (i.e. less deactivation) in either or both anterior and posterior medial areas associated with self-reflection when directed to focus on non-self-relevant thoughts during the distraction trials.
Methods
Participants
Participants self-reported being in good physical health, with no history of cardiovascular or neurological disorder, and normal, or corrected to normal, vision. Beck Depression Inventory (BDI-II; Beck et al., 1996) scores were obtained on the day of the scan. Control participants [n = 24 (11 females)] had BDI scores ≤8 (M = 4.04, s.d. = 2.65); MDE participants [n = 20 (9 females)] had BDI scores ≥21 (M = 27.85, s.d. = 6.23). All MDE participants met full criteria for a current MDE according to the Structured Clinical Interview for DSM-IV (SCID; First et al., 1998), administered at the end of the scan session. None of the participants in the control group met the criteria for either current or past MDE. Inter-rater reliability on the SCID was good (κ = 0.67, P < 0.0001). The groups did not differ on age [Mcontrol = 20.6 years (s.d. = 2.4 years), MMDE = 21.9 (s.d. = 2.9 years); P > 0.05] or education [reported in years; Mcontrol = 14.4 (s.d. = 1.9 years), MMDE = 15.3 (s.d. = 2.2 years); P > 0.10]. Four of the MDE participants self-reported taking psychotropic medications (lamictal, effexor, zyprexa, zoloft). Because the pattern of findings remained the same when participants taking medications were removed, we report the data with these participants included. The Human Investigation Committee of Yale University Medical School approved the protocol, and consent was obtained according to the Declaration of Helsinki (1991, Br Med J, 302, 1194). All participants were paid.
Design and procedure
The study was a mixed 2 (group: control, MDE) × 3 (condition: hopes, duties, distraction) design. Participants saw short phrases and were instructed to ‘focus on the idea expressed by the phrase and use your imagination to visualize or think about the idea …’. They were asked to press a button when they had ‘formed a really clear thought, or idea, or image’ and to keep thinking about the idea until a cross-hair appeared. There were six practice trials outside the scanner. During the scan, participants saw the cue ‘hopes and aspirations’ 12 times (‘hopes’ condition), the cue ‘duties and obligations’ 12 times (‘duties’ condition) and 12 different concrete ‘distraction’ cues (e.g. pattern on oriental rug, shape of USA; Nolen-Hoeksema, 2004). Cue types were pseudo-randomly intermixed in 3 runs of 12 trials each (four trials per condition). Each trial was 18 s, with the cue shown for 14 s and a cross-hair shown for 4 s.
Post-scan reports
Immediately after the scan session (which included Experiment 2, described later), participants moved to another room where they were asked to write a paragraph or two describing what they thought about during the scan when they saw the phrases ‘hopes and aspirations’ and ‘duties and obligations’, with order of reports counterbalanced. Two research assistants, blind to the participants’ MDE status, coded each report for both positivity and negativity (1 = not at all to 4 = very). Inter-rater reliability was good (κ = 0.83, P < 0.0001), and each participant was assigned the average of the scores of the two raters. One rater counted the words in each report and also gave each a global detail rating on a scale from 1 (no detail, very vague) to 5 (extremely detailed).
Visual analog scale of mood
Participants’ current mood was measured using a 14-item visual analog scale at three points in the procedure: before going into the scanner, between Experiments 1 and 2 and again after the post-scan report. Adjectives appeared one-at-a-time on the screen and participants indicated for each item how much it described how they were currently feeling on a scale from 0% (not at all) to 100% (very much). The scale was in 10% intervals, and participants pressed a button to indicate their response for each item. There were six relatively negative items (anxious, guilty, hopeless, irritable, sad and worthless), four positive items (calm, energetic, happy and mellow) and four filler items that we considered to be relatively neutral in this context (bored, hungry, talkative and tired) presented in a different pseudorandom order each time.
Individual difference measures
After the scan, participants filled out questionnaires that assessed depressive symptoms (BDI-II; Beck et al., 1996) and tendency to ruminate (Treynor et al., 2003). Participants were also administered the mood module of the SCID (First et al., 1998).
fMRI procedure
Anatomical images were acquired using a 1.5T Siemens Sonata scanner at the Magnetic Resonance Research Center at Yale University. Functional scans were acquired with a single-shot echoplanar gradient-echo pulse sequence [repetition time (TR) = 2000 ms, echo time (TE) = 35 ms, flip angle = 80° and field of view (FOV) = 24]. The 24 oblique axial slices (3.8 mm thick with an in-plane resolution of 3.75 × 3.75 mm) were aligned with the anterior commissure-posterior commissure (AC-PC) line. Each run began with 12 blank seconds to achieve steady state magnetization, and was followed by a 1-min rest interval. One volume was collected every 2 s, or nine full brain images for each trial (108 images per participant per condition).
fMRI analyses
After reconstruction, time series were shifted by sinc interpolation to correct for slice acquisition times. Data were motion-corrected using a six parameter automated algorithm (AIR; Woods et al., 1992). A 12 parameter AIR algorithm was used to co-register participants’ images to a common reference brain. Data were mean-normalized across time and participant and spatially smoothed (3D, 8 mm FWHM gaussian kernel). The data were analyzed using NeuroImaging Software (http://kraepelin.wpic.pitt.edu/nis/). We used voxel-based analysis of variance (ANOVA) with participant as a random factor and all other factors fixed. F-maps were transformed to Talairach space using Analysis of Functional NeuroImages (AFNI) (Cox, 1996), and areas of activation were localized using AFNI and Talairach Daemon software (Lancaster et al., 1997) and manually checked against atlases (Talairach and Tournoux, 1988; Duvernoy, 1999).
We first identified regions showing a condition (hopes, duties, distraction) × time within trial (images 1–9) interaction with a minimum of six contiguous voxels, each being significant at P < 1.0 × 10−21 (Forman et al., 1995), or a group (control, MDE) × condition × time interaction with a minimum of six contiguous voxels, each being significant at P < 0.001 (Forman et al., 1995). For the regions thus identified, subsequent analyses were conducted on percent signal change (from Time 1) for the region at Times 4–7, which is the period during which event-related hemodynamic responses tended to peak in this task. Here, we focus on the medial areas identified and, for these areas, we report results of planned group × condition contrasts in percent signal change when each self-focus condition was separately compared with distraction. We also conducted planned contrasts between conditions within each group, and between the groups for each condition. These contrasts were performed on regions initially identified using the stringent criteria described above, and had specific directional predictions (P < 0.05, one-tailed); therefore, we note all two-tailed effects significant at P < 0.10. Conditions for Experiment 2 will be described in the ‘Methods’ section, but the analysis strategy was identical to that used in Experiment 1. For completeness, Tables 1 and 2 report all areas identified in Experiments 1 and 2, respectively.
Table 1.
Regions of activation in Experiment 1
| Anatomical area | H | BA | X | Y | Z | max F |
|---|---|---|---|---|---|---|
| Hopes > duties > distraction | ||||||
| Medial frontal gyrus, anterior cingulate (Figure 1A) | M | 10, 32 | −5 | 48 | 4 | 20.85 |
| Duties > hopes > distraction | ||||||
| Precuneus, posterior cingulate, cuneus (Figure 1C) | M | 7,31,23,19 | −4 | −67 | 31 | 38.31 |
| Duties > hopes = distraction | ||||||
| Cuneus, lingual gyrus (Figure 1D) | M | 18 (19) | 11 | −80 | 1 | 3.13a |
| Hopes = duties > distraction | ||||||
| Superior, medial frontal gyri (Figure 1B) | M | 10 | −4 | 60 | 21 | 3.21a |
| Middle temporal gyrus, angular gyrus | L | 39,22 | −46 | −66 | 28 | 14.33 |
| Distraction > hopes = duties | ||||||
| Middle, inferior frontal gyri | R | 46,10 (45) | 45 | 40 | 13 | 11.99 |
| Inferior frontal gyrus, precentral gyrus | L | 44,6 | −49 | 4 | 30 | 12.75 |
| Inferior frontal gyrus (inferior precentral gyrus) | R | 44,6 | 46 | 7 | 31 | 15.10 |
| Inferior temporal gyrus, fusiform gyrus, middle temporal gyrus | L | 37,19,21 | −54 | −56 | −7 | 38.01b |
| Inferior, middle temporal gyri | R | 37 | 52 | −52 | −6 | 16.83 |
| Inferior parietal lobule | L | 40 | −49 | −37 | 47 | 18.21 |
| Inferior parietal lobule | R | 40 | 43 | −41 | 48 | 11.97 |
| Superior/middle occipital gyrus | L | 19/18 | −34 | −85 | 22 | 11.30 |
Areas were identified via either a condition × time interaction (with a minimum of six contiguous voxels each significant at P < 1 × 10−21; Forman et al., 1995) or a group × condition × time (six contiguous voxels, P < 0.001) in an initial whole-brain ANOVA. Talairach coordinates (X, Y, Z) are shown for the voxel with the maximum F-value in each area of activation. For identified areas, planned contrasts were performed on percent signal change from Time 1 at Times 4, 5, 6 and 7 (see ‘Methods’ section). Headings refer to patterns for Controls. Major anatomical regions and BA numbers are listed in descending order of approximate size, with areas of approximately equal size indicated by a slash (parentheses indicate a small extent relative to other areas listed). Different patterns for MDE group:aDiscussed in text;bDistraction > hopes > duties. H, hemisphere; L, left; M, medial; R, right; BA, Brodmann area.
Table 2.
Regions of activation in Experiment 2
| Anatomical area | H | BA | X | Y | Z | max F |
|---|---|---|---|---|---|---|
| Analytical > experiential > distraction | ||||||
| Medial frontal gyrus, anterior cingulate (Figure 2A) | M | 10,32 | −5 | 45 | −4 | 3.64a |
| Medial, superior frontal gyri (Figure 2B) | M | 9,10 | −4 | 57 | 21 | 3.88a |
| Medial, superior frontal gyri (anterior cingulate) (Figure 2C) | M | 9,10 (32) | 0 | 51 | 12 | 14.00a |
| Precuneus, cuneus, cingulate gyrus (Figure 3A) | M | 7,31,23 | −4 | −55 | 32 | 20.74a |
| Experiential > analytical > distraction | ||||||
| Cingulate gyrus (Figure 3B) | M | 23/24 | 0 | −21 | 34 | 11.79a |
| Analytical = experiential > distraction | ||||||
| Inferior frontal gyrus (precentral gyrus) | L | 45(47) | −46 | 23 | 7 | 3.08 |
| Inferior frontal gyrus | L | 47 | −43 | 27 | −1 | 12.06 |
| Middle, superior temporal gyri | L | 21,22 | −50 | −29 | −1 | 16.89 |
| Superior temporal, supramarginal, angular gyrus | L | 39,22,40 | −53 | −54 | 26 | 16.22 |
| Analytical = experiential = distraction | ||||||
| Lingual gyrus | L | 18 (19) | −16 | −68 | −1 | 3.36b |
| Distraction > experiential = analytical | ||||||
| Parahippocampal gyrus (hippocampus) | L | 36,37 | −24 | −36 | −10 | 24.08 |
| Parahippocampus (hippocampus), fusiform gyrus | R | 36(37) | 33 | −36 | −12 | 22.81 |
| Distraction > experiential > analytical | ||||||
| Middle, inferior frontal gyri | R | 46,45 | 49 | 29 | 16 | 15.25c |
| Middle frontal gyrus | R | 9/6,44 | 38 | 14 | 32 | 3.91d |
| Precentral gyrus, inferior frontal gyrus | R | 6,44 | 46 | 4 | 31 | 12.94d |
| Inferior, middle temporal gyri, middle occipital gyrus | L | 37,19 | −47 | −56 | −7 | 23.30 |
| Inferior, middle temporal gyri | R | 37,20 | 52 | −52 | −10 | 16.42c |
| Middle temporal gyrus | L | 37(21) | −47 | −53 | 1 | 3.57e |
| Inferior parietal lobule | L | 40 | −45 | −41 | 51 | 14.42 |
| Superior parietal lobule | L | 7 | −18 | −68 | 52 | 11.78c |
| Precuneus, superior parietal lobule | R | 7 | 27 | −60 | 51 | 13.35 |
| Superior occipital gyrus | L | 19 | −38 | −81 | 23 | 16.10 |
| Superior occipital gyrus | R | 19 | 38 | −78 | 25 | 12.58c |
Areas were identified via either a condition × time interaction (with a minimum of six contiguous voxels each significant at P < 1 × 10−21; Forman et al., 1995) or a group × condition × time (six contiguous voxels, P < 0.001) in an initial whole-brain ANOVA. For identified areas, planned contrasts were performed on percent signal change from Time 1 at Times 4, 5, 6 and 7 (see ‘Methods’ section). Headings refer to patterns for controls. Major anatomical regions and BA numbers are listed in descending order of approximate size, with areas of approximately equal size indicated by a slash (parentheses indicate a small extent relative to other areas listed). Different patterns for MDE group: aDiscussed in text;bA > E = D; cD > E = A; dD = E > A; eD = E = A. H, hemisphere; L, left; M, medial; R, right.
In addition, for each region in Figures 1–3, the mean percent change scores (Times 5–8 or 6–9) for each participant for each condition were correlated with their scores on the post-scan rumination questionnaire. We used slightly later time frames than used to assess peak activations in the main analysis (Times 4–7) because it seems likely that ruminative tendencies would be more related to differences seen later in trials. Scatter plots are provided for all significant correlations in the areas shown in Figures 1–3.
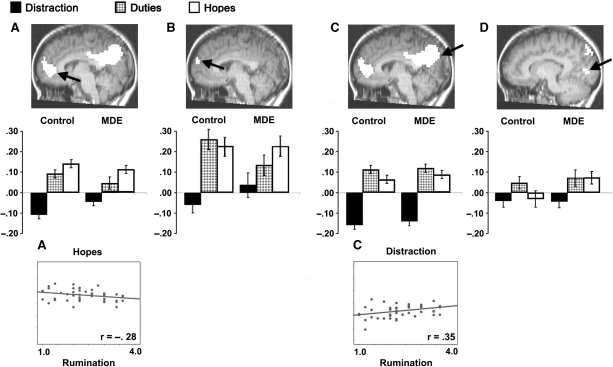
Fig. 1.
Experiment 1. Bar graphs show the mean percent change in bold signal value for control (left) and MDE (right) groups and scatter plots show significant correlations within conditions (distraction and hopes) between mean percent signal change and rumination score (see text). See Table 1 for details regarding coordinates, BA and maximum F for the region. (A) Area of anterior medial cortex showing hopes > duties > distraction for both groups, and MDE > controls in the distraction condition. (B) A more superior area of anterior medial cortex showing hopes = duties > distraction for both groups, with no significant condition differences between the groups. (C) Area of posterior medial cortex showing duties > hopes > distraction for both groups, with no significant condition differences between the groups. (D) Area of posterior medial cortex showing duties > hopes and duties >distraction, but hopes = distraction for controls; duties = hopes > distraction for the MDE group, and MDE > controls in the hopes condition.
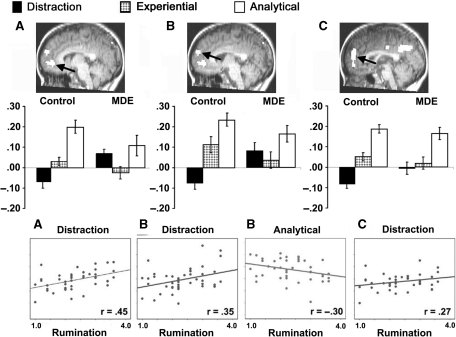
Fig. 2.
Experiment 2. Bar graphs show the mean percent change in bold signal value for control (left) and MDE (right) groups and scatter plots show significant correlations within conditions (distraction and hopes) between mean percent signal change and rumination score (see text). See Table 2 for details regarding coordinates, BA and maximum F for the region. (A and B) Areas of anterior medial cortex showing control > MDE in the analytical condition and MDE > control in the distraction condition, with no difference in the experiential condition. (C) Area of anterior medial cortex showing MDE > control in the distraction condition only.
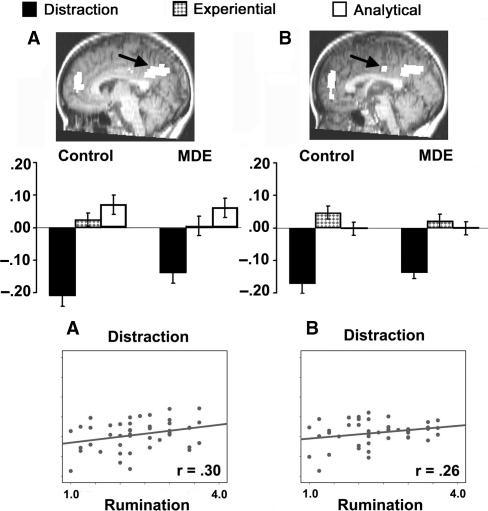
Fig. 3.
Experiment 2. Bar graphs show the mean percent change in bold signal value for control (left) and MDE (right) groups and scatter plots show significant correlations within conditions (distraction and hopes) between mean percent signal change and rumination score (see text). See Table 2 for details regarding coordinates, BA and maximum F for the region. (A) Area of posterior medial cortex showing MDE > controls in the distraction condition, with no other condition differences between the groups. (B) Area of posterior medial cortex showing no differences between MDE and controls for any of the conditions.
Results and discussion
Figure 1A (see also Table 1) shows an area of medial frontal gyrus and anterior cingulate [Brodmann areas (BA) 10, 32] identified in our initial whole brain analysis (see ‘Methods’ section). Subsequent planned comparisons between distraction and each of the two self-referential conditions showed that the difference in activity between hopes and distraction (F = 5.42, P = 0.025) and between duties and distraction (F = 6.56, P = 0.014) was greater for control than MDE participants. These interactions largely arose because the MDE group showed less deactivation in the distraction condition than the controls (F = 4.65, P = 0.04), and the groups did not differ in the other conditions (both P's > 0.10). Within the control group in this area, activity in the hopes condition was greater than (>) the activity in the distraction condition (F = 75.71, P < 0.001), duties > distraction (F = 50.12, P < 0.001) and hopes > duties (F = 4.28, P = 0.05). Within the MDE group in this area, hopes > distraction (F = 36.38, P < 0.001), duties > distraction (F = 8.20, P = 0.01) and hopes > duties (F = 8.23, P = 0.01).
Figure 1B shows an area of superior frontal gyrus, medial frontal gyrus (BA 10) somewhat superior to the region in Figure 1A. In subsequent comparisons of activity in this area, the difference between duties and distraction conditions was greater for the controls than the MDE group (F = 5.06, P = 0.030). The difference in this region between hopes and distraction did not differ between groups (P > 0.10), and the difference between groups was not significant for any of the conditions (all P > 0.10). Within the control group, hopes > distraction (F = 18.41, P < 0.001) and duties > distraction (F = 18.15, P < 0.001), but hopes and duties did not differ significantly (P > 0.10). In the MDE group, hopes > distraction (F = 10.03, P = 0.005) and hopes > duties (F = 3.94, P = 0.062), while duties and distraction did not differ (P > 0.10).
Thus, both groups showed a more inferior area of anterior medial PFC where activity was greater on hopes than duties trials and a more superior area of anterior medial PFC in which activity in the two self-referential conditions did not differ, replicating and extending Johnson et al. (2006) to MDE participants. Contrary to our expectations, MDE participants did not show less activity than controls during the hopes trials. However, as expected, the MDE group did show less deactivation than controls during distraction trials (Figure 1A).
Figure 1C shows an area of precuneus, posterior cingulate, cuneus (BAs 7, 31, 23, 19) where the difference between each of the two self-focus conditions and distraction did not differ between groups (both P's > 0.10), and the difference between groups was not significant in any of the three conditions (all P > 0.10). Collapsed across groups, hopes > distraction (F = 134.22, P < 0.001), duties > distraction (F = 161.66, P < 0.001) and duties > hopes (F = 6.09, P = 0.018). For the control group, hopes > distraction (F = 56.51, P < 0.001), duties > distraction (F = 82.88, P < 0.001) and duties > hopes (F = 3.62, P = 0.07). Similarly, within the MDE group, hopes > distraction (F = 94.39, P < 0.001), duties > distraction (F = 83.08, P < 0.001) and duties > hopes (F = 3.17, P = 0.09).
An additional posterior medial area [cuneus, lingual gyrus, BA 18 (19); Figure 1D] showed an interesting pattern: greater difference in activity between the hopes and distraction conditions for the MDE group than for controls (F = 5.33, P = 0.026). The groups did not differ when duties and distraction were compared (P > 0.10). The MDE group showed greater activity than the controls in the hopes condition (F = 4.53, P = 0.039), but the groups did not differ in the other two conditions (both P's > 0.10). For controls, hopes did not differ significantly from distraction (P > 0.10), while duties > distraction (F = 11.71, P = 0.002) and duties > hopes (F = 9.11, P = 0.006). For the MDE group, hopes > distraction (F = 8.47, P = 0.009) and duties > distraction (F = 7.09, P = 0.015), while hopes and duties did not differ (P > 0.10).
Thus, as in Johnson et al. (2006), both groups showed an area (Figure 1C) of posterior medial cortex where activity was greater in the duties than hopes condition. In addition, in this area, MDE and control groups looked very similar. In a more inferior and posterior area (Figure 1D), controls again showed greater activity for duties than hopes, but the MDE group showed equal activity for these two self-referential conditions.
Individual differences in rumination
Higher trait rumination scores were associated with less activity in anterior medial cortex during hopes trials (r = −0.28, P = 0.07, Figure 1A) and with more activity in posterior medial cortex during distraction trials (r = 0.35, P = 0.02, Figure 1B). During duties trials, rumination scores did not correlate with activity in any of the areas identified in Figure 1.
Post-scan reports
Participants reported thinking of a wide range of topics in response to the self-relevant cues. For hopes cues, topics included: help[ing] developing and poor countries, finding the cure for cancer, writing a screenplay, owning a large house, helping my uncle's business grow, winning scholarships, being worry-free about money/debt, be[ing] able to say intelligent things and hav[ing] people listen and respect me, find[ing] a research mentor for this summer, meet[ing] a woman my mother will like, and being offered a career in intelligence. For duties trials, topics included: keep[ing] parents and friends at home updated on my life, studying for finals, [attending] family reunions, accepting and seeing people for their best, helping my younger sister, volunteering, [thinking of] friends who expect me to be there and how I find it very difficult, [owing] bills, and [being married] and the level of commitment one must have-even during the rocky times. There were no significant group or condition differences in number of words (M = 100.5; all P > 0.10) or amount of detail (M = 3.20; all P > 0.10) in the post-scan reports. With respect to valence ratings, there was a valence × report type interaction (F = 108.79, P < 0.001). As might be expected, for the hopes reports positivity ratings (M = 3.02) were higher than negativity ratings (M = 1.20), while for the duties reports, negativity ratings (M = 1.85) were higher than positivity ratings (M = 1.29). A group main effect (MMDE = 1.97, Mcontrols = 1.71; F = 6.19, P < 0.05) was qualified by a group × valence interaction (F = 4.43, P < 0.05) because the MDE participants’ (M = 1.79) reports were rated as more negative than the control participants’ (M = 1.26) for both types of reports, with no difference in positive ratings (MMDE = 2.15, Mcontrols = 2.16).
In short, in Experiment 1, for control participants, we replicated two findings reported by Johnson et al. (2006): (i) a double dissociation where activity was greater in anterior medial cortex when participants thought about hopes than duties and greater in posterior medial cortex when participants thought about duties than hopes, and (ii) a dissociation within anterior medial PFC where a more inferior area showed hopes > duties and a more superior area showed hopes = duties. We expected that the MDE group would show less activity in anterior medial cortex during hopes trials and less deactivation in this region during distraction trials. While the second prediction was confirmed, the first was not (Figure 1A and B). These findings suggest that MDE participants were relatively successful in engaging positive self-referential processing when cued to do so, but were less successful in disengaging from self-referential processing in the distraction task. Thus, when MDE participants were specifically cued to think about hopes and aspirations and duties and obligations, the activity in anterior and posterior medial cortex suggests that they were doing so. The fact that MDE and control participants’ post-scan reports did not differ in positivity ratings is consistent with this interpretation. However, the means were in the expected direction of less activity in medial PFC during hopes trials in the MDE group (Figure 1A), and rumination scores were negatively correlated (P < 0.07) with activity in medial PFC during hopes trials. In addition, post-scan reports of MDE participants were rated as significantly more negative than those of controls. Taken together, these findings suggest that under the right cuing conditions, we might observe a significant reduction in medial PFC activity associated with depression. Specifically, we might expect larger differences in medial PFC activity between groups to emerge when participants are cued to think about themselves, but are not specifically cued to think about positively valenced topics such as hopes and aspirations. This hypothesis was tested in Experiment 2.
EXPERIMENT 2
When depressed participants are given emotionally neutral self-focused cues, they tend to ruminate about negative personal experiences more than non-depressed participants do (Lyubomirsky et al., 1999). Watkins (2008) distinguished between two types of self-focused rumination cues: ‘analytical cues’, which focus participants’ attention on self-evaluations (e.g. who you strive to be, why things turn out as they do), and ‘experiential cues’, which focus participants’ attention on their current state (e.g. current physical sensations, how alert you feel). Both types of cues direct participants’ attention to the self, but not specifically to positive or negative aspects (Lyubomirsky and Nolen-Hoeksema, 1993). Watkins and Teasdale (2001; also Rimes and Watkins, 2005) found that for depressed individuals, both types of cues maintain depressed mood, but analytical cues are especially likely to result in negative self-referential thought. That is, for depressed but not control participants, analytical cues result in self-focus that is evaluative, perseverative and negative. In addition, such cues prompt depressed individuals to retrieve more negative autobiographical memories, give more negative interpretations of current personal events and make more negative predictions about their future than control participants (Nolen-Hoeksema et al., 2008; Watkins, 2008).
These behavioral findings, in combination with the fMRI findings from Experiment 1, suggest that analytical cues, but perhaps not experiential cues, would result in less activity in medial PFC in MDE participants compared with controls. This is because MDE participants should be less likely to spontaneously generate positive self-focused thoughts in thinking about self-evaluations, and more likely to turn to negative rumination. We also expected to replicate and extend the results from Experiment 1 showing less deactivation in anterior medial cortex when MDE participants are given distraction cues. This would indicate that these participants have more difficulty disengaging from self-focused thought.
Methods
Participants were the same as in Experiment 1 (Experiment 2 followed Experiment 1 in the same scanning session). The study was a mixed 2 (group: control, MDE) × 3 (condition: analytical self-focus, experiential self-focus, distraction) design. Twelve of each cue type were randomly intermixed as in Experiment 1: new distraction cues (e.g. truckload of watermelons, shape of Italy), analytical cues (e.g. who you strive to be, why things turn out as they do, what your feelings mean, quality of your friendships) and experiential cues (e.g. current physical sensations, how alert you feel, how motivated you feel, how decisive you feel). The procedure, fMRI acquisition and analyses paralleled Experiment 1.
Results and discussion
Three areas of anterior medial cortex were identified (Figure 2 and Table 2). An area of medial frontal gyrus and anterior cingulate (BAs 10 and 32) is shown in Figure 2A. Comparisons of each self-referential condition with distraction resulted in group × condition interactions (analytical, F = 12.16, P = 0.001; experiential, F = 13.22, P = 0.001). As predicted, the MDE group showed less activity in this area than controls during the analytical condition (F = 2.99, P = 0.091) and more activity during the distraction condition (F = 14.43, P = 0.001); they did not differ in the experiential condition (P > 0.10). Within the control group, analytical > distraction (F = 31.76, P < 0.001), experiential > distraction (F = 7.18, P = 0.013) and analytical > experiential (F = 38.54, P < 0.001). In contrast, in the MDE group, the analytical and distraction conditions did not differ in this area (P > 0.10), but distraction > experiential (F = 6.22, P = 0.022) and analytical > experiential (F = 5.85, P = 0.026).
In an area of medial, superior frontal gyrus (BAs 9, 10) represented in Figure 2B, comparisons of each self-referential condition with distraction also resulted in group × condition interactions for analytical (F = 13.18, P = 0.001) and experiential (F = 10.83, P = 0.002) conditions. As with the region in Figure 2A, the MDE group showed less activity than controls during the analytical condition (F = 3.78, P = 0.059) and more activity than controls during the distraction condition (F = 10.76, P = 0.002) in this area; the groups did not differ in the experiential condition (P > 0.10). Within the control group, analytical > distraction (F = 56.49, P < 0.001), experiential > distraction (F = 12.30, P = 0.002) and analytical > experiential (F = 19.12, P < 0.001). Within the MDE group, neither analytical nor experiential differed from distraction (both P's > 0.10), but analytical > experiential (F = 7.05, P = 0.016).
A region of medial, superior frontal gyrus, extending into anterior cingulate [BAs 9, 10 (32)] is shown in Figure 2C. Comparisons of each self-referential condition with distraction resulted in a group × condition interaction for analytical (F = 3.31, P = 0.076) and experiential (F = 6.39, P = 0.015). The MDE group showed more activity than controls during the distraction condition (F = 5.25, P = 0.027), but the groups did not differ in the other conditions (both P's > 0.10). Within the control group, analytical > distraction (F = 60.18, P < 0.001), experiential > distraction (F = 18.91, P < 0.001) and analytical > experiential (F = 28.49, P < 0.001). Within the MDE group, analytical > distraction (F = 16.16, P < 0.001), experiential did not differ from distraction (P > 0.10) and analytical > experiential (F = 13.62, P = 0.002).
In short, relative to controls, for MDE participants, sub-regions of anterior medial PFC were less engaged during the analytical condition (Figure 1A and B) and less likely to be disengaged (i.e. deactivate) during the distraction condition (Figure 1A–C). The groups did not differ in any of these areas in the experiential condition.
Two areas of posterior medial cortex were also identified (Figure 3, Table 2). An area of precuneus and cuneus, extending into cingulate gyrus (BAs 7, 31, 23), is shown in Figure 3A. Comparisons of each self-referential condition with distraction found no significant group × condition interaction for the analytical condition (P > 0.10), but there was an interaction for the experiential condition (F = 3.38, P = 0.073). The MDE group showed greater activity than controls during the distraction condition (F = 4.40, P = 0.042), but the groups did not differ in the other conditions (both P's > 0.10). Within the control group, analytical > distraction (F = 35.64, P < 0.001), experiential > distraction (F = 28.18, P < 0.001) and analytical > experiential (F = 3.22, P = 0.086). Within the MDE group, analytical > distraction (F = 18.89, P < 0.001) and experiential > distraction (F = 25.12, P < 0.001), but analytical did not differ from experiential (P > 0.10).
An area of cingulate gyrus (BAs 23/24) is shown in Figure 3B. Comparisons of each self-referential condition with distraction resulted in no significant group × condition interactions (both P's > 0.10), and there were no group differences for the individual conditions (all P > 0.10). Within the control group, however, analytical > distraction (F = 17.35, P < 0.001), experiential > distraction (F = 36.40, P < 0.001) and experiential > analytical (F = 3.33, P = 0.081). Within the MDE group, analytical > distraction (F = 33.75, P < 0.001) and experiential > distraction (F = 27.87, P < 0.001), but analytical did not differ from experiential (P > 0.10).
Overall, in posterior medial cortex, the only difference between the control and MDE groups was less deactivation during distraction in the precuneus, cuneus area as shown in Figure 3A. In contrast to the pattern in anterior medial cortex, in posterior medial cortex there was little difference in activity between the two self-referential conditions or between groups in the self-referential conditions.
Individual differences in rumination
As expected, higher rumination scores were associated with less activity in anterior medial cortex during analytical trials (r = −0.30, P = 0.046, Figure 2B). In addition, higher rumination scores were associated with more activity during distraction trials in anterior medial cortex (r = 0.45, P < 0.001, Figure 2A; r = 0.35, P = 0.019, Figure 2B; r = 0.27, P = 0.072, Figure 2C), and in posterior medial cortex (r = 0.30, P = 0.045, Figure 3A; r = 0.26, P = 0.087, Figure 3B). Rumination scores were not significantly correlated with activity in regions shown in Figures 2 and 3 during experiential trials (P > 0.10).
Visual analog mood scale results
The mean mood scores obtained pre-scan (Time 1), between Experiments 1 and 2 (Time 2) and post-scan (Time 3) are presented in Table 3 (n = 18 in the MDE group because two participants were each missing one measure and thus were excluded from analysis). A 2 (group: control, MDE) × 3 (valence: negative, neutral, positive) × 3 (Time: 1, 2, 3) ANOVA on the mood scale scores showed a three-way interaction (F = 3.06, P = 0.018). As might be expected, compared with controls, the ratings of the MDE group were significantly more negative for the negative items, and significantly less positive for the positive items, at all three time points (all P's < 0.001). However, the full pattern of mood data argues against an interpretation that the current fMRI findings are based merely on a task-induced worsening of mood during the scans in the MDE group. Specifically, the MDE group's ratings for negative items became less negative over time: from Time 1 to 2, the ratings decreased 3.15% (P > 0.10) and from Time 2 to 3, they decreased 6.39% (t = 3.81, P = 0.001). Hence from Time 1 to 3, the MDE group's ratings on negative items decreased on average 9.54% (t = 2.69, P = 0.015). For control participants, the decrease from Time 1 to 3 was only 3.38%, which, although a reliable change (t = 2.48, P < 0.02), was marginally less than that of the MDE group (t = 1.79, P = 0.081). The MDE group showed no changes in their ratings of the positive items (all P's > 0.10). The control group showed a 7.5% decrease on the positive items from Time 1 to 2 (t = 3.60, P = 0.002), but a small increase (3.9%) from Time 2 to 3 (t = 3.89, P = 0.079); thus, the change from Time 1 to 3 was not significant (P > 0.10).1 Finally, although there was not much change in ratings of the filler items (e.g. hungry, tired, bored) over time in either group, the MDE group gave higher ratings to the filler items than did the control group at Time 1 (t = 2.23, P = 0.032) and Time 3 (t = 2.47, P = 0.02), suggesting that they may have been more sensitive to their bodily feelings than were the control participants before and after the scan session, but not during it.
Table 3.
Mean mood ratings (s.e. of the cell mean) for three time points
| Time 1 | Time 2 | Time 3 | |
|---|---|---|---|
| Pre-scan | Between Experiments 1 and 2 | Post-scan | |
| Negative Items | |||
| Control | 12.36 (2.76) | 10.42 (2.70) | 8.99 (2.85) |
| MDE | 43.15 (3.19) | 40.00 (3.11) | 33.61 (3.29) |
| Positive items | |||
| Control | 67.08 (2.77) | 59.58 (2.74) | 63.47 (2.35) |
| MDE | 42.64 (3.20) | 44.03 (3.16) | 41.67 (2.71) |
| Filler items | |||
| Control | 33.85 (2.67) | 35.73 (2.76) | 36.77 (2.76) |
| MDE | 42.92 (3.08) | 39.58 (3.19) | 47.18 (3.18) |
In sum, as predicted, when participants were induced to think about themselves and their life with cues that did not explicitly direct them toward thoughts with a particular valence, MDE participants showed less activity in anterior medial cortex than did controls. That this pattern was observed during analytical but not experiential trials is consistent with previous behavioral findings suggesting that analytical cues are more likely than experiential cues to induce rumination (Watkins, 2008). Rumination scores were also negatively correlated with activity in anterior medial cortex during analytical trials and positively correlated with activity in both anterior and posterior medial cortex during distraction trials. Thus, the results of Experiment 2 demonstrate that, when responding to valence-neutral cues that induce self-evaluative thought, depression is associated with reduced likelihood of engaging regions of anterior medial cortex that have been shown to be associated with positive thoughts about one's hopes and aspirations. The findings also indicate that depression is associated with less deactivation of medial regions associated with self-referential thought, suggesting difficulties disengaging from self-focused thought when the task demands (Siegle et al., 2002; Joorman, 2004, 2005).
Finally, the relative absence of activity associated with self-reflection in posterior medial cortex in both groups in Experiment 2, especially in the experiential condition, provides further evidence that this region is not simply a ‘self’ area. Rather it may be involved in more specific functions, such as the revival of episodic memories (Hassabis et al., 2007), that may occur more as one thinks about self-relevant agendas such as hopes and duties (Experiment 1; Johnson et al., 2006) than less concrete issues associated with self-evaluation (e.g. analytic cues such as considering the nature of one's relationships).
GENERAL DISCUSSION
To our knowledge, these are the first fMRI studies to compare medial cortex activity of MDE and control individuals contrasting self-focused cues about two types of personally relevant agendas (hopes, duties; Experiment 1) and contrasting self-referential cues that have been shown to differentially induce rumination in depressed individuals (analytic, experiential; Experiment 2).
In Experiment 1, activity in an inferior area of anterior medial cortex (Figure 1A) was greater in response to cues to think about hopes and aspirations than cues to think about duties and obligations, replicating previous findings (Johnson et al., 2006). Furthermore, activity in this region did not differ significantly between control and MDE participants. These findings suggest that MDE participants were able to recruit this area when explicitly given cues to engage the sorts of self-reflective processing previously shown to activate this region in control participants, and are consistent with behavioral studies showing that depressed individuals can generate positive memories and thoughts when guided to do so (Wenzlaff et al., 1988). However, behavioral studies also indicate that depressed individuals have difficulty generating positive memories and thoughts on their own (Wenzlaff et al., 1988), and findings from Experiment 2 provide neural evidence consistent with these behavioral findings.
In Experiment 2, a similar area of anterior medial PFC (Figure 2A) was more engaged in response to analytical self-focus cues (e.g. ‘why things turn out as they do’) than experiential cues (e.g. ‘current physical sensations’), consistent with findings from Experiment 1 that this region is not simply a self-focus region, but rather is especially sensitive to certain types of self-focus (see also Johnson et al., 2006; Mitchell et al., 2009). Further, control participants showed greater activation in medial PFC compared with MDE participants in the analytical cue condition. This pattern of activation would be expected if this sub-region of medial PFC is associated with positively valenced self-focus (Johnson et al, 2006; Sharot et al., 2007) and if analytical self-focus led to negative self-thought (rumination) in susceptible individuals (Watkins, 2008). That is, depressed individuals may show reduced medial PFC activity, compared with those without depression, because they are less likely or able to generate positive ideas in response to analytical cues, because the positive thoughts they do generate feel less positive, and/or negative thoughts offset the impact of positive thoughts.
The present findings provide additional evidence for different functional sub-regions of medial PFC (Ochsner et al., 2004; Mitchell et al., 2005; Amodio & Frith, 2006; Northoff et al., 2006). In particular, these results provide converging support for the conclusion that an area of inferior medial PFC is associated with more positive thought, while a more superior medial PFC area is associated with either more general self-focus or with less-affective aspects of self-focused thought (Johnson et al., 2006; see also Moran et al., 2006). Converging evidence for the hypothesis that inferior medial PFC is associated with positively valenced thoughts (Johnson et al., 2006; Sharot et al., 2007) was reported by D’Argembeau et al. (2008), who cued participants to think of positive and negative future events (generated by the same participants in a previous session) and found positive > negative in an area of inferior medial PFC close to the area we found in both experiments (0, −51, −4, compare with current Figures 1A and 2A). Nonetheless, the areas of anterior medial cortex associated with valence do vary some across self-referential studies (Gutchess et al., 2007; Yoshimura et al., 2009). Thus, differentiating functional sub-regions of anterior medial cortex with respect to valence and the interaction between valence and self-reference remains a challenge.
Investigators have also begun to identify sub-regions of anterior medial cortex that appear to be involved in other aspects of self-reflection. For example, Packer and Cunningham (in press) investigated whether greater medial PFC activity for hopes than duties might be related to the more distant time frame that is likely to be considered for hopes than for duties. They cued participants to the time frame to consider (e.g. this week, next year, 10 years from now) and found hopes > duties in an area (−6, 46, 1) very close to the inferior medial PFC area as shown in Figures 1A and 2A. In addition, this area was not sensitive to time frame. Rather, both Packer and Cunningham (in press) and D’Argembeau et al. (2008) reported evidence that an even more inferior PFC region (BA 11: 0, 28, −19; and −3, 45, −19, respectively) than found in the present study is differentially active when participants think about distant compared with near events. Packer and Cunningham also found an area of more superior medial PFC (−3, 49, 29) near the areas in Figures 1B (−4, 60, 21) and 2B (−4, 57, 21), which they associated with more domain general processes invoked when the task was more difficult (in their experiment near hopes and far duties were more difficult than far hopes and near duties). (Where published papers reported MNI coordinates, to facilitate comparison, we converted to Talairach using BioImage Suite http://www.bioimagesuite.org/Mni2Tal/index.html.).
It is notable that across five studies comparing hopes and duties conditions (Johnson et al., 2006, Experiments 1 and 2; Mitchell et al., 2009; Packer and Cunningham, in press, and the present Experiment 1), the z coordinates of local maxima in anterior medial regions showing hopes > duties (in young/control participants) ranged from −7 to 16 and the z coordinates of anterior medial regions showing hopes = duties ranged from 21 to 32. This pattern is consistent with suggestions of a ventral/dorsal division of functions in medial PFC with respect to more/less valenced self-processing (Ochsner et al., 2005; Johnson et al., 2006; Moran et al., 2006) and, more generally, similar to the ventral/dorsal division of anterior cingulate cortex into more/less emotional processing areas (Bush et al., 2000). Thus, the relatively reduced recruitment of the two areas of medial PFC found in Experiment 2 for the MDE group likely reflects a combination of more goal-specific factors (e.g. affective content, Figure 2A) and more general factors (e.g. the type or complexity of reflective processes engaged, Figure 2B), but not differences in the time frames considered by the two groups.
Posterior medial cortex has also been found to be active during self-referential processing (Johnson et al., 2002; Vogt and Laureys, 2005; Northoff et al., 2006), and more so when people are cued to think about their duties and obligations than their hopes and aspirations (Johnson et al., 2006). We replicated this latter finding in both control and MDE participants in Experiment 1 (Figure 1C). Control participants also showed greater activity on duties trials than hopes trials in a more inferior and posterior area, whereas MDE participants did not (Figure 1D). The similar activity in hopes and duties trials in the posterior medial area in Figure 1D in the MDE group suggests that the thoughts of MDE participants in response to cues meant to elicit hopes and aspirations were in some way more similar to those meant to elicit duties and obligations than was the case for controls. Activity in the posterior medial cortex is associated with autobiographical retrieval (Cavanna and Trimble, 2006; Hassabis et al., 2007), and thinking about duties may generate more episodic recall of past events (e.g. specific commitments made to others) than thinking about hopes (Johnson et al., 2006). Thus, one hypothesis is that the pattern seen in the area of posterior medial cortex shown in Figure 1D may reflect a greater tendency of MDE participants to think back to past episodes during attempts to think of future-oriented hopes. However, the area in Figure 1D is more posterior and inferior to those (such as the area in Figure 1C) typically associated with self-referential processing and with episodic memory. Thus, this interesting pattern needs to be replicated using more systematic manipulations in order to isolate its role in self-referential processing.
Anterior, as well as posterior, medial cortex has been associated with autobiographical memory and with imagining future events (Addis et al., 2007; Hassabis et al., 2007; D’Argembeau et al., 2008). Given that depression is not only associated with less positive thinking, but also with less specific, more general thoughts, both for autobiographical memories and for imagining future events, particularly when depression involves analytical rumination (Williams et al., 1996; Williams et al., 2000; Dickson and Bates, 2006; Watkins, 2008), the reduced activity in the MDE group in anterior medial cortex in the analytical condition of Experiment 2 might reflect more impoverished thoughts. However, it is of note that we found no evidence of reduced activity associated with depression in posterior medial cortex in either experiment. Recall also that the post-scan reports about what participants were thinking did not differ for the MDE and control groups in length or rated detail. Thus, the difference in anterior medial cortex activity in the analytical condition in Experiment 2, coupled with no under-recruitment of posterior medial cortex in the MDE group in either experiments, suggests a difference between groups not in ‘how much’ participants were thinking, but in ‘what’ they were thinking, i.e. a difference in specific content and/or valence. Further studies will be needed to dissociate the impact of the amount of detail in what is thought from the valence, specificity or other features of what is thought. Nevertheless, the fact that the MDE group did not show reduced activity relative to controls in posterior medial cortex during self-reflection in a range of cuing conditions represented in the two experiments argues for a relatively specific reduction in anterior medial cortex activity associated with depression.
In short, the overall pattern across these two experiments suggests that a primary difference between MDE and control individuals when engaging in self-reflection is an under-recruitment in MDE participants of anterior medial areas associated with positively valenced thoughts (e.g. hopes and aspirations) when cues are ambiguous in terms of valence and do not specifically elicit such thoughts. This fits with models of depression suggesting it is characterized by an underactive approach system, leading to low motivation and a failure to experience positive effect (Gray, 1982; Fowles, 1988; Davidson, 1998; Schaefer et al., 2006).
When Johnson et al. (2006) originally reported a double dissociation between the nature of self-relevant agendas (hopes vs duties) and the medial regions that are most active (anterior vs posterior medial cortex, respectively), they suggested a number of potential factors to explore that could contribute to this finding: (i) differences in content; (ii) differences in the component processes engaged (e.g. generating/discovering new relations vs retrieving episodic information); (iii) differences in affective/motivational significance; (iv) differences in subjective experience of self (e.g. instrumental control vs experience of awareness); and (v) differences in social/contextual factors (e.g. duties may be more likely to involve others; differences in perspective taking). Of course, these are not entirely orthogonal factors. For example, differences in the subjective experience of self should be related to differences in the component processes engaged (Johnson and Reeder, 1997). The present results add to other recent findings (e.g. Vogt and Laureys, 2005; Cavanna and Trimble, 2006; Johnson et al., 2006; Hassabis et al., 2007; Sharot et al., 2007; D’Argembeau et al., 2008; Packer and Cunningham, in press) that together suggest that specific sub-regions of medial cortex are differentially associated with factors such as type of processing engaged, type of goal, valence and time-frame considered. Such factors would be expected to show important group and individual differences and thus should be promising targets for further study.
It is also important to note that regions in anterior and posterior medial cortex are part of a network that has come to be called the ‘default network’ because activity is found in these regions when people do not have any particular cognitive task to do (Gusnard et al., 2001). Commonly, in fMRI studies, these areas are active at rest and ‘deactivate’ during performance of a cognitive task. In both Experiments 1 and 2, the MDE group showed less deactivation than did the control group in medial cortex during distraction trials, suggesting that they may be disengaging these default areas less than controls when presented with a non-self-referential, cognitive task. In individuals with major depression, the medial cortex regions associated with the default network, especially more inferior anterior regions including subgenual cingulate cortex, show hypermetabolism (e.g. Drevets et al., 2002; Mayberg, 2003b) and increased functional connectivity (e.g. Greicius et al., 2007) during the resting state (see Drevets et al., 2008; Gotlib and Hamilton, 2008, for recent reviews). These areas also demonstrate less deactivation during cognitive or emotion processing tasks in depressed, compared with control, participants (e.g. Vasic et al., 2008; Grimm et al., 2009; Sheline et al., 2009; see also, Drevets et al., 2008; Gotlib and Hamilton, 2008, for recent reviews). Moreover, such differences correlate with depressive symptoms. For example, amount of subgenual cingulate cortex connectivity within the default network correlates with the duration of current episode of depression (Grecius et al., 2007), and less deactivation during the processing of (negative) emotional pictures in ventromedial PFC correlates with subjective ratings of hopelessness and less deactivation in posterior cingulate cortex correlates with level of depression (i.e. scores on the Hamilton Depression Scale) (Grimm et al., 2009).
Because fMRI data are correlational, the present findings alone cannot differentiate whether: (i) the observed group differences in medial cortex activity signal differences in what is motivationally significant or salient during self-relevant thinking in individuals with depression, so that changes in focus or content lead to changes in medial cortex activity; or (ii) changes in the functioning of medial cortex in depression promote differences in the content or focus of self-relevant thinking. However, based on a range of neurophysiological evidence, including developmental trajectories, it has been suggested that default network connectivity is sculpted by experience (Sheline et al., 2009). Thus, it may be that long-term self-focused, negative ruminative thinking, such as that engaged by many depressed individuals, strengthens connectivity of certain medial areas of the default network (Greicius et al., 2007), making it more difficult to disengage when appropriate (e.g. during distraction tasks).
In any event, differences in activity within specific areas of the ‘default network’ may provide an index of the extent and type of self-focused thought that is spontaneously engaged, and these may differ systematically between populations. Our findings provide strong support for the idea that individuals with depression engage in self-focused thought even when the task is to engage in non-self-relevant processing (see also, e.g. Sheline et al., 2009, for a similar suggestion). These findings are in line with behavioral data that depressed individuals have difficulty withdrawing attention from self-relevant information when it would be appropriate to do so (Joormann, 2004, 2006; Donaldson et al., 2007). If deactivation of medial cortex is necessary for the activation of other areas in response to certain cognitive task demands (e.g. attention and memory tasks, Grady et al., 2006), then depression would be expected to disrupt cognitive performance, and there is behavioral evidence that it does so (Strack et al., 1985; Hertel, 1998; Nolen-Hoeksema et al., 2008).
Together, the findings discussed may help explain why, across studies of depression, the specific patterns of findings in medial cortex are mixed. For example, in medial PFC, studies sometimes show hypofrontality and sometimes hyperfrontality (Mayberg, 2003a; Phillips et al., 2003; Drevets et al., 2008; Gotlib and Hamilton, 2008 for reviews and discussion). Our results are generally consistent with earlier neuroimaging studies finding reduced task-related activity in medial PFC associated with depression (Bench et al., 1993; Drevets, 2000; Charney and Manji, 2004), and consistent with the idea that depression involves dysfunction in the ability to self-regulate emotion via the recruitment and maintenance of approach-related self-relevant agendas (as in Joormann and Siemer, 2004; Wenzlaff et al., 1988). But, our results also suggest that some variability in findings with depressed individuals across studies may in part reflect differences in the nature of the spontaneous thoughts engaged by participants during, for example, resting periods or baseline assessments.
Our results also suggest that some variability in findings with depressed individuals may depend in part on whether and what kind of self-reflective processing is relevant to the task at hand, and how well cues engage that specific processing. For example, if self-relevant processing of positive information such as hopes and aspirations is specifically cued, based on our findings, we would expect less difference between controls and depressed participants in medial PFC activity than in tasks that put fewer constraints on the nature of self-referential thinking. Group differences should be most likely in the unconstrained case because depressed participants are less likely to spontaneously generate positive thoughts and more likely to spontaneously generate negative thoughts (Wenzlaff et al., 1988). If self-relevant processing (positively or negatively valenced) is not appropriate to the task at all (as in many cognitive tasks), we would expect to find more medial PFC activity (i.e. less deactivation) associated with depression (Siegle et al., 2002), consistent with a failure to disengage from self-relevant processing when it is not appropriate. In other words, increases in activity in medial PFC associated with depression, relative to controls, would be expected if depressed participants are less able to disengage self-focused thought and engage processes required for other tasks.
Depression is a heterogeneous disorder. Studies of dysphoric and MDD/MDE participants along the lines of the present study, that manipulate the specific nature of both self-relevant and non-self-relevant processing, would help distinguish between individual differences in dysfunction of the self-relevant and self-regulatory processing networks that involve anterior and posterior medial cortex regions and could clarify individual differences in the circumstances under which these areas are engaged, which could be clinically useful. For example, in subclinical dysphoric populations, the extent to which medial PFC is engaged under specific instructions to think about hopes and aspirations or the extent to which engagement is predicted by rumination scores may provide an early biomarker of susceptibility to develop clinically significant depression. In clinically depressed patients, such responses may predict response to treatment. Alternatively, measures of spontaneous activity during ambiguous cues or during rest may provide better biomarkers of susceptibility and/or responsiveness to treatment. Such possibilities warrant further exploration. In addition, depression frequently co-occurs with other disorders (Davidson et al., 2002) that also involve emotional dysregulation or non-constructive self-reflection (e.g. anxiety disorders, borderline personality disorder, post-traumatic stress disorder; Gross and Munoz, 1995; Nolen-Hoeksema et al., 2008). Our understanding would be advanced by systematically investigating these co-occurring disorders to establish whether the current findings are specific to depression or are present in other forms of psychopatholgy as well.
Finally, we note that although discussions of depression tend to focus on areas, such as subgenual anterior cingulate, that are somewhat inferior to our most inferior area (Figures 1A and 2A), other anterior medial areas clearly show disruption in depression (besides the current studies, see also, e.g. Vasic et al., 2008; Grimm et al., 2009; Sheline et al., 2009). As we begin to discover more about the functional specificity of sub-regions of anterior medial cortex (e.g. Johnson et al., 2006; D’Argembeau et al., 2008; Packer and Cunningham, in press; current experiments), the differences in the sub-regions of medial cortex found to be affected in depression under different conditions should provide information about both this disorder and the functions of these regions. For example, it may be that the more inferior, subgenual region of medial PFC, which appears to be hyperactive in treatment-resistant depression and which responds to deep brain stimulation, plays a specific role in maintaining negative mood (Mayberg et al., 2005), whereas the regions of slightly more superior medial PFC observed in the present studies play a role in maintaining positive mood.
In conclusion, our findings suggest that depression is associated with lower probability of engaging regions of anterior medial PFC associated with positive thoughts such as hopes and aspirations when the cues to self-reflection are ambiguous. Furthermore, a tendency to ruminate is correlated with greater activation in anterior and posterior medial cortex during tasks where these regions typically deactivate, suggesting a persisting self-focus when it may not be appropriate. Exploring differential engagement and disengagement of specific regions in anterior and posterior medial cortex in different populations under different circumstances should help clarify the role that these regions normally play in self-reflection and emotion regulation and in the interaction between motivation, emotion and cognition more generally.
Acknowledgments
These studies were funded by National Institutes of Health (AG09253). We thank MR technologists Hedy Sarofin and Cheryl McMurray for assistance in collecting the MR data; Kathleen Muller for help with figures and scoring of the participants’ post-scan reports; and Anna Swan for help with report scoring.
Footnotes
As noted in the introduction to Experiment 2, we knew from existing behavioral literature (Watkins and Teasdale, 2001; also Rimes and Watkins, 2005) that the self-reflection manipulations used in Experiment 2 were likely to induce rumination in the MDE group. We thus tested everyone first in Experiment 1 to minimize potential carryover effects that might have attenuated MDE participants’ likelihood of recruiting medial cortex areas when specifically prompted with cues that we knew would recruit these areas in controls. Of course, there could have been carryover effects of the Experiment 1 manipulation on the nature of participants’ self-reflection in Experiment 2, as well. For example, if MDE participants failed to come up with compelling hopes in Experiment 1, or failed to feel hopeful about the ones they did generate, they may have had more negative thoughts in the analytical condition in Experiment 2 than they otherwise would have. If so, this would be consistent with findings that depression leads to a cycle of negative thinking that pervades even ostensibly neutral situations. However, the significant rise in mood scores in the MDE group suggests that priming of positive ideas might have worked against our predictions for Experiment 2. That is, being prompted to come up with specific hopes in Experiment 1 may have attenuated differences between groups in Experiment 2 by priming positive thoughts for MDE participants that would not otherwise have been accessed. Thus, the group difference we found in medial PFC in Experiment 2 actually might have underestimated the true (unprimed) difference in these groups in the nature of their spontaneous self-reflection.
Conflict of Interest: None declared.
REFERENCES
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of the minds: the medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. San Antonio, TX: The Psychological Corporation; 1996. Manual for the Beck Depression Inventory-II;?>. [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RSJ, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychological Medicine. 1993;23:579–90. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cantor N, Kihlstrom JF. Personality and Social Intelligence. Englewood Cliffs, NJ: Prentice-Hall; 1987. [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: Multiple pathways lead to increased risk and new opportunities for intervention. Science’s STKE (Electronic Resource: Signal Transduction Knowledge Environment) 2004;225:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu Z-L, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–30. [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Dickson JM, Bates GW. Autobiographical memories and views of the future: in relation to dysphoria. International Journal of Psychology. 2006;41:107–16. [Google Scholar]
- Dickson JM, MacLeod AK. Dysphoric adolescents’ causal explanations and expectations for approach and avoidance goals. Journal of Adolescence. 2006;29:177–91. doi: 10.1016/j.adolescence.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behaviour Research and Therapy. 2007;45:2664–78. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002;12:527–44. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-dimensional Sectional Anatomy with MRI, and Blood Supply. 2nd. New York: Springer; 1999. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (Version 2.0) New York: Biometrics Research Department; 1998. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: a motivational approach. Psychophysiology. 1988;25:373–91. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: current status and unresolved issues. Current Directions in Psychological Science. 2008;17:159–63. [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18:227–41. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Gray J. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press; 1982. [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–43. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–64. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience. 2007;27:14365–74. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel PT. Relation between rumination and impaired memory in dysphoric moods. Journal of Abnormal Psychology. 1998;107:166–72. doi: 10.1037//0021-843x.107.1.166. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Promotion and prevention: Regulatory focus as a motivational principle. Advances in Experimental Social Psychology. 1998;30:1–46. [Google Scholar]
- Johnson MK, Reeder JA. Consciousness as meta-processing. In: Cohen JD, Schooler JW, editors. Scientific Approaches to Consciousness. Mahwah, NJ: Erlbaum; 1997. pp. 261–93. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Attentional bias in dysphoria: the role of inhibitory processes. Cognition and Emotion. 2004;18:125–47. [Google Scholar]
- Joormann J. Inhibition, rumination, and mood regulation in depression. In: Engle RW, Sedek G, Hecker UV, McIntosh DN, editors. Cognitive Limitations in Aging and Psychopathology. New York: Cambridge University Press; 2005. pp. 275–312. [Google Scholar]
- Joormann J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: evidence from a negative priming task. Cognitive Therapy and Research. 2006;30:149–60. [Google Scholar]
- Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: difficulties in repairing sad mood with happy memories? Journal of Abnormal Psychology. 2004;113:179–88. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerlin JL, Rainey L, Freitas CS, Fox PT. The Talairach daemon, a database server for Talairach atlas labels. NeuroImage. 1997;5:S633. [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Self-perpetuating properties of dysphoric rumination. Journal of Personality and Social Psychology. 1993;65:339–49. doi: 10.1037//0022-3514.65.2.339. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Tucker KL, Caldwell ND, Berg K. Why ruminators are poor problem solvers: clues from the phenomenology of dysphoric rumination. Journal of Personality and Social Psychology. 1999;77:1041–60. doi: 10.1037//0022-3514.77.5.1041. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Markus H, Nurius P. Possible selves. American Psychologist. 1986;41:954–69. [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003a;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clinics of North America. 2003b;13:805–15. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychology and Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–82. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The response style theory. In: Papageorgiou C, Wells A, editors. Depressive Rumination: Nature, Theory, and Treatment. New York: Wiley; 2004. pp. 107–23. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1748–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA. Neural correlates of reflection on goal states: The role of regulatory focus and temporal distance. Social Neuroscience. in press doi: 10.1080/17470910902750186. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Rimes KA, Watkins E. The effects of self-focused rumination on global negative self-judgments in depression. Behaviour Research and Therapy. 2005;43:1673–81. doi: 10.1016/j.brat.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural reactivity to positive social stimuli in pre and post-treatment depression. Biological Psychiatry. 2006;60:974–86. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–5. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings for the National Academy of Sciences of the United States of America. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Strack S, Blaney PH, Ganellen RJ, Coyne JC. Pessimistic self-preoccupation, performance deficits, and depression. Journal of Personality and Social Psychology. 1985;49:1076–85. doi: 10.1037//0022-3514.49.4.1076. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain- 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research. 2003;27:247–59. [Google Scholar]
- Vasic N, Walter H, Sambataro F, Wolf RC. Published online by Cambridge University Press, 10 October 2008. 2008. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. doi:10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. In: Laureys S, editor. Progress in Brain Research. Vol. 150. The Netherlands: Elsevier; 2005. pp. 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: effects of self-focus and analytic thinking. Journal of Abnormal Psychology. 2001;110:353–7. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Wegner DM, Roper DW. Depression and mental control: the resurgence of unwanted negative thoughts. Journal of Personality and Social Psychology. 1988;55:882–2. doi: 10.1037//0022-3514.55.6.882. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imageability of the future. Memory & Cognition. 1996;24:116–25. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Teasdale JD, Segal ZV, Soulsby J. Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. Journal of Abnormal Psychology. 2000;109:150–5. doi: 10.1037//0021-843x.109.1.150. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S-I, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain and Cognition. 2009;69:218–25. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]